Drinking Water Treatment Notes.docx
DRINKING WATER TREATMENT
Overview and Introduction
Ensuring the provision of safe and high-qualitydrinking water involves understanding and implementing various quality standards, treatment processes, and considerations based on the source of the water. Below is an in-depth exploration of these topics, drawing from authoritative sources such as "Water and Wastewater Engineering: Design Principles and Practice" by Mackenzie Davis and "Water Quality & Treatment: A Handbook on Drinking Water" by the American Water Works Association (AWWA).
Quality Requirements and Quality Aims of Drinking Water
Quality requirements refer to the mandatory standards that drinking water must meet to ensure safety and public health. These are often established by governmental or international regulatory bodies and specify maximum contaminant levels for various substances, including microorganisms, chemicals, and radionuclides. For instance, the U.S. Environmental Protection Agency (EPA) sets National Primary Drinking Water Regulations that enforce legally enforceable standards.
Quality aims, on the other hand, are aspirational targets set by water utilities or health organizations to achieve water quality that exceeds the basic regulatory requirements. These aims focus on optimizing water aesthetics (such as taste, odor, and color), minimizing the presence of emerging contaminants, and enhancing overall consumer satisfaction. While not legally binding, quality aims represent best practices and reflect a commitment to continuous improvement in water quality management.
Biological, Chemical, and Physical Treatment Processes
Drinking water treatment employs a combination of biological, chemical, and physical processes to remove contaminants:
Biological Processes: These involve the use of microorganisms to degrade organic matter and are more common in wastewater treatment. However, certain drinking water treatments, such as slow sand filtration, utilize biofilms to remove pathogens and organic compounds.
Chemical Processes: Chemical treatments include coagulation and disinfection. Coagulation involves adding chemicals (e.g., aluminum sulfate) to destabilize and aggregate suspended particles, facilitating their removal. Disinfection, typically achieved through chlorination, ozone, or ultraviolet light, aims to inactivate pathogenic microorganisms, ensuring the microbiological safety of the water.
Physical Processes: These processes physically remove contaminants and include sedimentation, filtration, and aeration. Sedimentation allows suspended particles to settle out of the water column. Filtration, through media such as sand or membranes, removes particulate matter and pathogens. Aeration introduces air into the water to expel dissolved gases (like radon) and oxidize dissolved metals (such as iron and manganese), facilitating their removal.
Typical Unit Processes Involved
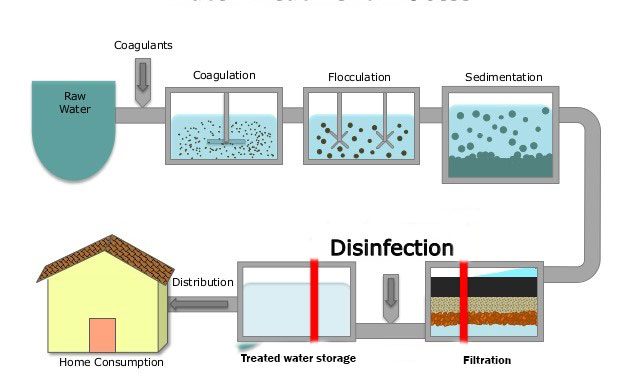
The treatment of drinking water typically involves a series of unit processes, each designed to address specific contaminants:
Screening: Removes large debris (e.g., sticks, leaves) from raw water sources.
Coagulation and Flocculation: Addition of coagulants to destabilize particles, followed by gentle mixing to form larger aggregates (flocs).
Sedimentation: Allows flocs to settle out, reducing turbidity.
Filtration: Passes water through filters (sand, gravel, or membranes) to remove remaining particles and microorganisms.
Disinfection: Applies chemical or physical agents to inactivate pathogens.
pH Adjustment: Modifies the water's pH to prevent corrosion in the distribution system and improve disinfection efficacy.
Fluoridation: In some regions, fluoride is added to prevent dental cavities.
Water Sources and Their Differences
The source of water significantly influences the treatment approach:
Groundwater: Typically has low turbidity and microbial contamination due to natural filtration through soil and rock. However, it may contain dissolved minerals like iron, manganese, or arsenic, necessitating specific removal processes.
Surface Water: Includes rivers, lakes, and reservoirs. It is more susceptible to contamination from runoff, industrial discharges, and microbial pathogens, often requiring comprehensive treatment, including coagulation, filtration, and disinfection.
Seawater: Characterized by high salinity, making desalination (via processes like reverse osmosis or distillation) essential. Desalination is energy-intensive and requires management of brine by-products.
Reclaimed Water: Treated wastewater intended for reuse. It undergoes advanced treatment processes, including microfiltration, reverse osmosis, and advanced oxidation, to ensure safety for applications such as irrigation or even potable use in some regions.
Water Treatment Plants
The design and operation of water treatment plants are influenced by several factors:
Water Characteristics: The quality and variability of the source water dictate the necessary treatment processes The specific characteristics of the raw water source influence the design and selection of treatment processes. Some of the key water characteristics include:
Turbidity and Suspended Solids
High turbidity (common in surface water) requires extensive pre-treatment, such as coagulation, flocculation, and sedimentation, before filtration. Low-turbidity groundwater may require minimal treatment, often only disinfection.
Microbial Contamination (Bacteria, Viruses, and Protozoa)
Surface water sources are more prone to microbial contamination and require comprehensive disinfection (chlorination, UV, ozone). Groundwater is typically less contaminated microbiologically, but in some cases (e.g., shallow wells or karst formations), disinfection is necessary.
Organic Matter and Natural Organic Material (NOM)
High organic content (from decayed vegetation, algae, etc.) can cause taste, odor, and disinfection by-product (DBP) formation when chlorine reacts with organics. Treatment includes activated carbon filtration, advanced oxidation, and enhanced coagulation.
Dissolved Minerals (Hardness, Iron, Manganese, Arsenic, Fluoride, etc.)
