Immunology exam 1 notes
Fetal and Neonatal immunity
Uterus is an Immune privileged site
fetus and mother are partial MHC mismatch, suggesting the gravid uterus is immune privileged site.
Physical separation of maternal and fetal tissues
Antigenic immaturity of the fetus
Immune tolerance
Maternal immunosuppression induced by pregnancy hormones and seminal fluid
Physical separation of fetal and maternal circulation
(F) fetal endothelium
(C) chorionic epithelium
(E) Endometrial epithelium
(M) Maternal endothelium
Epitheliochorial
Pig, horse, cow
F, C, E, M
Endotheliiochorial
Dog, cat
F, C, M
Hemochorial
F, C
In hemochorial placentation, maternal immune cells are in direct contact with fetal tissue
Maternal immune tolerance
CD4+ regulatory T cells (Treg)
Accumulate in decidua and elevated in maternal circulation during pregnancy
Treg insufficiency or dysfunction associated with infertility, miscarriage.
Placental MHC class I expression
mainly primates
CD8+ T cells
FasL expression
induces apoptosis of activated lymphocytes
Factors capable of inducing apoptosis in immune cells that reach conceptus/fetus
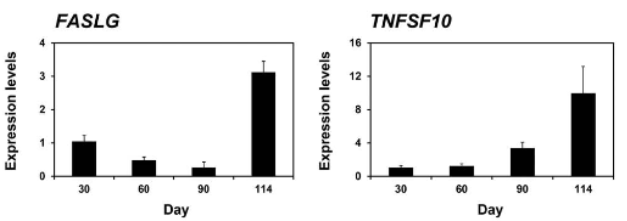
Expression in conceptus/fetus
FasLG
TNFSF10
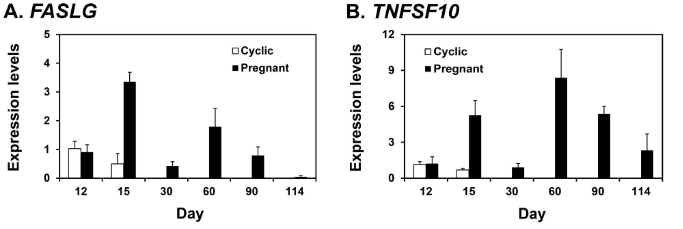
Expression in endometrium
FasLG
TNFSF10
Fetal immune Development
Thymus is the first lymphatic organ to develop
B cells appear after development of spleen and lymph nodes
Antibodies appear in late fetal, or early postnatal period.
Gradual increase in the use of gene conversion or somatic mutation to increase antibody diversity
Cytokine production low in the fetus and neonate.
Immune response to Intrauterine infection
Infections that may be mild or inapparent in the mother, may be severe or lethal in the fetus
Blue tongue, BHV-1, BVD
In general, the response to these viruses is determined by the state of immunological development of the fetus
Neonatal Immunity dependent on rate of immune development
Longer gestation = more responsive at birth
Domestic species
acquired immune system fully developed at birth, but naive. Thus, immune response is slow to respond and not fully functional until several weeks of age.
Neonatal Immunity
All responses are primary; no memory responses when born
Neonatal immune system development stimulated by intestinal microbiota
Neonatal immune response skewed toward Th2 response
result of pregnancy hormones?
Newborns vulnerable to infections for first few weeks of life
protection provided by passive immunity
Passive Immunity
Maternal Antibody Transfer
colostrum
Maternal Ig crossover through placenta
Short-term immunity which results from the introduction of relevant antibodies from another animal
Colostrum
“first milk”
mammary secretions accumulated over the last few weeks of pregnancy
Rich in IgG, IgM, IgA
Provides humoral immunity (passive immunity) to neonate
contains immune cells
contains cytokines
Colostrum - Mechanism of Entry
Absorption
newborn intestine has low protease activity
colostrum has trypsin inhibitors
Newborn enterocytes have receptors (FcRn) that bind colostral antibody
Antibody endocytosed and absorbed into peripheral circulation
Time-dependent absorption of colostral antibodies
Highest Ab uptake first 6 hours of life
Negligible uptake after 24 hours = gut closure
Exact time of gut closure varies, depending on feeding
Feeding colostrum speeds up gut closure
Delayed feeding colostrum slows down gut closure
Predominant immunoglobulin in colostrum and milk varies by host species
Host
colostrum
Milk
Ruminants
IgG
IgG
Monogastrics
IgG
IgA
Primates
IgA
IgA
Don’t forget that IgG, IgM and IgA are ALL present in colostrum
IgG transfer (and reliance on colostrum) varies according to placental type
IgG transfer (not IgM, IgA, or IgE)
Placental Type
In utero
Colostrum
Species
Epitheliochorial
None
100%
Horse, pig, cow, sheep
Endotheliochorial
5 - 10%
90 - 95%
Dog, cat
Hemochorial
90%
10%
Primates, rodents
Situations that you might encounter
Failure of Passive Transfer (FPT)
Neonatal Isoerythrolysis (NI)
Neonatal sepsis
Failure of Passive Transfer (FPT)
Affected: calves and foals
Cause: insufficient absorption of maternal antibodies
Quality, quantity, and timing all contribute
Clinical presentation: failure to thrive, weakness, recurrent infection
