Chapter 10- Chemical Calculations
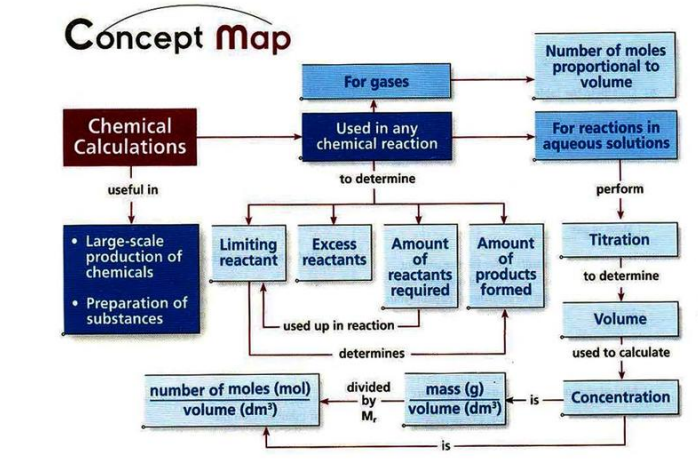
TITRATION
- The titrant is placed in the burette. Solution of unknown concentration is introduced in a conical flask using a pipette. A few drops of indicator is added to the conical flask.
- The titrant is allowed to react completely with the solution of unknown concentration.
- When the end-point is reached, the indicator permanently changes colour. This is when the titration stops.
- The concentration is then calculated.
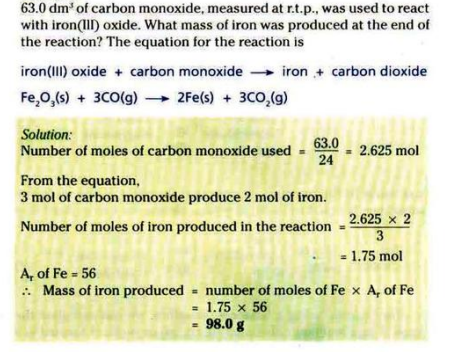
FORMULAE FOR CHEMICAL CALCULATIONS
\n