Hard water (high calcium & magnesium) requires softening processes. High iron & manganese need oxidation and filtration. Arsenic and fluoride require adsorption, ion exchange, or membrane filtration.
Salinity and Total Dissolved Solids (TDS)
Seawater has high salinity and requires desalination (reverse osmosis, distillation). Some brackish groundwater also requires desalination.
pH and Alkalinity
Extreme pH levels can lead to corrosion (low pH) or scaling (high pH). pH adjustment (lime addition, CO₂ stripping) may be necessary.
Temperature
Cold water slows down chemical reactions and biological activity, affecting coagulation, filtration, and disinfection efficiency. High temperatures can promote bacterial growth and impact taste/odor.
Factors Influencing Planning: Considerations include the population served, projected water demand, regulatory requirements, and future growth. The plant's capacity must accommodate peak usage and potential expansions. Longevity is addressed through the selection of durable materials and adaptable process designs. The planning of a water treatment plant is influenced by a combination of technical, economic, environmental, and regulatory factors.
Population and Water Demand
A WTP must be designed to meet current and future demand. Population growth projections help determine the required capacity. Seasonal fluctuations in water use (e.g., higher summer demand) must be considered.
Regulatory Standards and Compliance
Compliance with local and international drinking water quality standards (e.g., EPA, WHO, EU standards) determines treatment processes. Stringent regulations may necessitate advanced treatment technologies (e.g., membrane filtration, advanced oxidation).
Future Expansion and Scalability
Plants should be designed with flexibility for future expansion as populations grow. Modular designs allow additional treatment units to be added later.
Energy and Operational Costs
Energy-intensive processes (e.g., reverse osmosis, advanced oxidation) need careful consideration. Plants may incorporate energy recovery systems or use gravity-fed designs to reduce pumping costs.
Longevity and Infrastructure Durability
Materials used in construction (e.g., corrosion-resistant pipes, reinforced concrete) impact long-term maintenance. Well-maintained WTPs can last 50+ years, but some components (membranes, filters) need regular replacement.
Environmental Impact and Sustainability
Waste disposal (e.g., sludge from coagulation, brine from desalination) must be managed properly. Sustainable practices (e.g., rainwater harvesting, use of solar power) can reduce environmental impact.
Site Selection and Land Availability
The plant should be located close to the water source but away from flood-prone areas. Large WTPs need sufficient land for treatment units, storage, and expansion.
Impact of Water Sources on Plant Setup: The source dictates the treatment train. Each water source presents unique challenges and requires different treatment approaches.
Groundwater Treatment Plants
Generally require minimal treatment because groundwater is naturally filtered.
Key processes:
Aeration (to remove dissolved gases like hydrogen sulfide)
Iron and Manganese Removal (oxidation + filtration)
Softening (if hardness is high)
Disinfection (chlorination or UV)
Wellhead protection is critical to prevent contamination from agricultural runoff or industrial pollutants.
Surface Water Treatment Plants
Require extensive treatment due to higher turbidity and microbial contamination.
Key processes:
Coagulation, Flocculation, and Sedimentation (to remove suspended solids)
Filtration (sand filtration, membrane filtration)
Disinfection (chlorine, ozone, or UV)
Taste and Odor Control (activated carbon)
More infrastructure is needed for raw water intake, screening, and pre-treatment.
Seawater Desalination Plants
Designed to remove high levels of dissolved salts and contaminants.
Key processes:
Pre-treatment (coagulation, filtration) to remove particulates before desalination
Reverse Osmosis (RO) or Distillation (removes salt and dissolved solids)
Post-treatment (remineralization, pH adjustment) to make the water safe and palatable
Brine Disposal (managing the concentrated salt waste)
Desalination is energy-intensive and expensive but essential for coastal regions with limited freshwater resources.
Reclaimed Water Treatment Plants
Used to treat wastewater for reuse (non-potable or potable applications).
Key processes:
Primary and Secondary Treatment (similar to conventional wastewater treatment)
Tertiary Treatment (filtration, advanced oxidation) to remove remaining contaminants
Reverse Osmosis or Ultraviolet Disinfection (for high-purity applications)
Used in drought-prone areas for indirect potable reuse or direct potable reuse after advanced treatment..
Coagulation, Flocculation and Clarification
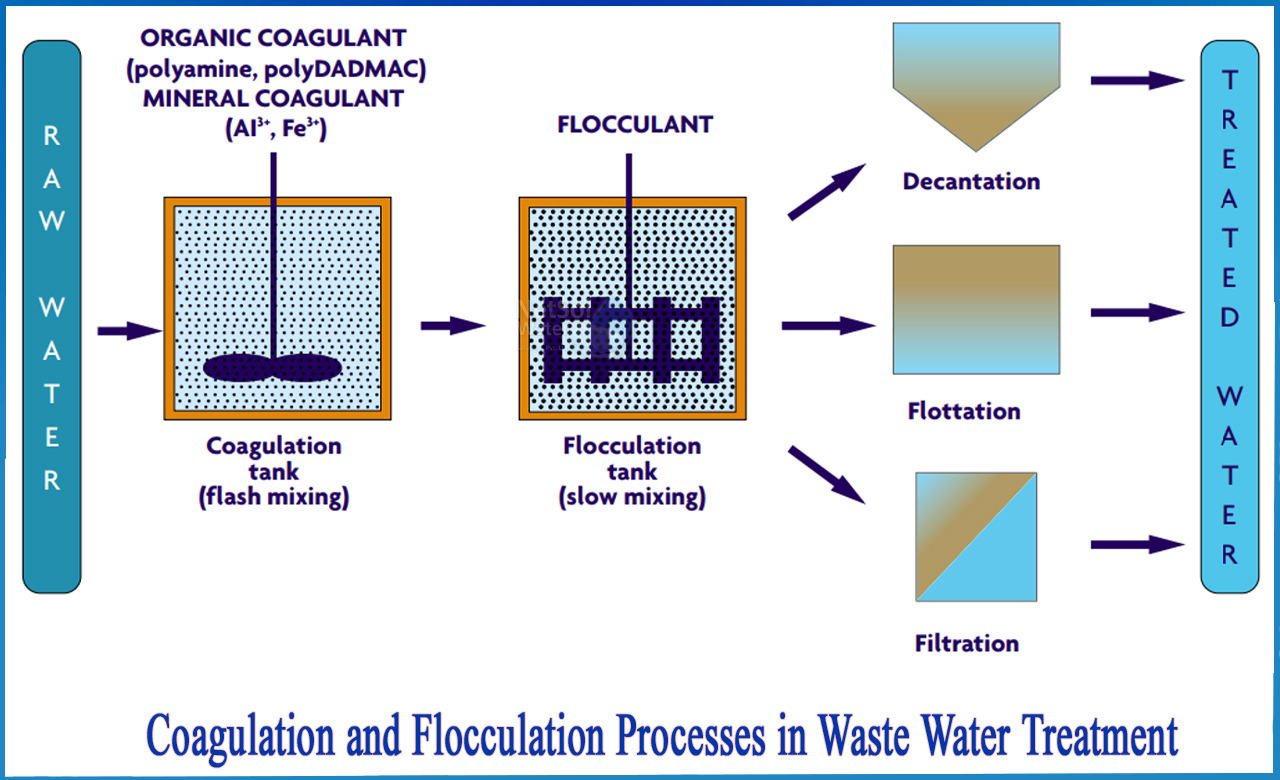
Coagulation, flocculation, and clarification are critical processes in water treatment, working sequentially to remove suspended particles and impurities, thereby enhancing water quality.