Prevention: ensure adequate ingestion of high-quality colostrum ASAP after birth
38% incidence of FPT (Negative test result)
Increased disease incidence and poorer outcomes in cases of FPT
Neonatal Isoerythrolysis
Affected: Foals
Pathogenesis
Dam sensitized to foreign RBC antigens from sire (Aa, Qa) and alloantibodies produced
Antibodies concentrated in colostrum
If ingested by foal prior to gut closure, antibodies will target and lyse RBCs
More common in multiparous mares
Diagnosis
circumstantial, indirect coombs test is definitive
Treatment
Supportive care and alternative source of nutrition for foal, strip mare of colostrum; transfusion if PCV <12% using washed RBCs from mare
Neonatal Isoerythrolysis
Affected: CAts
Pathogenesis
Dam produces alloantibodies to RBC antigens from sire
Queen (blood type B) have naturally occurring ABs to type A antigens
Queen is multiparous or had unmatched transfusion
If ingested by kittens prior to gut closure, antibodies will target and lyse RBCs
Diagnosis
Circumstantial, indirect Coombs test is definitive
Treatment
Supportive care and alternative source of nutrition
Neonatal/early life infection - exemplars
Feline Neonatal Sepsis
Wide variety of opportunities
E. coli, Klebsiella, Pseudomonas, Streptococcus, Enterobacter, Salmonella)
Secondary to other factors
poor nutrition, parasitism, heritable defects in immunity
Equine Neonatal sepsis
Wide variety of opportunists
E.coli, Klebsiella, Pseudomonas, streptococcus, Enterobacter, Salmonella)
Secondary to other factors
inadequate or poor quality colostrum, prematurity, dirty environment
Canine Neonatal Sepsis
14.8% incidence with 25.6% mortality among affected
75% occur during first week of life
Wide variety of opportunists
E. coli, Staphylococcus, Streptococus)
Often secondary to umbilical trauma, but other routes of infection possible.
Bovine Neonatal Sepsis
Wide variety of opportunists
E. coli, Klebsiella, Campylobacter, Salmonella
Often secondary to FPT
Often occurs via gastrointestinal exposure
Critical Concepts
Fetal immune system is immature and fetal environment is protective, but immunosuppressed
Result of in utero viral infection depends on timing and viral pathogenicity
Degree of passive transfer of IgG in utero (and dependence on colostrum) varies according to placentation type.
Neonatal immune system is immature, resulting in “immunity gap” as passive immunity wanes.
Bacterial infections during neonatal period are primarily opportunistic infections due to predisposing factors
Immune Response to Microbes - Intro and extracellular bacteria
Why do we have an immune system?
The principal physiologic function of the immune system is defense against infectious microbes.
The principal function of the veterinarian in defense against microbes is to take advantage of and aid the immune system.
Who are the enemies
Parasites
protozoa
Helminths
Ectoparasites
Intracellular bacteria and fungi
Extracellular Bacteria
Viruses
What do we have in our immune arsenal? ****
Physical barriers
skin/epithelium
mucus
peristalsis
“Immune” cells
Neutrophils, eosinophils
Mast cells
Monocytes/Macrophages
Dendritic cells (DCs)
NK cells/Innate lymphoid cells (ILCs)
T cells
B cells
Epithelial and other cells
Cell products
Complement
Antibody
Cytokines
The pathogen’s perspective
Make contact
skin, mucosal surfaces, tissue, blood
Colonize and replicate
Infect = disease
Breach physical and innate barriers
Spread or remain localized and cause damage
Some release products (toxins)
Establish extracellular or intracellular niche)
Immunity to infection
Defense is mediated by both innate and adaptive immune mechanisms ***
Somewhat artificial separation
not completely sequential
Different types of microbes stimulate distinct responses and effector mechanisms ***
efficient use of complex system
Oversimplification of immune repertoire ***
Innate
physical barrier front line
Innate response (macrophages, granulocytes, NK cells, complement, etc.) - killing
DCs (innate) present antigen to T cells (adaptive)
Adaptive
T cells kill and/or help innate and B cells
Antibody has direct effects and supports innate
Cytokines (innate and adaptive) used to communicate
All are recruited to where they need to be
lymph node (production) or site of infection (action)
chemokines and adhesion molecule interactions.
