Ap Bio Unit 1
1.1 structure of water and hydrogen bonding
High electronnegtive= attracts more electrons
Low electronegativity= Less ability to attract electrons
Nonpolar bond
Atoms share electrons equally
Nonpolar molecules are hydrophobic
Polar bond
Atoms with different electronegativity do not share electrons equally: one atom has a more negative charge, and the other is more positive
Polar molecules are hydrophilic
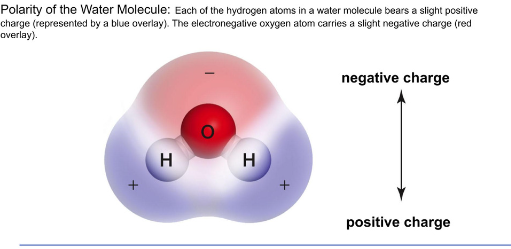
Water:
Unique properties of liquid water raided because of the water molecules’ polarity
Universal Solvent
The oxygen atom has a slightly negative electronegativity
Hydrogen atoms are slightly positive
Cohesion and adhesion
Cohesions: Water moles tick on one another
Adhesion: When water molecules cling to the other surface
High surface tension
High heat capacity
Changes in density based on temperature
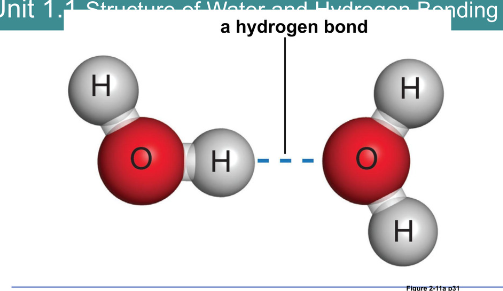
Hydrogen bond:
Weak attraction between a highly electronegative atom and a hydrogen atom
Hydrogen atoms take part in a separate polar covalent bond
Do not form molecules and are not chemical bonds
Stabilize the strictures of large biological molecules
Acids
Donate hydrogen ions in a water solution
pH below 7
Bases
Accept hydrogen ions in a water solution
pH above 7
Buffer
Srt of chemicals (weak acids or base and its salt) that can keep the pH of a solution stable
1.2 Elements of Life
Carbon
4 electrons in its outer energy level
Can bond up to four other atoms to complete its outer shell
Can bond two, three, or four atoms
Form polar or nonpolar
can form chains or ring
Be assembled and remolded many organic compounds
Work as a framework of many sugars, starches, and fats
Organic