Coagulation
Coagulation is the initial step in water treatment aimed at destabilizing colloidal particles that are typically too small to settle out of suspension. These particles possess negative surface charges, causing them to repel each other and remain dispersed. The coagulation process involves adding chemicals, known as coagulants, to neutralize these charges, allowing particles to aggregate into larger formations.
Common Coagulants:
Aluminum Salts: Such as aluminum sulfate (alum).
Iron Salts: Including ferric sulfate and ferric chloride.
Polymers: Synthetic organic polymers that can enhance coagulation efficiency.
Mechanism:
Upon addition, coagulants dissolve and dissociate in water, releasing positively charged ions. These ions neutralize the negative charges on colloidal particles, reducing repulsive forces and promoting particle aggregation. Simultaneously, coagulants hydrolyze to form insoluble precipitates that can enmesh fine particles, further aiding in their removal.
Flocculation
Following coagulation, flocculation gently mixes the water to promote the formation of larger aggregates called flocs. This process involves slow stirring, enabling particles to collide and stick together, forming flocs that are heavy enough to settle out of the water. The efficiency of flocculation depends on factors like mixing intensity and duration, which must be carefully controlled to optimize floc formation without causing shear that breaks apart the flocs.
Process Details:
Gentle Mixing: Slow stirring encourages particle collisions without causing floc breakage.
Flocculant Addition: High-molecular-weight polymers (flocculants) may be introduced to bridge particles, strengthening floc structures.
Time and Energy: Optimal mixing intensity and duration are crucial to form robust flocs suitable for removal.
Clarification
Clarification encompasses processes that separate flocculated particles from water, resulting in clarified effluent. The primary methods include sedimentation and flotation.
Sedimentation:
Gravity Settling: Flocs, being denser than water, settle to the bottom of sedimentation basins over time.
Design Considerations: Basin dimensions and detention time are engineered to optimize particle settling.
Flotation:
Air Introduction: Fine air bubbles are dispersed in water, attaching to flocs and causing them to rise to the surface.
Surface Skimming: Accumulated flocs are removed from the water surface.
Application: Particularly effective for removing low-density particles or when treating waters with specific contaminants.
Study Questions
How does water quality change during coagulation and why? How are alkalinity and pH related to coagulation? Are there other parameters that change during coagulation?
During coagulation, the addition of coagulants neutralizes the charges on suspended particles, leading to their aggregation into larger flocs. This process reduces turbidity and removes color and organic matter from the water. The reaction of coagulants with water can consume alkalinity and lower pH levels; for instance, alum addition produces hydrogen ions, decreasing pH. Other parameters that may change include the concentration of dissolved metals introduced by the coagulant and the potential formation of by-products.
How is coagulant and its dose selected? How does water quality impact the selection?
Selecting an appropriate coagulant and its dosage involves considering the specific characteristics of the water, such as turbidity, pH, alkalinity, temperature, and the nature of suspended particles. Jar tests are commonly conducted to simulate treatment processes and determine the optimal type and amount of coagulant. Water with low turbidity may require lower coagulant doses, while waters with high organic content might need coagulants that effectively remove dissolved organic matter.
What are the reasons to apply coagulation (followed by flocculation) for groundwater treatment vs. for surface water treatment?
Surface waters often contain higher levels of suspended solids, organic matter, and microorganisms, necessitating coagulation and flocculation to remove these impurities. Groundwater, typically clearer with fewer suspended particles, may not require coagulation and flocculation unless it contains specific contaminants like iron, manganese, or color-causing organic compounds. In such cases, these processes help in precipitating and removing dissolved substances.
How does water quality change during flocculation and why?
During flocculation, the gentle mixing encourages the formation of larger flocs from the destabilized particles produced in coagulation. This results in increased particle size, making them more amenable to removal in subsequent clarification steps. While turbidity decreases as particles aggregate, the overall chemical composition of the water remains largely unchanged during flocculation.
What kind of structures and equipment are used in coagulation and flocculation processes? What are their purposes?
Coagulation typically employs rapid mix tanks or flash mixers, where coagulants are quickly and uniformly dispersed into the water to ensure immediate and even distribution. Flocculation involves flocculation basins equipped with slow mixers or paddle agitators that gently stir the water, promoting the aggregation of particles into flocs without causing them to break apart.
Why is it important to select the right velocity gradient and mixing time for flocculation and coagulation?