Goal is clearance or at least containment
Response
Action
Innate (0 - 4h)
Microbe removal
second exposure - more efficient due to memory
Early induced (4 - 96h)
Effector cell recognition, activation
microbe removal
Late adaptive (>96h)
Naive T and B cell recognition, activation
clonal expansion, differentiation
microbe removal
Protective immunity
preformed T and B cell recognition, activation
microbe removal
Memory
Memory T and B cell recognition
rapid clonal expansion, differentiation
Microbe removal
Why do we have lymph nodes? (spatial response)
Adherence to epithelium
local infection, penetration of epithelium
local infection of tissues
lymphatic spread
adaptive immunity
Protection against infection
Normal flora and local chemical factors inhibit microbial growth phagocytes activated (especially in lung)
Wound healing induced antimicrobial proteins and peptides, phagocytes, and complement destroy invading microorganisms.
Complement activation, dendritic cells migrate to lymph nodes, phagocytes action, NK cells activated, cytokines and chemokines produced.
Pathogens trapped and phagocytosed in lymphoid tissue. Adaptive immunity initiated by migrating dendritic cells
Infection cleared by specific antibody, T-cell dependent macrophage activation and cytotoxic T cells

Immunity to extracellular bacteria
mucosal spaces, circulation, tissue spaces
Physical defenses have been breached
Disease
Tissue destruction
endotoxins (LPS)
Exotoxins
Adhesion/Effacement
Inflammation
Non-specific defense
Physical ***
skin
mucociliary system
Intestinal motility
Temperature
Chemical/enzymatic
lysozyme
pH
Iron competition
Defensins
Histamine
AA metabolites
Clotting cascade
Kinin System
Complement
Innate immunity to extracellular bacteria
Immediate
components
neutrophils, monocytes/macrophages, DCs and NK cells/ILCs
Cooperation with adaptive critical ***
SCID - control many infections with innate system, highlighting the latter’s importance, however, can’t always sterilize
Neutrophils first line ***
Macrophage phagocytosis and activation
Opsonization (via ab, complement fragments) increases activation over 1000-fold ***
cytokine production
stimulates inflammation
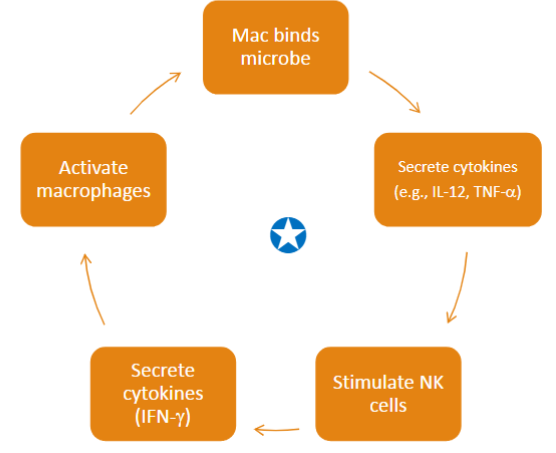
**** repeats
Mac binds microbe
Secrete cytokines (IL-12, TNF-a)
Stimulates NK cells
Secrete cytokines (IFN-y)
Activate macrophages
Complement
Alternative and mannose pathways
Stimulate inflammation (C3a, C5a) ****
Opsonins (C3b, C4b) ****
Inflammation/vascular changes
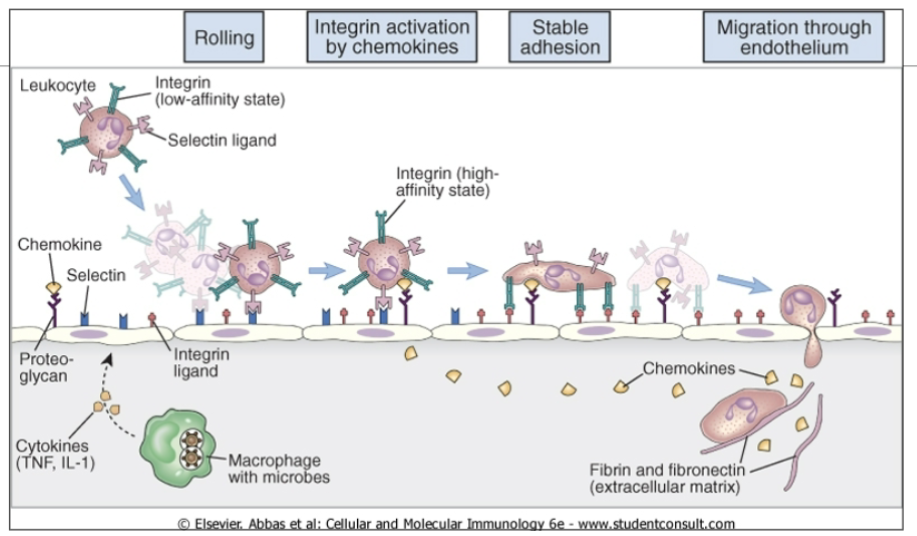
recruit more immune cells ***
With inflammation, adhesion molecules (E and P selections and VCAM-1) upregulated on endothelium
More neuts and monos attracted
monos —> macs — > activated macs
chemokines also recruit
Innate response proceeds
Leukocyte migration
Tissue Dendritic Cells (DCs)
DC recognition of bacteria
Toll-like, mannose, scavenger receptors, etc
Take up antigens
Increased expression of MHCII and co-stimulatory molecules
Then what?