Molecules are complex molecules of life built on a framework of carbon atoms
Carbohydrates
Lipids
Proteins
Nucleic acids
Monomers
Molecules are used as subunits to build larger molecules
Polymers
Larger molecules that are chains of monomers
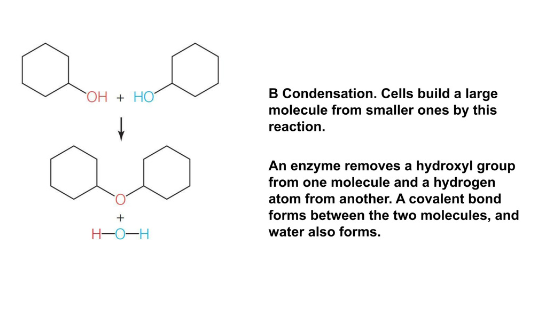
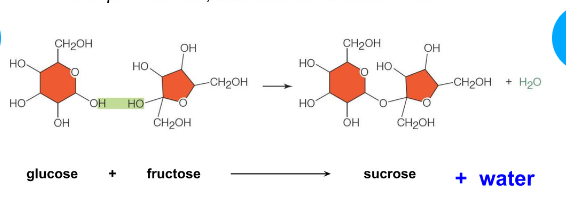
Dehydration synthesis (condensation reaction)
Joins monomers into polymers
water is realize
Covalent bonding of two molecules to form a larger molecule
Water forms as a product
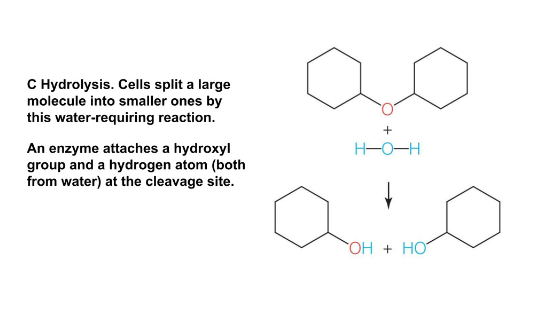
Hydrolysis
Breaks polymers into monomers
Water is added to break polymers into monomers
Reverse of condensation
Cleavage creation splits larger molecules into smaller ones
Water is split
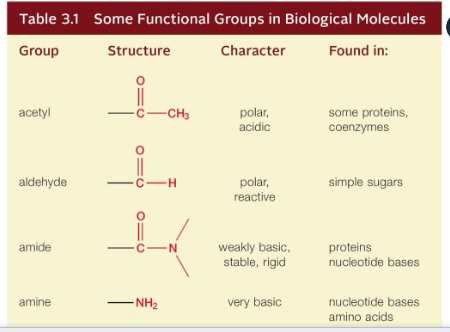
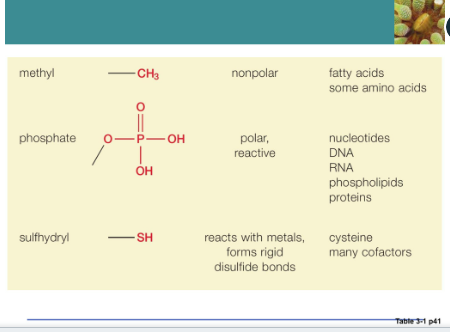
Function groups:
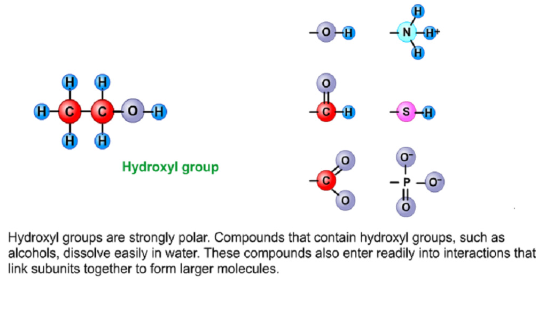
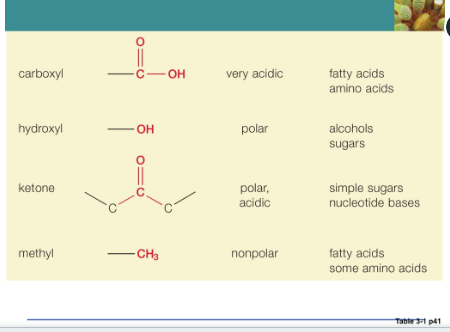
Hydroxyl
Strong polar
Alcohols is an example of a hydroxyl
Interaction that links subunits together to form larger molecules
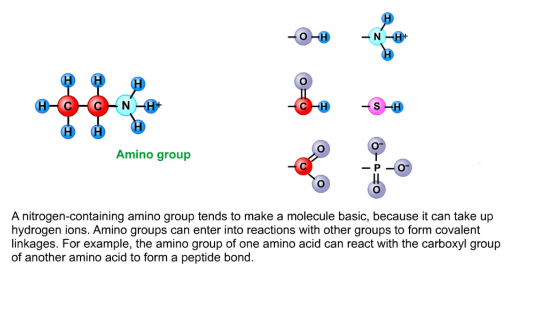
Amino groups
Nitrogen-containing amino groups tend to make a molecule basic
Takes up hydrogen ions
Enter into reactions with other groups to form covalent linkages
Amino groups can react with a carboxyl group of another amino acid to form a peptide bond
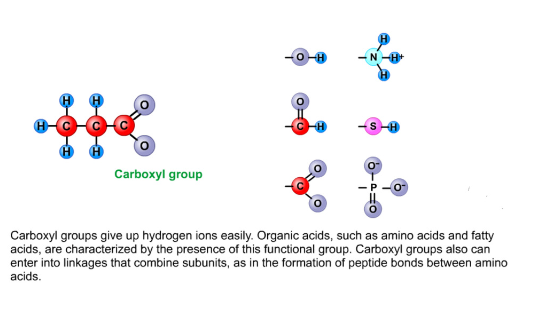
Carboxyl group
Give up hydrogen ions easily
Organic acids like amino or fatty acids are characterized by the presence of it
Carboxyl group can enter to combine subunits
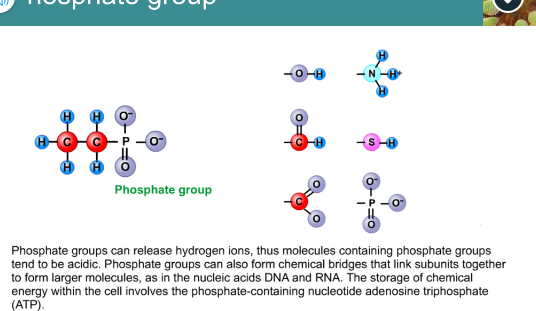
Phosphate group
Related hydrogen ions
Form chemical bridges that link subunits together to form larger molecules
Storage of chemical energy within the cell involves the phosphate-containing nucleotide (ATP