The velocity gradient (intensity of mixing) and mixing time are critical parameters that influence the effectiveness of coagulation and flocculation. In coagulation, rapid mixing with a high velocity gradient ensures the swift and uniform dispersion of the coagulant, facilitating effective particle destabilization. During flocculation, a lower velocity gradient and appropriate mixing time allow for gentle collisions between particles, promoting the formation of robust flocs. Incorrect mixing conditions can lead to inadequate floc formation or the breakup of flocs, reducing treatment efficiency.
How does water quality change during clarification and why?
Clarification significantly reduces turbidity by removing flocs formed during coagulation and flocculation. This process also decreases the concentrations of suspended solids and associated contaminants, such as pathogens and organic matter, resulting in clearer water. The removal of these impurities is primarily due to physical separation mechanisms like sedimentation or flotation.
What kind of structures and equipment are used in flotation and sedimentation processes? What are their purposes? How are flocs removed during clarification?
Sedimentation processes utilize settling tanks or clarifiers designed to provide a calm environment where flocs can settle to the bottom under gravity, forming sludge that is periodically removed. Flotation processes employ dissolved air flotation (DAF) units, where air is dissolved under pressure and then released to form fine bubbles that attach to flocs, causing them to rise to the surface. The floated material is then skimmed off. Both methods aim to separate flocs from the water, enhancing clarity and quality.
What can you tell about the reasons to select sedimentation vs. flotation?
Sedimentation is typically chosen when flocs are dense and settle easily under gravity. It is more effective for treating high-turbidity water with a significant amount of heavy suspended solids.
Flotation (especially Dissolved Air Flotation, DAF) is preferred when particles or flocs are lighter and do not settle efficiently. It is commonly used for waters containing algae, oil, grease, or natural organic matter, as well as in wastewater treatment.
The choice between sedimentation and flotation depends on raw water characteristics, the type of contaminants present, and the overall treatment objectives.
Aeration, Oxidation and Air Stripping
These three processes—aeration, oxidation, and air stripping—are widely used in water treatment to improve water quality by removing dissolved gases, oxidizing contaminants, and stripping volatile compounds.
Aeration
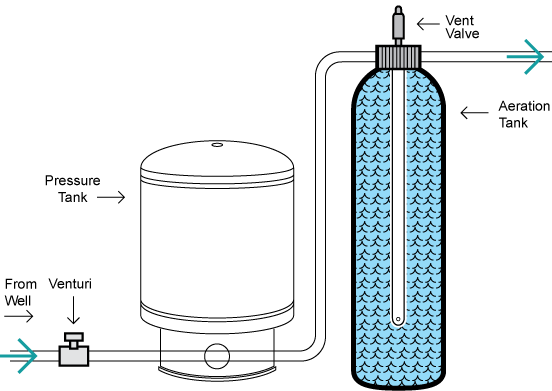
Definition and Purpose
Aeration is the process of bringing water and air into close contact to promote the transfer of gases between them. It is used primarily for:
Removing dissolved gases like carbon dioxide (CO₂), hydrogen sulfide (H₂S), and methane (CH₄).
Oxidizing dissolved iron (Fe²⁺) and manganese (Mn²⁺) to facilitate their removal.
Increasing dissolved oxygen (DO) levels to improve taste and odor.
Reducing volatile organic compounds (VOCs) and radon.
Types of Aeration Systems
Diffused Aeration
Air is introduced through fine bubbles using diffusers at the bottom of a tank. Provides efficient gas transfer but requires high energy input.
Cascade Aeration
Water is allowed to flow over steps or weirs, increasing contact with air. Effective for stripping gases like CO₂ and H₂S.
Tray Aerators
Water is distributed over perforated trays, increasing surface area exposure to air. Common in groundwater treatment for gas removal.
Spray Aeration
Water is sprayed into the air using nozzles, allowing gas exchange. Helps with radon and VOC removal.
Packed Tower Aerators
Uses packing media to enhance air-water interaction. Highly effective for removing volatile contaminants like trihalomethanes (THMs).
Oxidation
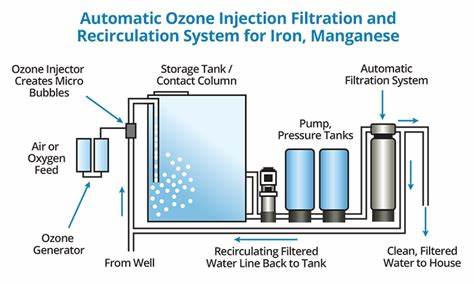
Definition and Purpose
Oxidation in water treatment refers to the addition of an oxidizing agent to chemically alter contaminants, making them easier to remove. It is used to:
Convert dissolved iron (Fe²⁺) and manganese (Mn²⁺) into precipitates that can be filtered out.
Break down organic matter (reducing disinfection byproduct formation).
Destroy pathogens and bacteria.
Remove taste, odor, and color compounds (e.g., hydrogen sulfide, algal toxins).
Common Oxidizing Agents
Oxygen (O₂) from Aeration
Used primarily for iron oxidation but is not strong enough for manganese oxidation.
Chlorine (Cl₂)
Used for disinfection and oxidation of iron, manganese, and hydrogen sulfide.
Forms disinfection byproducts (DBPs) with natural organic matter.
Ozone (O₃)
A very strong oxidant that breaks down organic matter and destroys microorganisms.
Used for advanced treatment and taste/odor control.
Potassium Permanganate (KMnO₄)
Excellent for manganese removal in addition to iron.
Often used with filtration systems.
Hydrogen Peroxide (H₂O₂)
Used in advanced oxidation processes (AOPs).
Often combined with UV for breaking down organic pollutants.