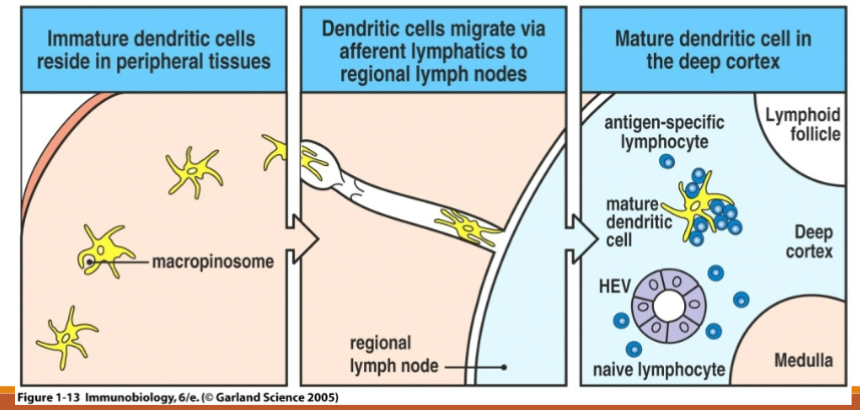
Antigen presentation to adaptive
Need to find specific T cells so travel to lymph node (adhesion molecule and chemokine mediated) ****
Immature dendritic cells reside in peripheral tissues
Dendritic cells migrate via afferent lymphatics to regional lymph nodes
Mature dendritic cell in the deep cortex
Hinge for dendritic cells
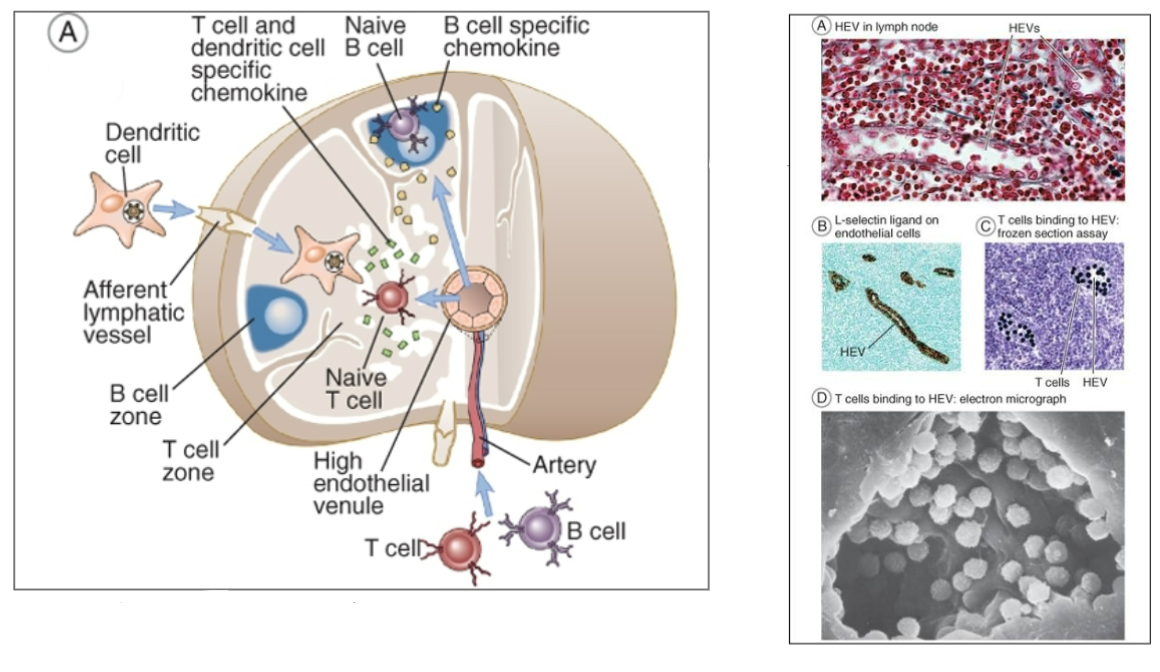
DC with antigen and costimulators upregulated travels to the T cell zone of the lymph node
Circulating naive T cells find lymph nodes by HEV adhesion molecules (e.g., L-selectin) and chemokines (e.g., CCR7)
migrate in and check out the antigens presented by DCs
No DC/T cell match - move one
Match - DC present antigen to T cell —> T cell activation
Activated T cell changes surface marker (decrease S1P1) to stay put for clonal Expansion and differentiation
Pretty efficient dating system
Effector T cells - where to now?
