CHEMISTRY: THE MATHEMATICS OF FORMULAS AND EQUATIONS(1)
Formula Mass
Since compounds are represented by formulas, the mass of the smallest unit of the compound is the formula mass, which is the sum of the atomic masses of all the atoms present.
Formula mass can be calculated for a formula unit of the compound.
Gram formula Mass of a substance = formula mass expressed in grams
Finding Formula Mass
Example problem: What is the formula mass of K₂CO₃?
To find formula mass we want to determine the number of atoms of each element from the formula
Element | Number of atoms |
|---|---|
K | 2 |
C | 1 |
O | 3 |
Refer to the periodic table for atomic mass of each element, and multiply it by the number of atoms. This will lead us to find total mass for each element.
K : 2 x 39.1 amu = 78.2 amu
C: 1 x 12.0 amu = 12.0 amu
O: 3 x 16.0 amu = 48.0 amu
Add the total mass of each element to find formula mass.
78.2 + 12.0 + 48.0 = 138.2 amu
Percentage Composition
Formulas represent the composition of a substance.
The percentage composition of a substance represents the composition as a percentage of each element compared with the total mass of the compound.
Finding percentage Mass
sample problem: What is the percentage of oxygen in K₂CO₃?
Determine the Formula mass of K₂CO₃
K : 2 x 39.1 amu = 78.2 amu
C: 1 x 12.0 amu = 12.0 amu
O: 3 x 16.0 amu = 48.0 amu
78.2 + 12 + 48 = 138.2 amu
Divide the mass of oxygen by the formula mass and then multiply by a 100%
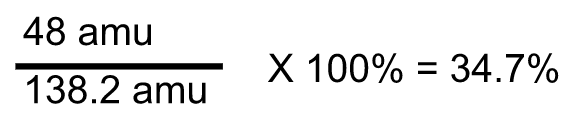 Our percentage of oxygen in K₂CO₃ is 34.7%
Our percentage of oxygen in K₂CO₃ is 34.7%
Mole
Chemists use a specific collective noun to define a particular usable number of particles.
The number 6.022 x 10^23 is called Avogadro's number. This is used to define the number of particles in a mole of a substance.
Although it is impossible to count a mole of particles, the mass of one mole of a substance can be found by determining its gram formula mass. This quantity contains 6.02 x 10^23 power particles of a substance.
Therefore the gram formula mass of any substance is the mass of one mole of that substance.
Abbreviation for mole is mol.
Converting grams to moles
Moles = number of grams x 1 mol/gram formula mass
Converting moles to grams
grams = number of moles x gram formula mass/1 mol
Finding grams of moles
How many grams are present in 40.5 mol of sulfuric acid(H₂SO₄)?
Calculate the formula mass of sulfuric acid
H: 2 atoms x 1 amu/atom = 2 amu
S: 1 atom x 32.1 amu/atom = 32.1 amu
O: 4 atoms x 16 amu/atom = 64 amu
Formula mass = 98.1 amu
Convert the formula to grams
98.1 g
Use the gram formula mass to convert the given number of moles to grams
Number of grams = moles x gram formula mass/1 mol
Grams H₂SO₄ = 40.5 mol x 98.1g/1 mol
Grams H₂SO₄ = 3970 g