Air Stripping
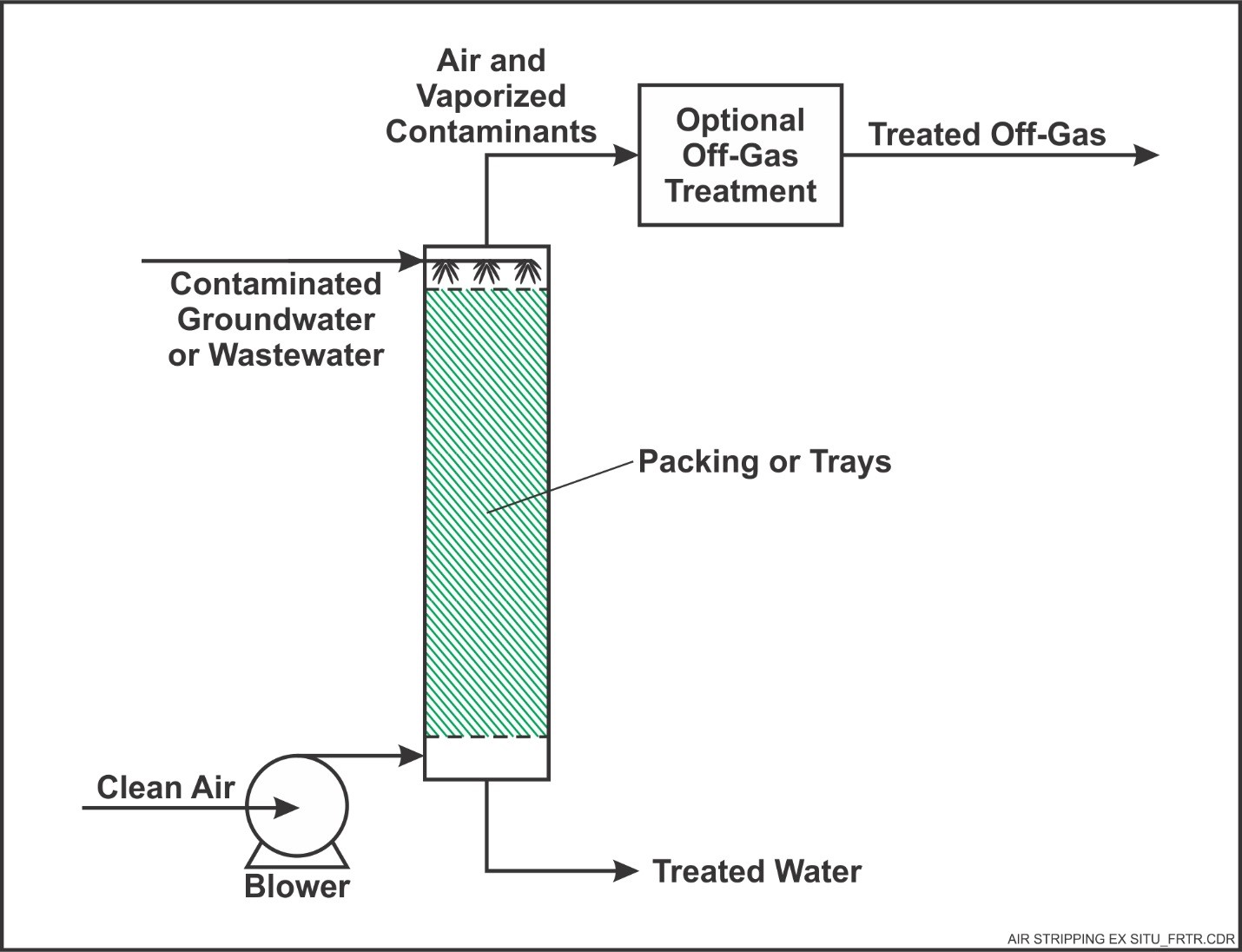
Definition and Purpose
Air stripping is a mass transfer process where volatile contaminants are removed from water by exposing it to air. It is effective for:
Removing volatile organic compounds (VOCs) such as benzene, trichloroethylene (TCE), and tetrachloroethylene (PCE).
Reducing ammonia levels in water.
Stripping radon and hydrogen sulfide.
Types of Air Stripping Systems
Packed Tower Air Strippers
Water flows down through a column filled with packing media while air is blown upward.
Provides high surface area for gas exchange.
Diffused Aeration Strippers
Air is bubbled through water to remove volatile compounds.
Less efficient for high VOC concentrations.
Spray Stripping Systems
Water is sprayed into the air, allowing volatile contaminants to escape.
Used in open or enclosed settings.
Study Questions
Why is ozone a more powerful oxidant than oxygen?
Ozone (O₃) is a stronger oxidant than oxygen (O₂) because it has a higher oxidation potential (+2.07V vs. +1.23V for oxygen). Ozone is unstable and readily breaks down into oxygen and reactive oxygen species, which aggressively react with contaminants. It reacts more quickly with organic and inorganic compounds, making it highly effective in advanced water treatment.
What factors influence oxidant consumption?
Water Quality (Presence of iron, manganese, organic matter, and microorganisms).
pH Levels (Higher pH can affect oxidation rates).
Temperature (Oxidation reactions generally proceed faster at higher temperatures).
Type of Oxidant Used (Chlorine, ozone, permanganate, etc.).
Contact Time and Mixing Conditions.
Why should manganese be oxidized with other oxidants and not oxygen, which is useful for iron?
Iron (Fe²⁺) can be oxidized by dissolved oxygen (O₂) alone, forming Fe(OH)₃ precipitate. Manganese (Mn²⁺) requires a stronger oxidant like chlorine, permanganate, or ozone because it forms more stable soluble complexes that do not readily oxidize with oxygen. If only oxygen is used, manganese oxidation is too slow and incomplete.
Why does Henry’s Law coefficient determine whether a compound can be removed by stripping?
Henry’s Law states that the solubility of a gas in liquid is proportional to its partial pressure in air. A compound with a high Henry’s Law constant (e.g., benzene, trichloroethylene) is more volatile and can be efficiently removed by air stripping. Low-volatility compounds (low Henry’s constant) remain in water and require alternative removal methods.
What is the role of packing in stripping columns?
Packing increases the surface area for air-water contact, improving the efficiency of gas transfer. It enhances mass transfer by creating turbulence, allowing better mixing between air and water. It prevents short-circuiting of water flow, ensuring uniform treatment.