Back to the site of infection
decrease L-selection, decrease CCR7
Increase VLA-4 (and more) - binds to VCAM1 on inflamed endothelium
Or stay behind to help B cells

T cell functions
Macrophage activation ****
T cel diff to Th1 (IL-12 driven) —> IFN-y
B cell help ****
antibody production
Th1 —> opsonizing antibody isotypes (mice
Th17 functions
TH17 produce IL-17
IL-6+ TGF-b promote differentiation (mice)
IL-23 promote maintenance (mice)
IL-17
Neutrophil proliferation, maturation and chemoattraction ***
induce proinflammatory cytokines, chemokines and MMPs
Anti-bacterial defense
ILC3s - innate counterpart that produces IL-17
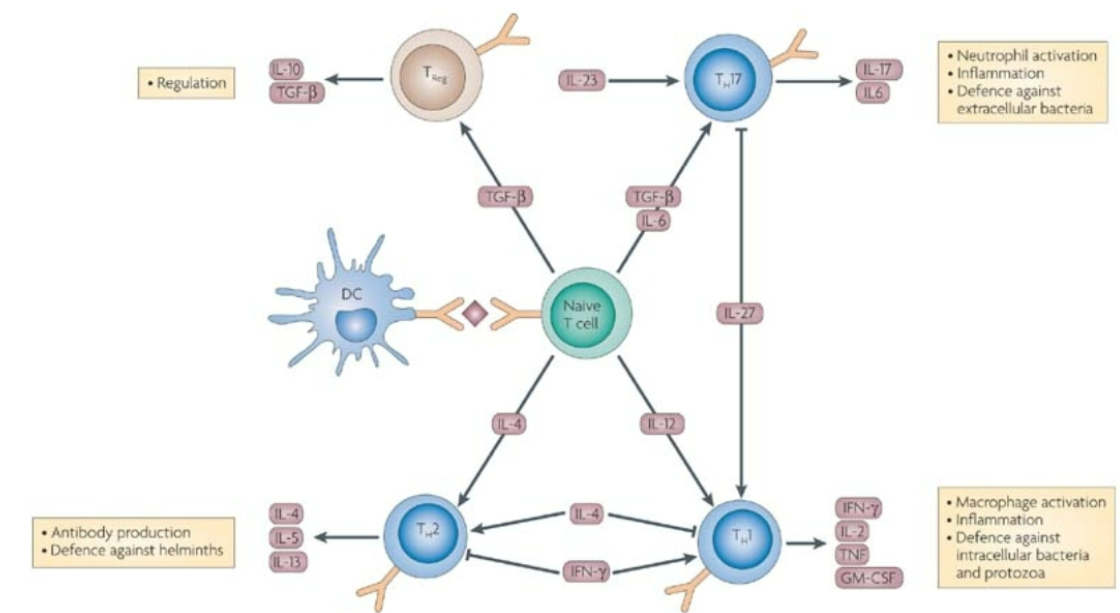
T Cell polarization
Regulation
Treg
IL-10
TGF-b
Antibody production & defense against helminths
Th2
IL-4
IL-5
IL-13
Neutrophil activation, inflammation, defence against extracellular bacteria
Th17
IL-17
IL-6
Macrophage activation, inflammation, defence against intracellular bacteria and protozoa
Th1
IFN-y
IL-2
TNF
GM-CSF
What about B cells?