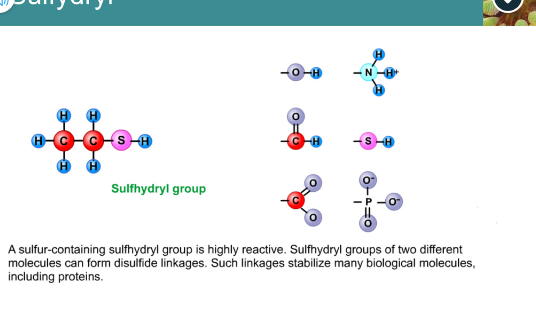
Sulfhydryl group
Highly reactive
Two different molecules can form disulfide linkages
Stabilize many biological molecules
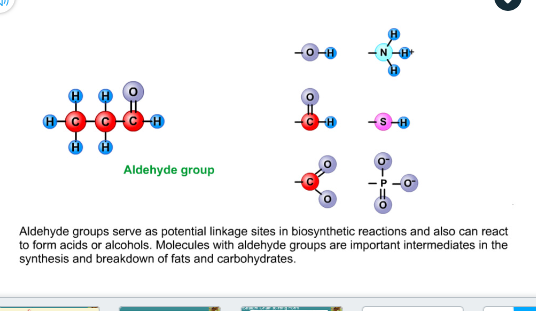
Aldehyde group
Serve as a potential linkage site in biosynthetic reaction
React to form acids or alcohols
Important in the synthesis and breakdown of fats and carbohydrates
1.3
Carbohydrates
ose means sugar
1:2:1 ratio (Carbon, Hydrogen, and Oxygen)
CH2O)n is the formula and n is the number of carbon in the molecule
MOnosaccharides
Simpliest carbohydrates
Used as an energy source
Backbones of 5 oe 6 carbons
Very Soluble in water
Ex, Glucose
Oligosaccharides
Short chains of monosaccharides
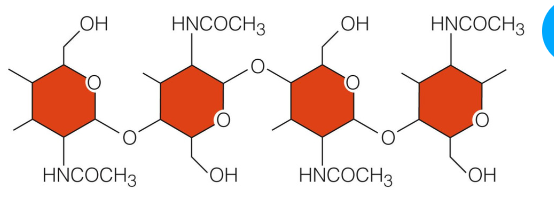
Poly saccharide
Straight or branched chains if many sugar monomers
The most common are cellulose, starch, and glycogen
Consists of glucose monomers
Different patterns of covalent bonding and different chemical properties
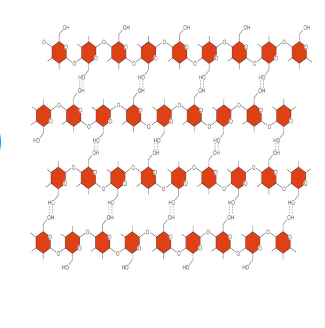
Cellulose
Major structure in plants
Long, straight chains of glucose monomers
Do not dissolve in water
In our vegetables
Starch
Energy reservoir in plants
Covalent bonding pattern between monomers make a chain that coils up into a spiral
Does not dissolve easily in water but is less stable then cellulose
Imporant in human food
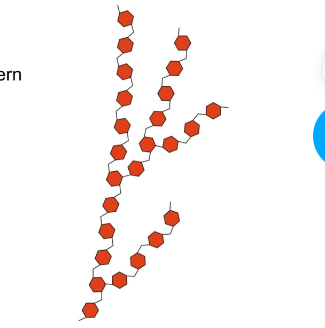
Glycogen
Polysaccharides
Covalent bonding pattern forms highly branched chains of glucose monomers
Energy rsevior in animals cells stores in muscle and liver cells
Chitin
Nitrogen containing polysaccharide
Strengthen the hard parts of animals like crabs a
Also the strong cells walls of fungi
Lipids