What are the reasons to apply aeration, oxidation, and stripping for groundwater vs. surface water treatment?
Process | Groundwater Treatment | Surface Water Treatment |
Aeration | Removes CO₂, H₂S, and methane; raises DO levels | Primarily for taste and odor control, reducing volatile organics |
Oxidation | Oxidizes Fe²⁺ and Mn²⁺; disinfects if required | Disinfection, taste/odor control, removal of organic contaminants |
Stripping | Removes radon, VOCs, and dissolved gases | Reduces volatile disinfection byproducts (THMs), controls taste/odor |
Filtration and Adsorption
Filtration and adsorption are two critical processes in drinking water treatment. Filtration physically removes suspended solids, pathogens, and particulate matter, while adsorption removes dissolved organic and inorganic contaminants via surface interactions with an adsorbent material.
Filtration
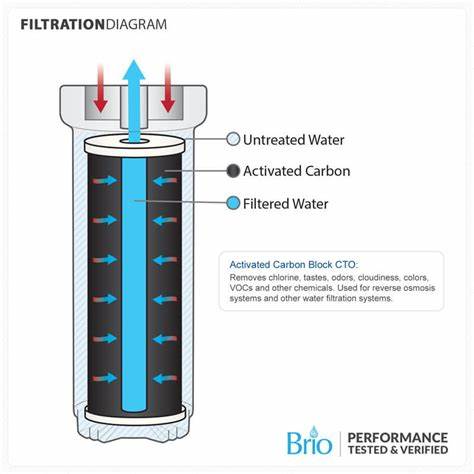
Definition and Purpose
Filtration is a physical separation process that removes suspended solids, microorganisms, and certain dissolved substances from water by passing it through a porous medium. It is one of the most commonly used unit processes in water treatment and is often applied after coagulation, flocculation, and sedimentation.
Types of Filtration Systems
Granular Media Filtration
Uses sand, anthracite, or granular activated carbon (GAC) as filter media.
Effective for removing turbidity, suspended solids, and some bacteria/protozoa.
Can be monomedia (single layer) or dual/multimedia (multiple layers of varying densities).
Slow Sand Filtration
Uses a thick bed of fine sand and relies on biological activity for contaminant removal.
Highly effective for microbial removal but has slow flow rates.
Rapid Sand Filtration
Uses a coarser sand bed with higher filtration rates.
Requires backwashing to clean the filter.
Membrane Filtration
Includes microfiltration (MF), ultrafiltration (UF), nanofiltration (NF), and reverse osmosis (RO).
Removes pathogens, dissolved salts, and fine particulates.
Pressure and Gravity Filters
Pressure filters operate under high pressure, commonly used in small water systems.
Gravity filters use natural water head pressure and are widely used in large-scale treatment plants.
Filter Media Types and Their Functions
Sand: Primary filtering medium for removing particulates.
Anthracite: Used in dual/multimedia filters to improve flow rates and trap larger particles.
Granular Activated Carbon (GAC): Provides both filtration and adsorption to remove organic compounds, taste, and odor compounds.
Membranes (RO, NF, UF): Remove dissolved salts, pathogens, and organic matter.
Adsorption
Definition and Purpose
Adsorption is a process where contaminants adhere to the surface of an adsorbent material rather than being absorbed into it. It is commonly used for removing:
Natural organic matter (NOM), disinfection byproducts (DBPs), and synthetic organic compounds (SOCs).
Taste and odor compounds like geosmin and MIB.
Heavy metals like arsenic and lead.
Types of Adsorption Systems
Granular Activated Carbon (GAC) Adsorption
GAC is used in filter beds or adsorption contactors.
Effective for removing organic contaminants and taste/odor compounds.
Requires periodic regeneration or replacement.
Powdered Activated Carbon (PAC) Adsorption
PAC is dosed directly into the water and removed during sedimentation or filtration.
Used as a temporary or emergency measure.
Ion Exchange Resins
Used to remove specific ions such as nitrate, sulfate, and heavy metals.
Advanced Adsorption Techniques
Zeolites, biochar, and other synthetic materials are being explored for water treatment.
Study Questions
What should water quality be like prior to granular filtration vs. GAC filtration? What do these filter types remove?
Granular filtration requires water with low turbidity and suspended solids, typically following coagulation, flocculation, and sedimentation.
Removes particulates, pathogens (Giardia, Cryptosporidium), and some organic matter.
GAC filtration works best with pre-treated water that has already been filtered for turbidity and particulates.
Removes organic contaminants, taste/odor compounds, and some disinfection byproducts.
Application for groundwater vs. surface water:
Groundwater: Less turbidity, so adsorption (GAC) is more common for removing dissolved contaminants.
Surface water: Typically requires both filtration and adsorption due to higher levels of turbidity and organics.
What is the advantage of multimedia/dual media filters over monomedia filters?
Dual or multimedia filters (e.g., anthracite over sand) provide better filtration efficiency by allowing water to penetrate deeper before clogging occurs.
They handle higher turbidity loads without excessive head loss.
Monomedia filters clog faster and require more frequent backwashing.
Why is the use of GAC in filter beds favored over dosing of PAC when necessary?
GAC has a longer contact time, allowing for more efficient contaminant removal.
PAC is dosed in bulk and removed quickly, making it less efficient and requiring higher doses.
GAC can be regenerated and reused, reducing long-term costs.
PAC is better for temporary or seasonal treatment, such as during algal blooms.
What are adsorption isotherms and what are they used for?
Adsorption isotherms describe how a solute interacts with an adsorbent under equilibrium conditions.
They are used to predict adsorption efficiency, optimize filter design, and determine breakthrough times.
Common models:
Langmuir Isotherm: Assumes monolayer adsorption on a uniform surface.
Freundlich Isotherm: Describes multilayer adsorption on heterogeneous surfaces.
How does operation of GAC filters differ from granular filters? What are filter components used and their purposes?