Some migrate to follicles, form germinal centers and undergo somatic hypermutation to optimize and diversify specificity of antibody (EXPAND)
ultimately produce antibody (as plasma cells***)
Migration is chemokine-mediated
B cells also directly bind antigen through membrane Ig —> cross-linked —> upregulates co-stimulatory molecules for ag presentation (B7-1, B7-2, and CD-40 )
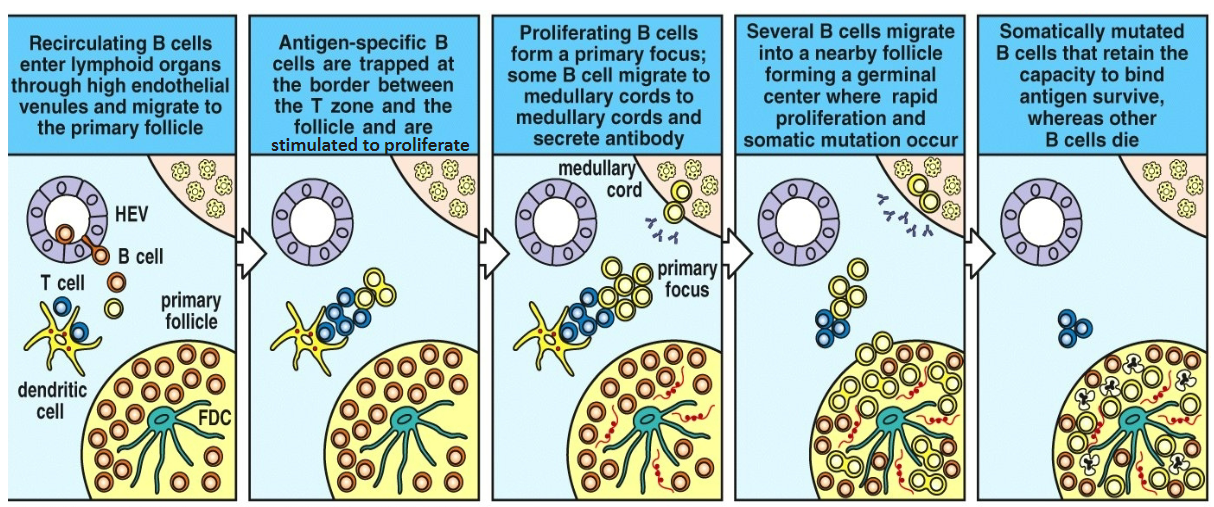
Recirculating B cells enter lymphoid organs through high endothelial venules and migrate to the primary follicle
Antigen-specific B cells are trapped at the border between the T zone and the follicle and are stimulated to proliferate
Proliferating B cells form a primary focus; some B cells migrate to medullary cords to medullary cords and secrete antibody
Several B cells migrate into a nearby follicle forming a germinal center where rapid proliferation and somatic mutation occur
Somatically mutated B cells that retain the capacity to survive, whereas other B cells die
Adaptive immunity to extracellular bacteria
B cells produce antibody
T-independent antigens (polysaccharides; limited T cell activation)
