
5.Ethers
1. By dehydration of alcohols
Alcohols undergo dehydration in the presence of protic acids (H2SO4 , H3PO4 ).
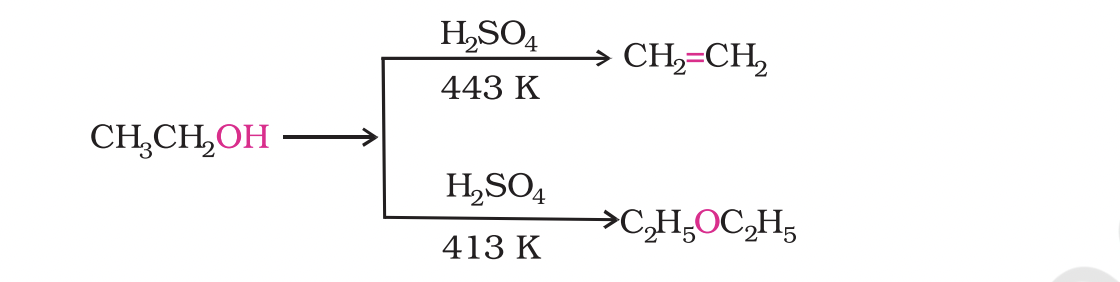
The formation of the reaction product, alkene or ether depends on the reaction conditions. For example, ethanol is dehydrated to ethene in the presence of sulphuric acid at 443 K.
At 413 K, ethoxyethane is the main product
The formation of ether is a nucleophilic bimolecular reaction (SN2) involving the attack of alcohol molecule on a protonated alcohol, as indicated below:
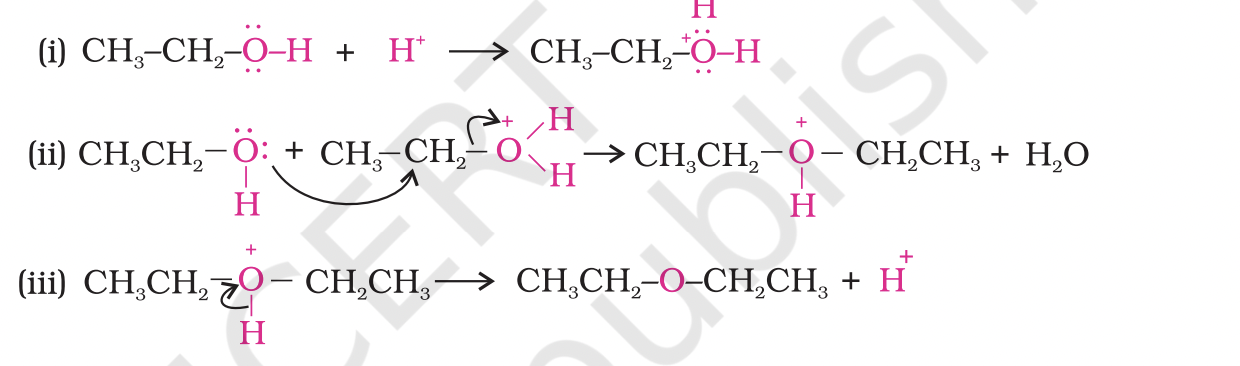
Acidic dehydration of alcohols, to give an alkene is also associated with substitution reaction to give an ether.
The method is suitable for the preparation of ethers having primary alkyl groups only.
The alkyl group should be unhindered and the temperature be kept low.
Otherwise the reaction favours the formation of alkene.
The reaction follows SN1 pathway when the alcohol is secondary or tertiary about which you will learn in higher classes.
However, the dehydration of secondary and tertiary alcohols to give corresponding ethers is unsuccessful as elimination competes over substitution and as a consequence, alkenes are easily formed.
2. Williamson synthesis
It is an important laboratory method for the preparation of symmetrical and unsymmetrical ethers.
In this method, an alkyl halide is allowed to react with sodium alkoxide.
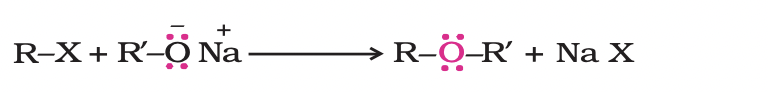
Ethers containing substituted alkyl groups (secondary or tertiary) may also be prepared by this method.
The reaction involves SN2 attack of an alkoxide ion on primary alkyl halide.
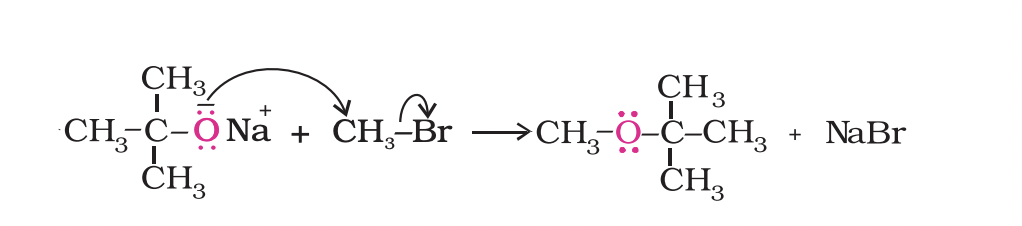
Better results are obtained if the alkyl halide is primary.
In case of secondary and tertiary alkyl halides, elimination competes over substitution.
If a tertiary alkyl halide is used, an alkene is the only reaction product and no ether is formed.
For example, the reaction of CH3ONa with (CH3)3C–Br gives exclusively 2-methylpropene.
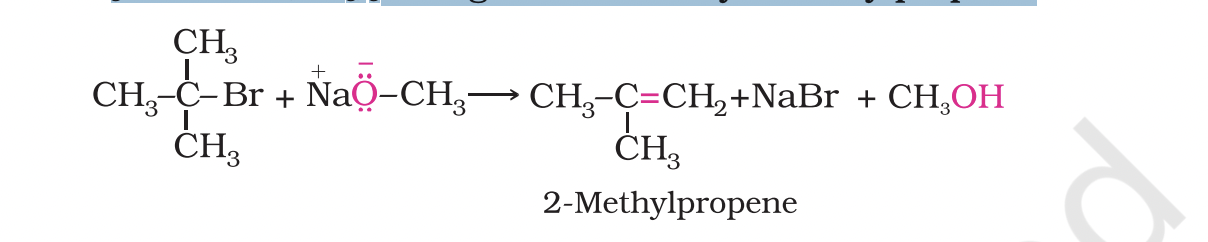 -
-It is because alkoxides are not only nucleophiles but strong bases as well.
They react with alkyl halides leading to elimination reactions.
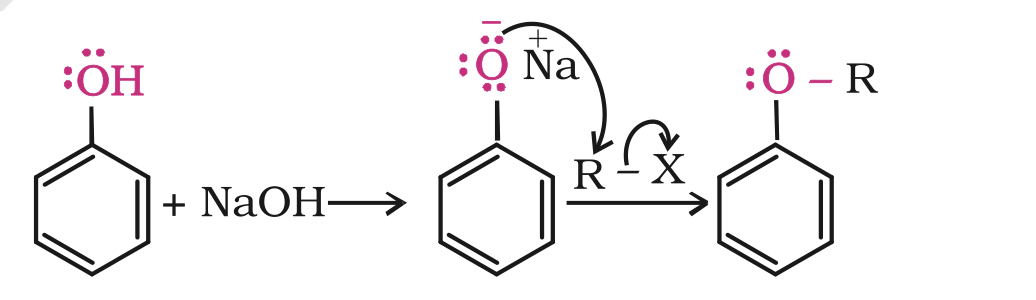
Phenols are also converted to ethers by this method. In this, phenol is used as the phenoxide moiety
5.Ethers
1. By dehydration of alcohols
Alcohols undergo dehydration in the presence of protic acids (H2SO4 , H3PO4 ).
The formation of the reaction product, alkene or ether depends on the reaction conditions. For example, ethanol is dehydrated to ethene in the presence of sulphuric acid at 443 K.
At 413 K, ethoxyethane is the main product