Filter Type | Operation | Components and Purposes |
Granular Media Filters | Removes particulates; requires frequent backwashing | - Sand/anthracite layers: Trap particles - Underdrains: Support filter media and allow for even flow distribution - Backwashing system: Cleans clogged filters |
GAC Filters | Adsorbs organic contaminants; requires periodic replacement | - GAC media: Adsorbs organics, tastes, and odors - Flow control valves: Maintain contact time - Regeneration system: Restores adsorption capacity |
pH, Alkalinity and Hardness Adjustment
Water treatment processes often require adjusting pH, alkalinity, and hardness to ensure treated water meets regulatory standards and remains non-corrosive or non-scaling. These adjustments are crucial for maintaining water quality, preventing pipe corrosion, and optimizing other treatment processes like coagulation, disinfection, and filtration.
pH Adjustment
Definition and Importance
pH is a measure of hydrogen ion (H⁺) concentration, indicating how acidic or basic water is. The pH scale ranges from 0 (highly acidic) to 14 (highly basic), with 7 being neutral.
Reasons for pH Adjustment
Preventing Corrosion and Scaling:
Low pH (<7) can make water corrosive, damaging pipes and leaching metals like lead and copper.
High pH (>8.5) can cause scaling due to calcium carbonate precipitation.
Optimizing Coagulation and Disinfection:
Coagulants like aluminum sulfate (Al₂(SO₄)₃) work best within a pH range of 5.5 to 7.5.
Chlorine disinfection is more effective at lower pH levels (6-7) due to the higher presence of hypochlorous acid (HOCl).
Enhancing Biological and Chemical Reactions:
Many biological and chemical processes (e.g., oxidation, adsorption) depend on a stable pH range.
Chemicals for pH Adjustment
To Increase pH (Neutralize Acidity):
Lime (Ca(OH)₂)
Soda ash (Na₂CO₃)
Caustic soda (NaOH)
To Decrease pH (Neutralize Alkalinity):
Carbon dioxide (CO₂)
Sulfuric acid (H₂SO₄)
Hydrochloric acid (HCl)
Alkalinity Adjustment
Definition and Importance
Alkalinity is the water’s capacity to neutralize acids, primarily controlled by the presence of bicarbonates (HCO₃⁻), carbonates (CO₃²⁻), and hydroxides (OH⁻). It acts as a buffer against pH changes, making water more stable.
Reasons for Alkalinity Adjustment
Ensuring pH Stability: Higher alkalinity helps resist rapid pH changes due to acidic or basic inputs.
Optimizing Coagulation and Flocculation: Coagulants consume alkalinity, so it must be adjusted to maintain effectiveness.
Preventing Corrosion and Scaling: Low alkalinity (<20 mg/L as CaCO₃) can lead to corrosion, while high alkalinity (>100 mg/L) can cause scaling.
Chemicals for Alkalinity Adjustment
To Increase Alkalinity:
Lime (Ca(OH)₂)
Sodium bicarbonate (NaHCO₃)
Soda ash (Na₂CO₃)
To Decrease Alkalinity:
Carbon dioxide (CO₂)
Sulfuric acid (H₂SO₄)
Hardness Adjustment
Definition and Importance
Hardness refers to the concentration of dissolved calcium (Ca²⁺) and magnesium (Mg²⁺) ions in water. It is classified as:
Soft Water: <60 mg/L as CaCO₃
Moderately Hard: 60–120 mg/L as CaCO₃
Hard: 120–180 mg/L as CaCO₃
Very Hard: >180 mg/L as CaCO₃
Reasons for Hardness Adjustment
Reducing Scaling in Pipes and Appliances: Hard water forms scale deposits in plumbing and water heaters.
Improving Soap and Detergent Efficiency: Hard water reduces soap’s ability to lather, leading to excessive use of detergents.
Preventing Corrosion: Some hardness is beneficial to form a protective layer inside pipes, reducing corrosion risks.
Chemicals for Hardness Adjustment
To Increase Hardness (Prevent Corrosion):
Calcium carbonate (CaCO₃)
Magnesium hydroxide (Mg(OH)₂)
To Decrease Hardness (Prevent Scaling):
Lime softening (Ca(OH)₂)
Soda ash (Na₂CO₃)
Ion exchange (using sodium-based resins)
Reverse osmosis (RO)
Study Questions
How do temperature and pH impact the speciation of inorganic carbon in water?
Inorganic carbon exists as CO₂, HCO₃⁻ (bicarbonate), and CO₃²⁻ (carbonate).
At low pH (<6): Carbon dioxide (CO₂) dominates.
At neutral pH (6-8.5): Bicarbonate (HCO₃⁻) is the main species.
At high pH (>9): Carbonate (CO₃²⁻) forms, which can precipitate as calcium carbonate (CaCO₃).
Higher temperatures reduce CO₂ solubility, leading to a shift towards HCO₃⁻ and CO₃²⁻.
Explain the difference between an open and closed system? Give examples of both.
Open System: Water is exposed to the atmosphere, allowing gas exchange (e.g., aeration tanks, rivers).
Closed System: No gas exchange occurs (e.g., pressurized pipes, deep groundwater aquifers).
Explain reasons to increase or decrease water hardness and alkalinity.
Increase Hardness/Alkalinity:
To prevent corrosion in soft, aggressive water.
To provide a protective mineral layer in pipes.
Decrease Hardness/Alkalinity:
To reduce scaling in industrial and domestic water systems.
To improve detergent efficiency in washing.
What chemicals are applied to increase alkalinity and hardness? What if the purpose would be to decrease these parameters?
To Increase:
Alkalinity: Lime (Ca(OH)₂), Soda ash (Na₂CO₃), Sodium bicarbonate (NaHCO₃)
Hardness: Calcium carbonate (CaCO₃), Magnesium hydroxide (Mg(OH)₂)
To Decrease:
Alkalinity: Carbon dioxide (CO₂), Sulfuric acid (H₂SO₄)
Hardness: Lime softening, Ion exchange, Reverse osmosis
How does the use of pH, alkalinity, and hardness adjustment chemicals differ in groundwater and surface water treatment?