T-dependent antigens (proteins)
Bacteria and toxin targeted
Antibody function
Opsonization ***
Complement activation (classical) ***
Neutralization (toxins) ***
Adaptive immunity to extracellular bacteria
IgM and IgG for most bacteria
Opsonization and complement activation; some neutralization
IgA at mucosal surfaces (driven by IL-4, IL-5, TGF-b)
neutralization
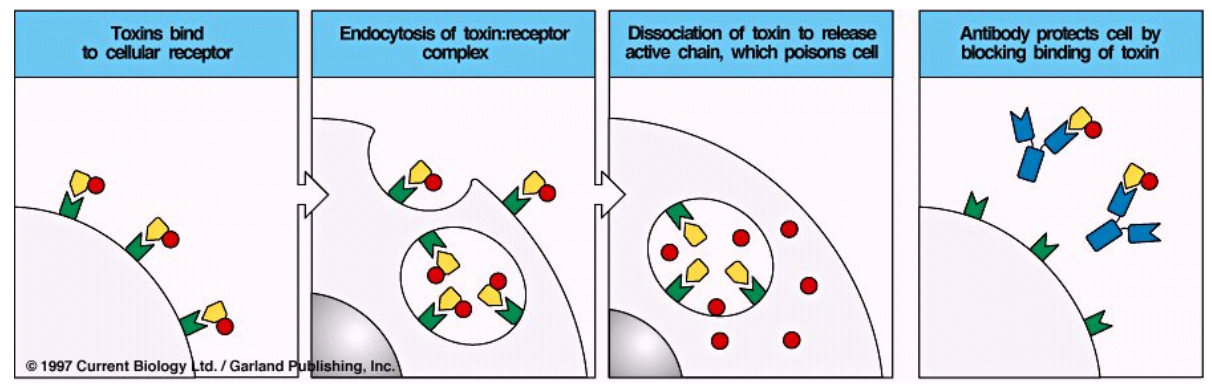
Toxin neutralization
Toxins bind to cellular receptor
Endocytosis of toxin:receptor complex
Dissociation of toxin to release active chain, which poisons cell
Antibody protects cell by blocking binding of toxin

Immune response to microbes - intracellular bacteria and fungi
Intracellular bacteria
some bacteria can live in phagocytes
mycobacterium spp. or facultative intracellular bacteria
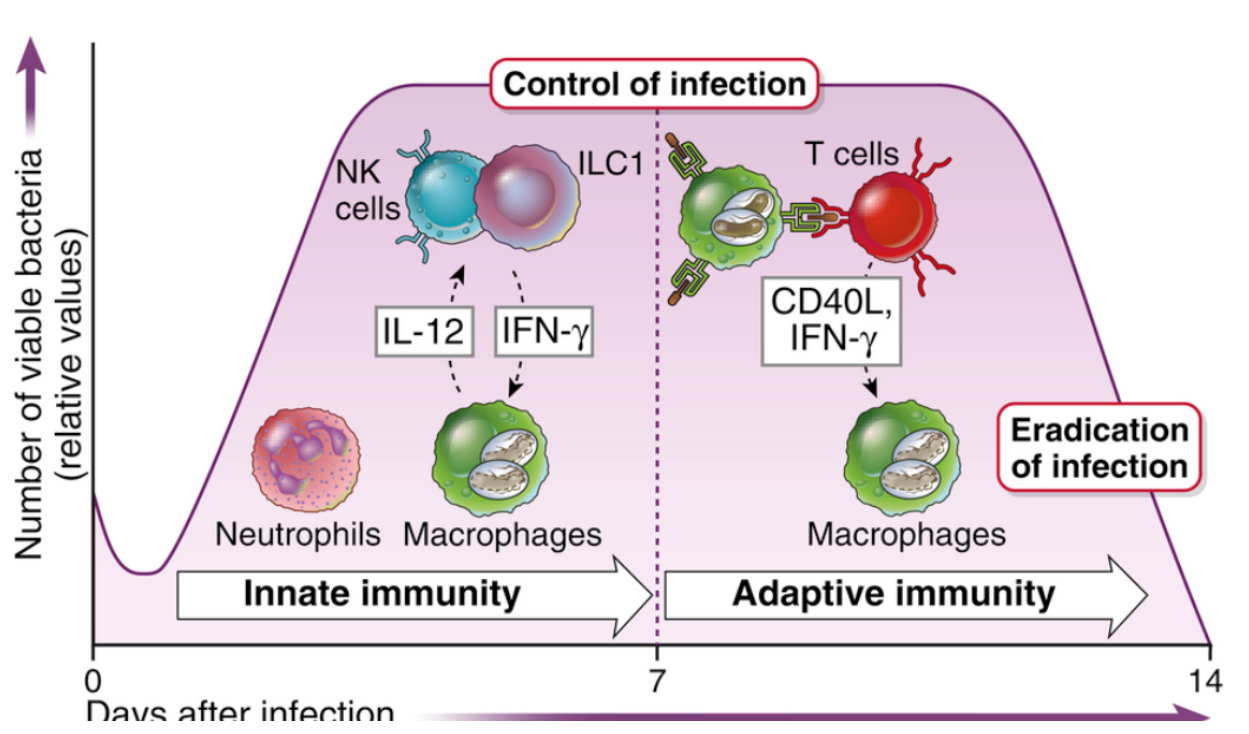
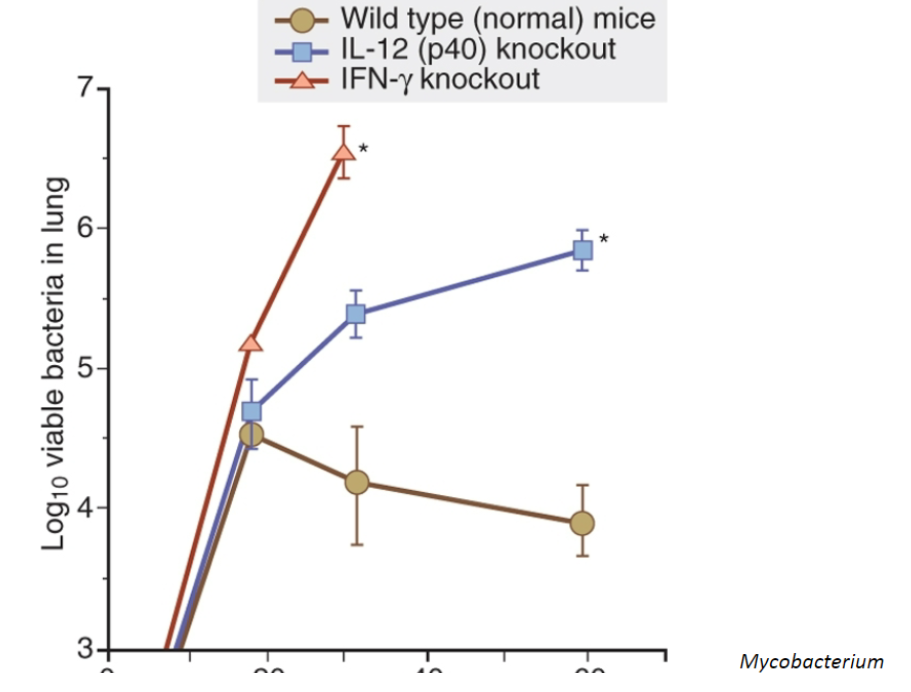
Activated macrophages critical in defense ***
can form giant cells
T cell component - primarily CD4+ Th1 cells provide help to macs (IFN-y, CD40L co-stim, isotype switching, etc)
NK cells - IFN-y production ****
ILC1s - IFN-y & TNF-a production
Humoral role?