Fat, oils, or waxy organic compounds that are insoluble in water
Function as the body's major energy reservoir
Structural foundation of cell membrane
Sime organic compound with carboxyl group joined to a backbone of 4-36 carbon atoms
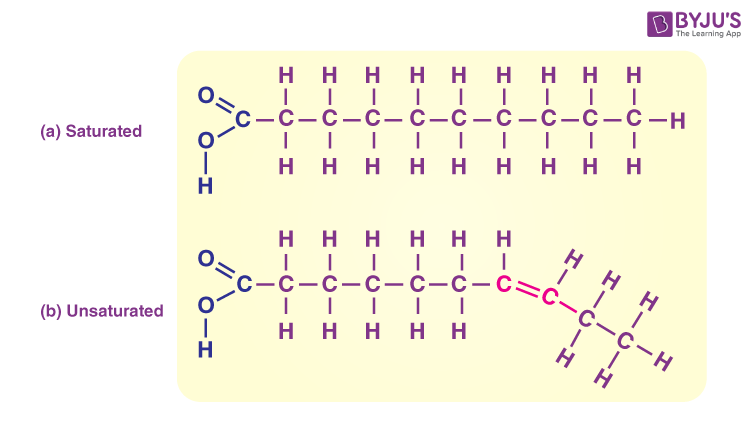
Saturated fatty acids (Animal fats)
Fatty acids with only single covalent bonds
Molecules are packed tightly; solid at room temperature
Unsaturated fatty acids (Vegetable Oils)
Fatty acids with one or two more double bonds
MOlecules are liquid at room temp
Triglycerides
Natural fats with three fatty acids attached to glycerol
The most abundant energy source in vertebrates
Concentrated in adipose for insulation and cushioning
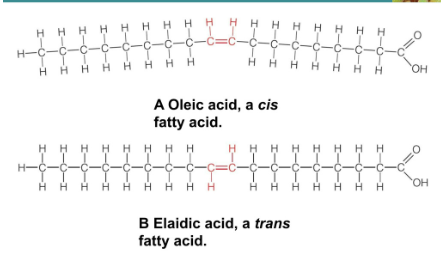
Trans Fats
Partially hydrogenated vegetables formed by the chemical hydrogenation process
Double bond straightens the molecule
Pack tightly; solid at room temp
Bonds can be either cis or trans, depending on how hydrogen is arranged
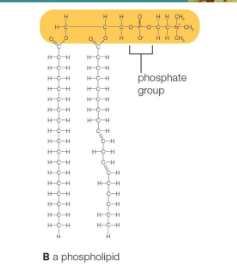
Phospholipids
Molecules with a polar head containing phosphate and two nonpolar fatty acid tails
Heads are hydrophilic and tails are hydrophobic
From lipid bilayers with hydrophobic tails sandwiched between hydrophilic heads
Steroids
Lipids with a rigid backbone of four carbon rings and no fatty acid tails
Protein
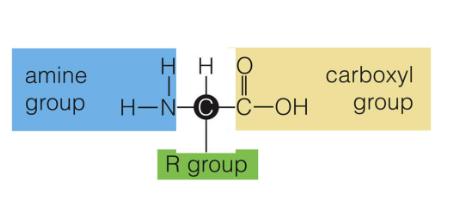
An organic compound composed of one or more chains of amino acids
A small organic compound with an amine group (-NH3+) or a carboxyl group (COO) and one or more R group
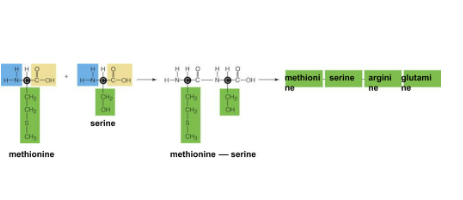
Polypeptide
Chain of amino acids bonded together by peptide bonds
Condensation reaction between the amine group of one amino acid and the carboxyl group of another amino acid
