THE RATE AND EXTENT OF CHEMICAL CHANGE
6.1 Rate of Reaction
Calculating Rates of Reactions
Rates of reactions can be measured using the amount of reactant used, or the amount of product formed over time.
Formulas for rate of reaction:
Rate of Reaction = Amount of Reactant Used / Time
Rate of Reaction = Amount of Product Formed / Time
Quantity of reactant or product can be measured by mass in grams or by volume in cm3.
Units of rate of reaction may be given as g/s or cm3/s.
You can also express the quantity of reactants or products in terms of moles (instead of mass or volume) and therefore, the units for the rate of reaction in this case would be mol/s.
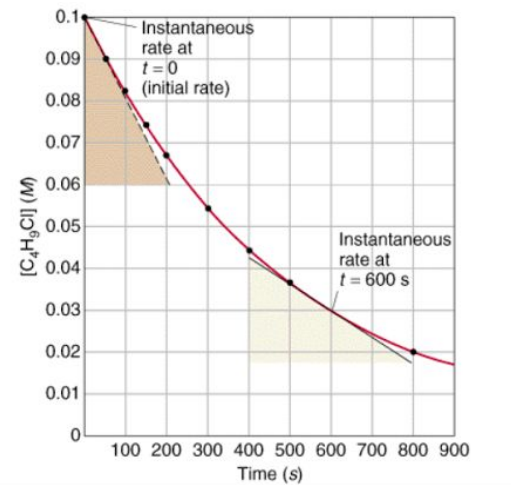
To find the rate of reaction graphically:
To measure the rate of reaction, draw tangents to curves and use the slope of the tangent.
Calculate the gradient of a tangent to the curve on these graphs as a measure of the rate of reaction at a specific time.
Factors Which Affect the Rates of Chemical Reactions
Factors:
Concentration
Pressure
Surface Area
Temperature
Catalysts
Collision Theory and Activation Theory
Collision Theory → Chemical reactions can occur only when reacting particles collide with each other and with sufficient energy.
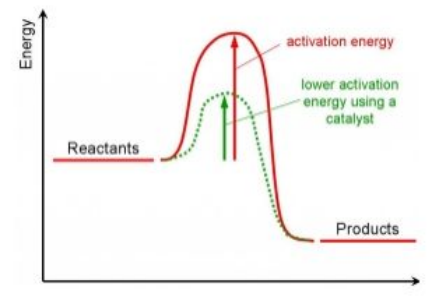
Activation Energy → The minimum amount of energy that particles must have to react.
Increasing the concentration of reactants in a solution, the pressure of reacting gases, and the surface area of solid reactants increases the frequency of collisions and so increases the rate of reaction.
Increasing the temperature increases the frequency of collisions and makes the collisions more energetic, and so increases the rate of reaction.
Catalysts
Catalysts → Substances that speed up the chemical reactions without being changed or used up during the reaction.
Enzymes act as catalysts in biological systems.
Catalysts are not included in the equation for a reaction.
Catalysts decrease the activation energy; this increases the proportion of particles with enough energy to react.
Catalysts provide a different pathway for a chemical reaction that has a lower activation energy.
6.2 Reversible Reactions and Dynamic Equilibrium
Reversible Reactions
In some chemical reactions, the products of the reaction can react to produce the original reactants.
These are called reversible reactions.
The direction of the reaction can be changed by changing the conditions.
E.g. In some cases (see below), in order to favor the forward reaction, you need to use hot conditions, and conversely to favor the reverse reaction you need to use cool conditions.
Use the symbol ⇌ instead of → to represent a reversible reaction.
E.g. The Haber Process: Hydrogen + Nitrogen ⇌ Ammonia (heat is released)
Energy Changes and Reversible Reactions
If a reversible reaction is endothermic in one direction, it is exothermic in the opposite direction.
The same amount of energy is transferred in each direction.
Just for one way, the energy will be lost and for the other, the same amount will be gained.
Equilibrium
When a reversible reaction occurs in a closed system, equilibrium is reached, when the reactions occur at exactly the same rate in both directions.
The Effect of Changing Conditions on Equilibrium
The relative amounts of all reacting substances at equilibrium depend on the conditions of the reaction.
If a system is at equilibrium and a change is made to any of the conditions, then the system responds to counteract the change (Le Chatelier’s principle).
The Effect of Changing Concentration
If the concentration of one of the reactants or products is changed, the system is no longer at equilibrium, and the concentrations of all the substances will change until equilibrium is reached again.
If concentration of reactants is increased: the position of equilibrium shifts towards products so more product is produced until equilibrium is reached again.
If concentration of products is increased: the position of equilibrium shifts towards reactants so more reactant is produced until equilibrium is reached again.
The Effect of Temperature Changes on Equilibrium
If temperature is increased: the equilibrium moves in the direction of the endothermic reaction.
E.g. if the forward reaction is endothermic and temperature is increased, equilibrium shifts right to produce more product.
If temperature is decreased: the equilibrium moves in the direction of the exothermic reaction.
The table below summarizes how temperature changes affect the the yield (amount of products produced) of a particular reaction, depending on whether the forward reaction is exothermic or endothermic:
Exothermic | Endothermic | |
|---|---|---|
An increase in temperature… | Decreases yield of reaction | Increases yield of reaction |
A decrease in temperature… | Increases yield of reaction | Decreases yield of reaction |
The Effect of Pressure Changes on Equilibrium
In gaseous reactions, an increase in pressure will favor the reaction that produces the least number of molecules as shown by the symbol equation for that reaction.
If pressure is increased: equilibrium shifts to side of equation with fewer moles of gas (e.g. N2 + 3H2 ⇌ 2NH3, left side has 4 moles of gas (1+3) and right has 2 moles of gas. If you increase the pressure equilibrium moves right as there are fewer moles of gas on the right hand side, making more product).
If pressure is decreased: equilibrium will shift to the side of the equation with more moles of gas (e.g. for previous example equilibrium would move left, making more reactants).
If a reaction produces a… | …larger volume of gas (more moles) | …smaller volume of gas (fewer moles) |
|---|---|---|
An increase in pressure… | Decreases yield of reaction | Increases yield of reaction |
A decrease in pressure… | Increases yield of reaction | Decreases yield of reaction |