contributes, but can’t succeed alone
extracellular stages
Intracellular bacteria, part 2
some intracellular bacteria end up in the cytoplasm (escape the phagosome) and thus can be presented by MHC1 to CD8+ T cells that help to kill the cell
Examples: Listeria, Chlamydia, Rickettsia
NK cells —> IFN-y —> activates macs —> cytokines —> CD4+ and CD8+ cells —> cycles —> clearance
Both CD4 and CD8 involved, thus both MHC I and II presentation occurring (CD8 perhaps more important due to intracytoplasmic lifestyle)
Nafe’s rule of 8: CD4 MHC2 = 8 and CD8 MHC1 = 8

A few more words about intracellular bacteria
Immunopathology - immune response is the problem ***
Granuloma - hallmark of mycobacterial disease
resist macrophage killing and decreases IFN-y mediated activation
Are granulomas good or bad???
organism walled off but not cleared
Can stimulate fibrosis (TNF-a and TGF-b)
In some cases, alternatives are more problematic.
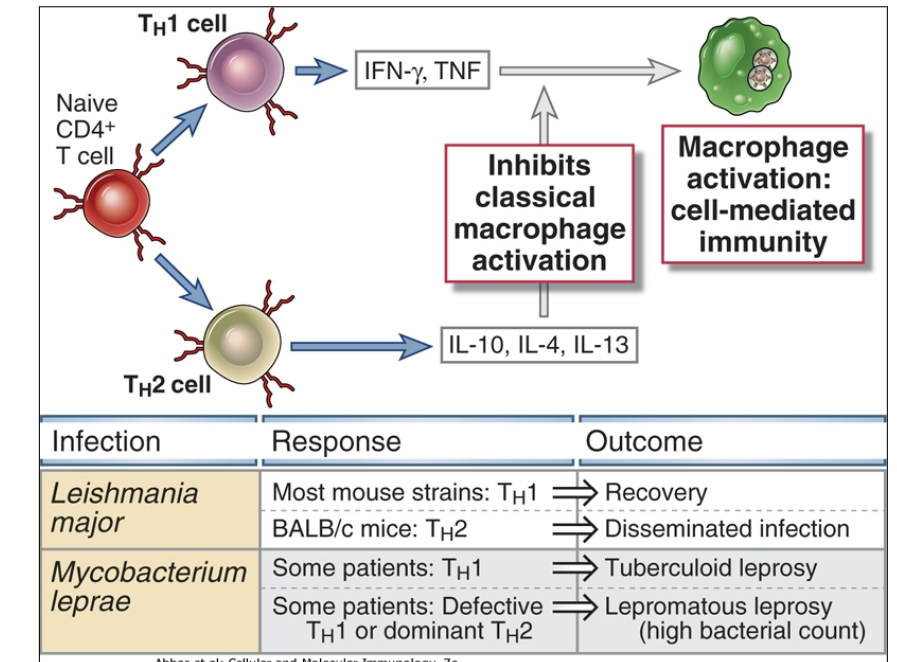
Leprosy
Two forms
Tuberculoid (Th1 predominates) - potent mac activation - controls bud does not eradicate - symptoms milder and associated with inflammatory response
Lepromatous form - (Th2 predominates and Th1 immunity is suppressed) - marked increase in bacterial numbers = more severe disease
Sheep with Johne’s = similar phenomenon
Th1 may transition to Th2
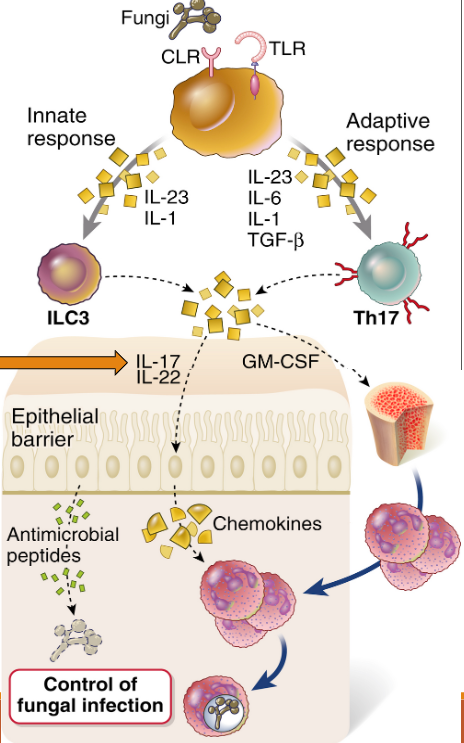
Immunity to fungi
often environmental “opportunists”
Disease in immunocompromised hosts (esp. innate but both involved; e.g., AIDS patient susceptible)
Granulomatous to ppyogranulomatous inflammation predominates ***
giant cells
Innate (macs, neuts & ILCs) critical ***
cytokines and antimicrobial peptides
Adaptive (CD4+) important too
CD8+ iff intracellular fungus such as Histoplasma sp.
Antibody response occurs, but role is debatable
Th1 & Th17 = good; Th2 = non-protective ***