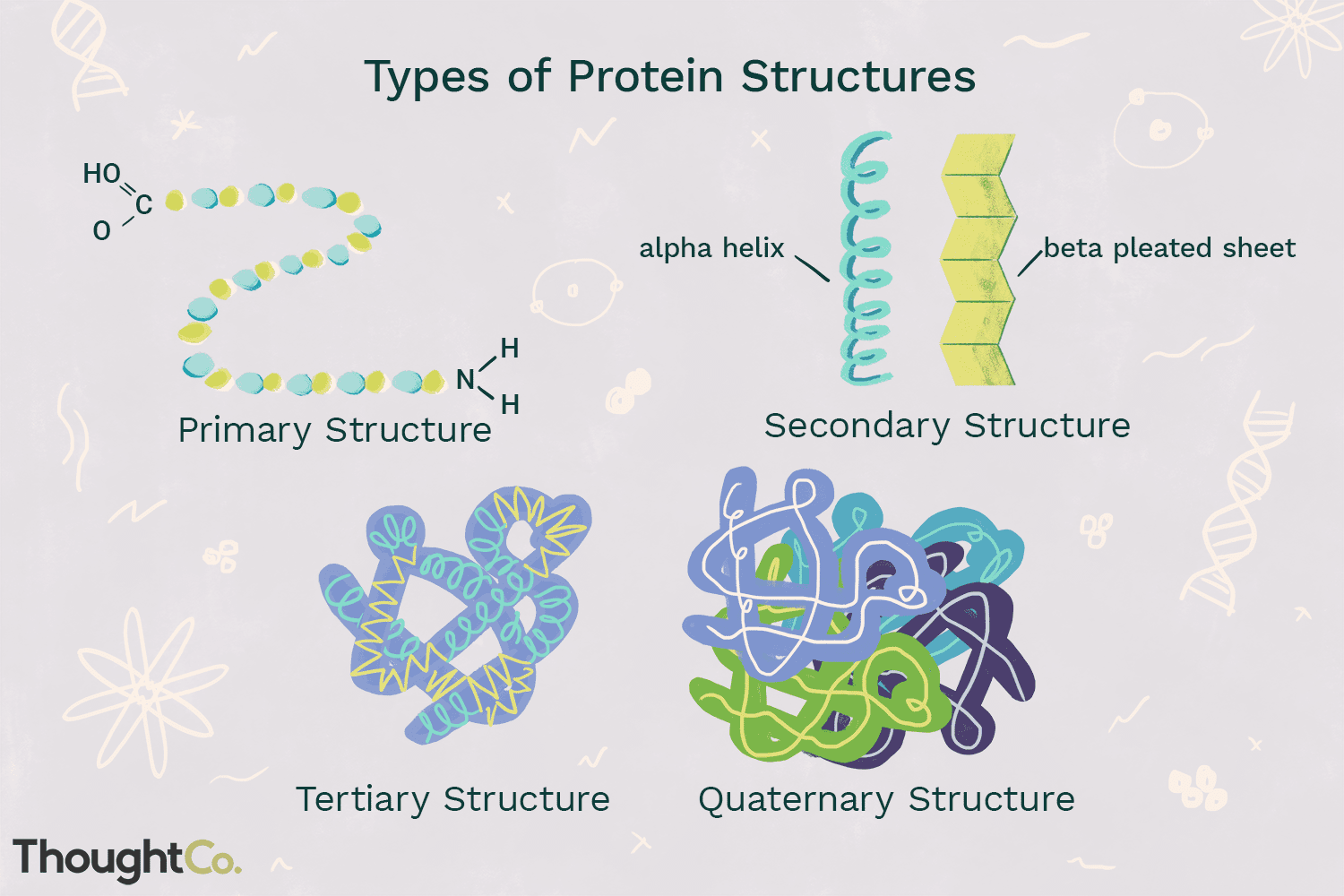
Protein Structure
Primary Structure
Unique amino acid sequence of a protein
Secondary Structure
Polypeptide chain golds and form hydrogen bonds between amino acids
Tertiary Structure
Secondary structure is compacted into structurally stable units called domains
Form a function protein
QUatemary structure
Proteins consist of two or more folded polypeptide chains in close association
Ex.Hemoglobin
Proteins must contain their correct three-dimensional shape
Change in protein shape may have drastic consequences or could be denatured
Head
Changes in pH
Salts and detergent can disrupt the structure of it
Prion
Misfolded proteins cause diseases
Mad cow disease
Creutzfeld-Jakob
Scrapie in sheep
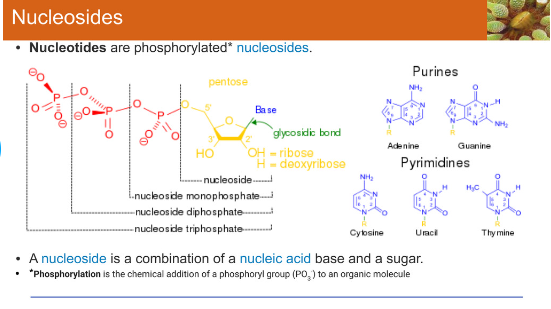
Nucleotides
Small organic molecule consisting of sugar with a five-carbon ring
Nitrogen-containing base
One or more phosphate group
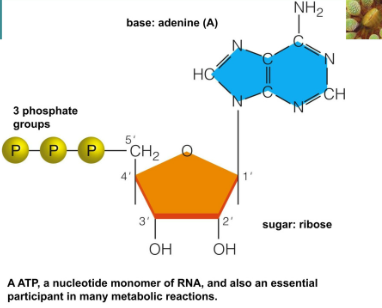
ATP (Adenosine triphosphate)
A nucleotide with three phosphate groups
Important in phosphate group energy transfer
Nucleic Acids
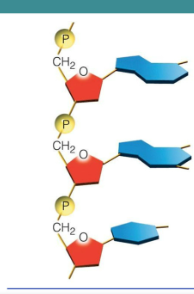
Polymers of nucleotides
sugar one is attached to a phosphate group of the next
RNA and DNA are nucleic acids
RNA (ribonucleic acid)
Contains four kinds of nucleotdies, monomers, including ATP
Consits of:
Ribose
Phosphate
One of the four nirogenous bases: Adeinine, Uracil, Guanine, or Cytosine
Imporant in protein synthesis
Messgenger, trasnfer, and ribosomal are three types of RNA
DNA (Deoxyribonucleic acid)
Nucleotides consists of
Sugar
Phosphate
One of the four nitrogen bases
Adenine
Thymine
Guanine
Cytosine
Two chains of the nucleotides twist together into a double hlex helped by hydrogen bonds