Groundwater:
Often has higher hardness and alkalinity, requiring softening treatments.
pH adjustments may be needed if groundwater is acidic due to CO₂ presence.
Surface Water:
More variable pH, requiring buffering with alkalinity adjustments.
May need pH lowering for effective coagulation.
Disinfection
Disinfection is a critical process in drinking water treatment, aimed at eliminating or inactivating pathogenic microorganisms to ensure water safety. It is the final barrier against waterborne diseases such as cholera, typhoid, and cryptosporidiosis.
The effectiveness of disinfection depends on water quality, disinfectant type, contact time, and pathogen resistance. Disinfection also plays a role in preventing microbial regrowth in the distribution system.
Types of Disinfection Methods
Chemical Disinfection
Chemical disinfectants destroy microorganisms by damaging their cell membranes, enzymes, or genetic material (DNA/RNA).
Chlorination (Chlorine-based Disinfection)
Types of chlorine disinfectants
Free chlorine (hypochlorous acid, HOCl)
Chloramines (combined chlorine)
Chlorine dioxide (ClO₂)
Key Features:
Strong disinfection capability, effective against bacteria and viruses.
Provides residual protection in the distribution system.
Forms disinfection byproducts (DBPs), such as trihalomethanes (THMs) and haloacetic acids (HAAs).
Ozonation (O₃)
A powerful oxidant that destroys microorganisms by breaking down their cell walls.
More effective than chlorine for inactivating protozoa like Cryptosporidium and Giardia.
No residual disinfectant in the distribution system, requiring secondary disinfection.
Can lead to the formation of bromate (BrO₃⁻), a potential carcinogen.
Chlorine Dioxide (ClO₂)
Does not form THMs but can produce chlorite (ClO₂⁻) and chlorate (ClO₃⁻), which have regulatory limits.
Effective at lower doses than free chlorine.
Used as a primary disinfectant, but not for secondary disinfection.
Physical Disinfection
Physical methods kill or inactivate pathogens without adding chemicals.
Ultraviolet (UV) Disinfection
Uses UV light (wavelength 254 nm) to damage microbial DNA, preventing reproduction.
Effective against Cryptosporidium and Giardia, which are resistant to chlorine.
No residual disinfectant, requiring secondary disinfection.
Boiling
Used in emergency situations to destroy pathogens.
Not practical for large-scale water treatment.
Primary vs. Secondary Disinfection
Primary Disinfection
Aimed at initial pathogen removal/inactivation at the treatment plant.
Methods: Chlorination, Ozonation, UV, Chlorine Dioxide.
Effectiveness depends on disinfectant concentration, contact time, and water quality.
Secondary Disinfection
Maintains a disinfectant residual in the distribution system to prevent regrowth.
Methods: Chlorination (free chlorine), Chloramines.
Chloramines are preferred for long distribution networks as they decay slower than free chlorine.
Factors Affecting Disinfection Efficiency
Water Quality Parameters
Natural Organic Matter (NOM): Reacts with chlorine to form disinfection byproducts (DBPs).
pH: Affects chlorine efficiency (HOCl is most effective at pH 5-7).
Temperature: Higher temperatures improve reaction rates but increase DBP formation.
Turbidity: Protects microorganisms from disinfectants.
Ammonia: Reacts with chlorine to form chloramines, reducing free chlorine availability.
Disinfectant Contact Time (CT Concept)
Higher CT values improve pathogen inactivation.
Study Questions & Answers
Explain free chlorine, combined chlorine, and total chlorine.
Free Chlorine: The portion of chlorine available for disinfection (HOCl and OCl⁻).
Combined Chlorine: Chlorine bound with ammonia, forming chloramines, which are weaker but more stable.
Total Chlorine: The sum of free and combined chlorine.
Explain log survival ratio. How can it be calculated?
The log survival ratio represents the microbial inactivation level.
Formula:
Example: If water contains 10⁶ (1,000,000) bacteria before disinfection and 10 bacteria after, the log removal is:
Explain primary and secondary disinfection. Where are they used?
Primary Disinfection: Used at the treatment plant to eliminate pathogens (e.g., chlorine, ozone, UV).
Secondary Disinfection: Used in distribution networks to maintain residual protection (e.g., chloramines, free chlorine).
How can you apply Chick-Watson law in the design of disinfection facilities?
Chick-Watson Law: Describes microbial inactivation rate:
Where:
Nt is microorganism count after time t
N0 is the initial microorganism count
k is the decay constant
C is the disinfectant concentration
t is contact time
Importance: Knowing which pathogens are present helps in choosing the correct disinfectant and determining the required CT value.
What is the role of NOM in DBP formation? Compare disinfectants.
NOM reacts with chlorine to form DBPs (e.g., THMs, HAAs).
Comparison of DBP formation:
Chlorine: Forms THMs and HAAs.
Chlorine Dioxide: Produces chlorite and chlorate.
Ozone: May form bromate if bromide is present.
UV & Chloramines: Minimal DBP formation.
Which other water characteristics impact disinfection effectiveness?
Turbidity: Shields pathogens, reducing disinfection efficiency.
pH: Affects chlorine efficiency.
Temperature: Affects reaction rates and DBP formation.
Which disinfectants should be applied for secondary disinfection? What options exist for primary disinfection?
Secondary Disinfection:
Chloramines (preferred for long networks due to slow decay).
Free chlorine (effective but decays faster).
Primary Disinfection:
Chlorine, Ozone, UV, Chlorine Dioxide.
Compare disinfectant use in groundwater vs. surface water treatment.
Groundwater:
Often lower microbial contamination, so chlorine or chloramines are sufficient.
May need oxidation (e.g., chlorine for iron/manganese removal).
Surface Water:
Higher contamination → Requires stronger disinfection (e.g., ozone, UV).
More NOM → Higher risk of DBP formation.