Electron Configuration
Bohr Model
Electrons live in “shells” that revolve around the nucleus of the atom
The first shell can hold 2 electrons
Every subsequent shell can hold 8 electrons
The last shell is known as the valence shell and is involved with all chemical reactions (bonds form by the sharing of electrons)
Heisenberg Uncertainty Principle
In reality, electrons are going everywhere — we don’t know where electrons are, and if they are in neat shells
states that it’s near impossible to calculate both the position and velocity of a particle at the same moment in time
Suborbitals
Orbitals are clouds of probability where an electron might be found → an area where they are they may be
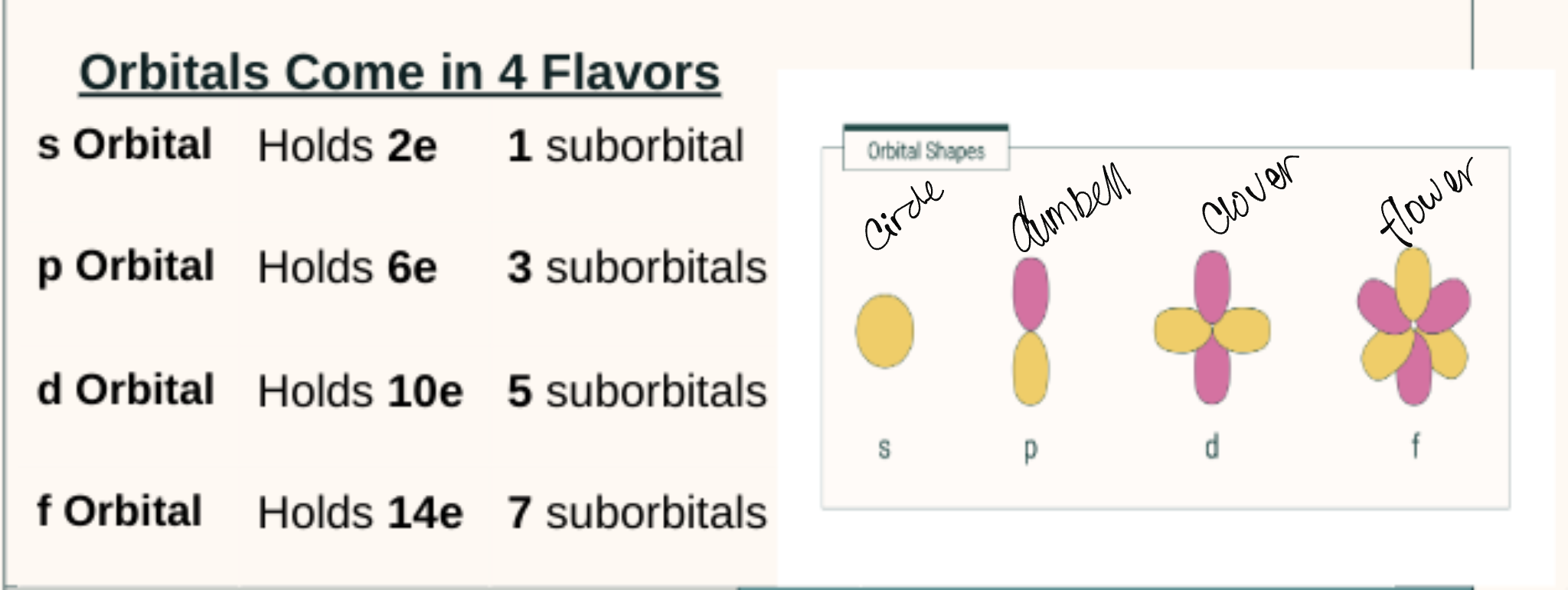
but also arranged by the different levels of energy
Rules of Suborbitals
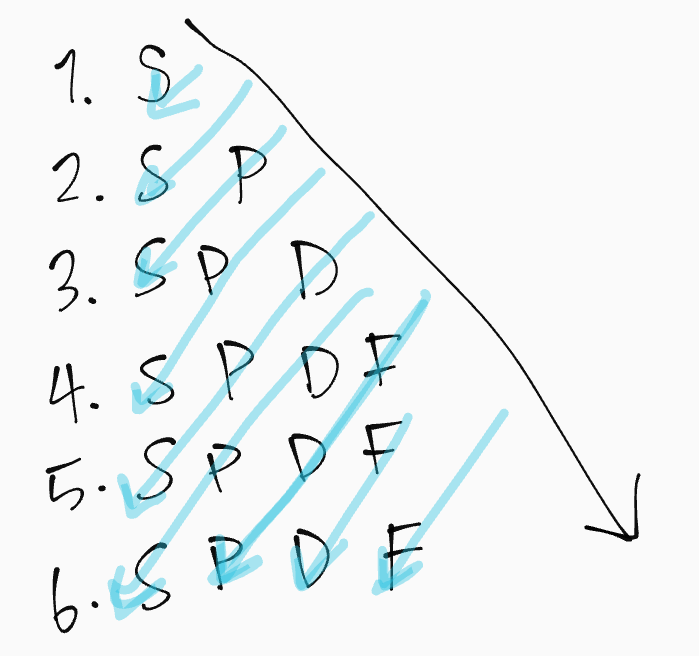
Aufbau Principle (build up)
Electrons fill the lowest energy level and orbital first, and then “build” their way up
Pauli Exclusion Principle
Each suborbital can hold a maximum of 2 electrons
Each electron is typically denoted with an up or a down arrow
A full orbital will have one up, and one down arrow
Hund’s Principle
Each suborbital in a energy level is filled with one electron, before any other orbital is filled
Typically, the first electron is shown as an up arrow (↑) and the second electron with a down arrow (↓)
a down arrow MUST be paired with a up arrow beforehand
start pairing them if you run out of boxes, ensuring that all electrons are accounted for in the correct order according to Hund's rule.
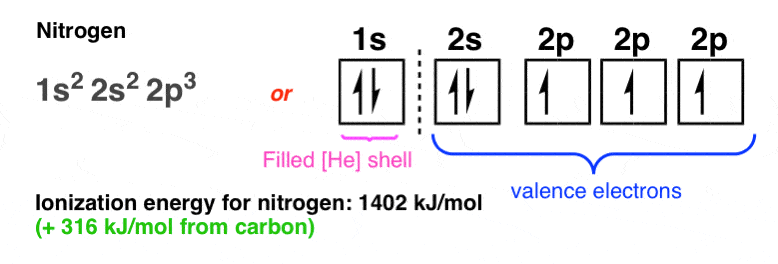
Naming
To name the electron configuration, list every energy level, every orbital, and how many electrons are in each orbital
Shorthand
For writing the notation for longer molecules — use the noble gas method
Instead of writing out the full electron configuration, we can instead list the most recent noble gas, and then the following orbitals
1s2 2s2 2p6 3s2 3p6 3d10 4s2 4p6 4d10 5s2 5p6 6s2 becomes…
[Xe] 6s2