GCSE Chemistry Revision "Limitations of Bonding Diagrams"
Dot and Cross Diagrams
Definition: Shows electrons from different atoms using different marks (dots and crosses).
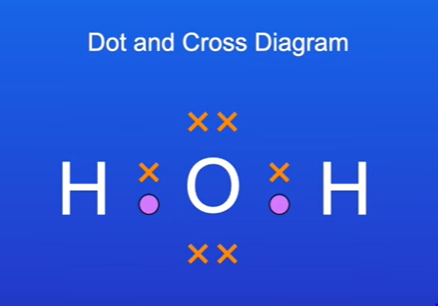
Advantage:
because we use dots to represent the electrons from one atom and we use crosses to represent the electrons from another atom, it's very clear where the electrons are coming from.
Disadvantages:
dot and cross diagrams such as this one don't tell us about the shape of the molecule.
Unable to represent non-bonding outer electrons(delocalised e-); for example, water has four outer electrons on oxygen that are not involved in bonding.
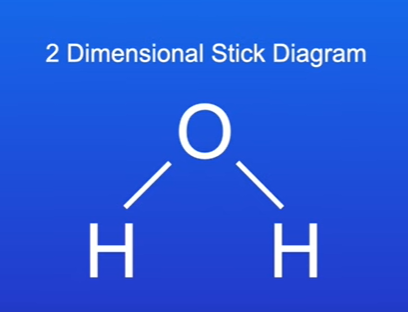
Two-Dimensional Stick Diagrams
Definition: Represents covalent bonds as sticks between atoms.
Advantages:
Simplistic view of bonding.
Disadvantages:
Because a covalent bond is shown as a stick, we cannot tell which electron in the covalent bond came from which atom.
Stick diagrams also give us no idea of outer electrons that are not in bonds. For example, in the water molecule, four outer electrons on the oxygen atom are not in covalent bonds.
Finally, two-dimensional stick diagrams do not give us accurate information on the shape of the molecule.
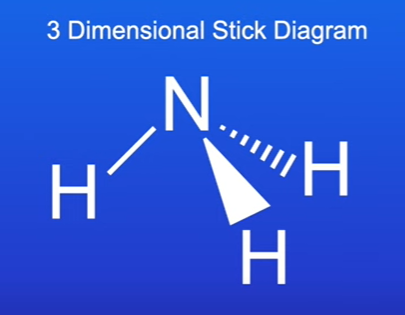
Three-Dimensional Stick Diagrams
Definition: Provides a spatial representation of molecules in three dimensions.
Advantages:
it shows us the shape of the molecule, offering a better representation of molecular geometry.
Example: Ammonia molecule represented in this format.
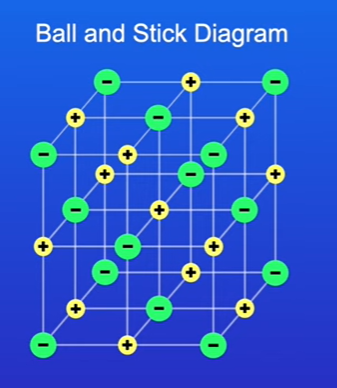
Ball and Stick Diagrams for Giant Ionic Structures
Definition: Shows ionic lattices where ions are represented as spheres connected by sticks to indicate bonds.
Advantages:
it allows us to clearly see the ions in three dimensions
Disadvantages:
Misrepresents spacing; ions appear to be widely spaced, but they are in fact closely packed in reality.
the ions are shown as widely spaced in diagram, but in reality, the ions are packed together.
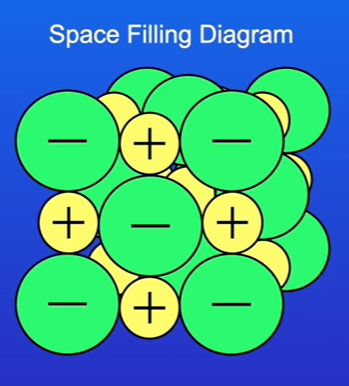
Space-Filling Diagrams
Definition: Represents the relative sizes of the ions to provide an idea of the overall volume of the ionic structure.
Advantages:
Clearly illustrates how closely packed the ions are in the structure.
Disadvantages:
Difficult to visualize three-dimensional packing accurately
In summary: give us a better idea of how closely packed the ions are; however, it can be difficult to see the three-dimensional packing with a space-filling diagram.
Limitations of Diagrams
One problem with both ball and stick diagrams and space-filling diagrams: They only show a tiny part of the giant crystal lattice. In reality, a crystal lattice is a giant structure, so both of these diagrams give the impression that these structures are much smaller than they actually are.
Understanding these diagrams' limitations is crucial for accurate chemical modeling.