Inherited information necessary to buikld an organism
Codee in the order of nucleotide bases
Pyrimidines
In DNA they have cytosine and thymine
RNA: Cyotine and uracil
One ring structure
Purines (Larger then pyrimidines
Two ring structure
1.6 Nucleic Acids
Nucleotides are the monomers of nucleic acids like DNA and RNA
Some have a metabolism
Nucleotide
Small organic molecule consisting of a sugar with a five-carbon ring
Nitrogen-containing base
One or more phosphate groups
Essentials for replication of DNA
Transcription of RNA in rapidly diving stages
Either synthesized from small molecules and amino acids or acquired in the diet
Essential in providing the cellular energy sources (ATP and GTP)
Involved in numerous other metabolic roles
Chains of nucleotides is a nucleic acid
Sugar of one nucleotide is covalently bonded to the phosphate group of the next
Forming sugar-phosphate backbone
ATP
Energy carrier in cells
It consists of 3 phosphate groups (High energy)
Adliene
Sulfur
Hydrolysis
Removing something
Ex. During ATP, the third phosphate gets removed for energy forming Diphosphate
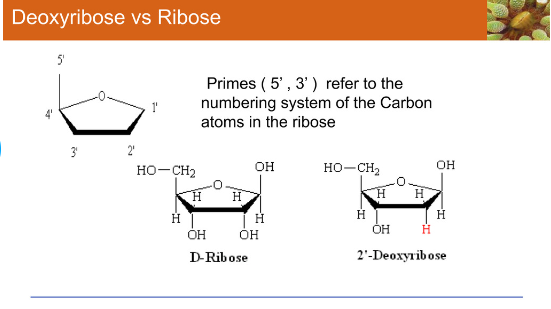
Ribose
Primes (5’3) refer to the numbering system
Carbon atoms in the ribose
RNA
the main type of RNA include
Messenger
Transfer
Ribosomal
RNA contains four kinds of nucleotide monomers
Adenine and Guanine (Purines)
Cytosine and URacil (Pyrimidine)
Important in protein synthesis
DNA
Two chains of nucleotides twist together into a double helix
Held by hydrogen bonds between base pairs
Base pairs are easily separated during replication and transcription
Contains all the information built into an organism
coded in the order of nucleotide bases
Covalent bonds (Backbone) of DNA