The formation of ether is a nucleophilic bimolecular reaction (SN2) involving the attack of alcohol molecule on a protonated alcohol, as indicated below:

Acidic dehydration of alcohols, to give an alkene is also associated with substitution reaction to give an ether.
The method is suitable for the preparation of ethers having primary alkyl groups only.
The alkyl group should be unhindered and the temperature be kept low.
Otherwise the reaction favours the formation of alkene.
The reaction follows SN1 pathway when the alcohol is secondary or tertiary about which you will learn in higher classes.
However, the dehydration of secondary and tertiary alcohols to give corresponding ethers is unsuccessful as elimination competes over substitution and as a consequence, alkenes are easily formed.
2. Williamson synthesis
It is an important laboratory method for the preparation of symmetrical and unsymmetrical ethers.
In this method, an alkyl halide is allowed to react with sodium alkoxide.
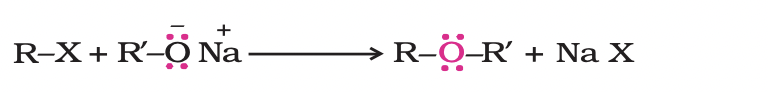
Ethers containing substituted alkyl groups (secondary or tertiary) may also be prepared by this method.
The reaction involves SN2 attack of an alkoxide ion on primary alkyl halide.
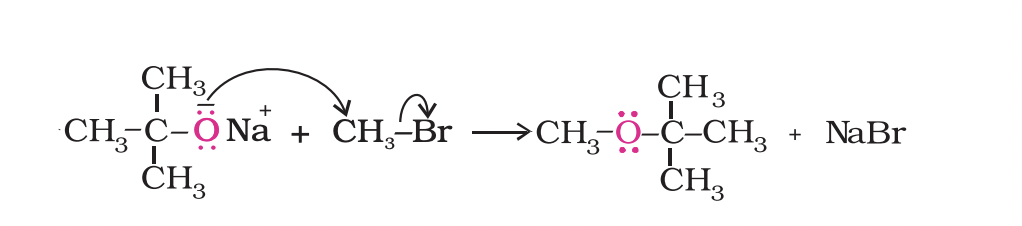
Better results are obtained if the alkyl halide is primary.
In case of secondary and tertiary alkyl halides, elimination competes over substitution.
If a tertiary alkyl halide is used, an alkene is the only reaction product and no ether is formed.
For example, the reaction of CH3ONa with (CH3)3C–Br gives exclusively 2-methylpropene.
 -
-It is because alkoxides are not only nucleophiles but strong bases as well.
They react with alkyl halides leading to elimination reactions.
Phenols are also converted to ethers by this method. In this, phenol is used as the phenoxide moiety

 Knowt
Knowt