T2b Preservatives Effects Ocular Surface
Overview of Preservatives in Ocular Health
Preservatives are used in ocular medications to prolong shelf life but can adversely affect the ocular surface.
Effects of Drugs on Corneal Epithelium
Drug effects categorized by damage potential:
No Damage:
Atropine
Chloromycetin
Gentamicin
Methylcellulose
Polyvinyl alcohol
Saline
Moderate Damage:
Pilocarpine
Fluorescein
Rose Bengal stain
Important Damage:
BAK (Benzalkonium Chloride)
Topical anaesthetics
Benzalkonium Chloride (BAK) and Corneal Damage
Histological analysis reveals:
BAK reduces epithelial thickness
Induces nuclear condensation and vacuole formation
Dose-dependent decrease in cellular viability
Comparison of Preservatives
BAK vs Polyquad vs SofZia:
SofZia preserved Travoprost shows greater cell survival compared to travoprost preserved with BAK.
Travoprost containing polyquad performed better than its BAK preserved formulation
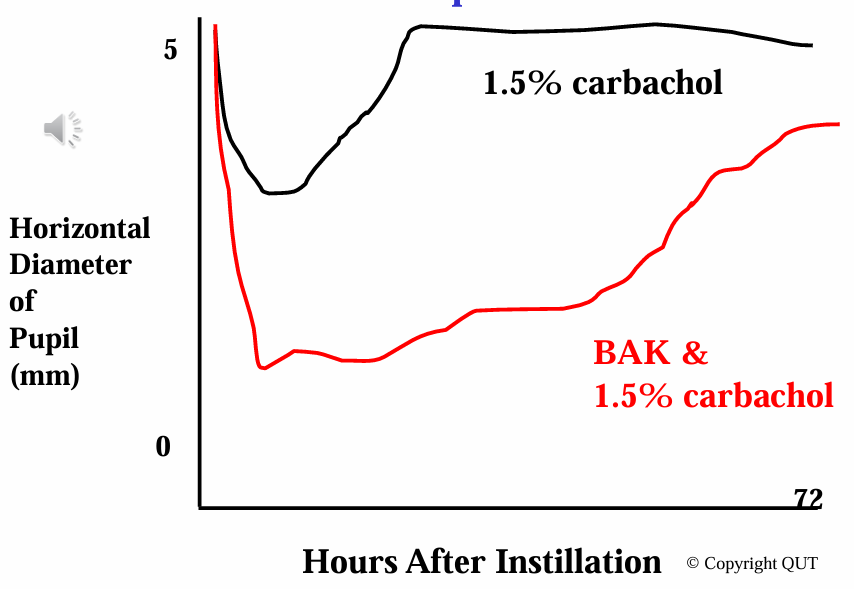
Ocular Absorption and Benzalkonium Chloride
Examination of pupil diameter post-instillation suggests:
Influence of BAK on ocular absorption.
Use of Preservatives in Medication
Should preservatives be included in certain medications?
Dry Eye Products:
No!
Preservatives worsen compromised tear film.
Shift toward unit dose products and ointments.
Ocular Anti-inflammatory Agents:
Preferably preservative-free due to increased susceptibility of inflamed eyes to surface damage.
Glaucoma Medications:
Long-term, asymptomatic treatment where ocular irritation can lead to discontinuation; however, BAK boosts drug absorption to the posterior segment.
Ocular Medicamentosa
Defined as chemical irritation or ocular tissues caused by topically applied drugs or preservatives, or by environmental or occupational substances
Notable irritants include:
Brimonidine
BAK
Prostaglandin analogues
Related factors:
Drug count, dosage/frequency, treatment duration, age, preexisting ocular surface health.
Symptoms include:
Ocular pain, stinging, burning, photophobia, redness, lid swelling, blurred vision.
Signs include:
Punctate staining, oedema of cornea/conjunctiva.
Treatment options:
Cessation of irritating medication, use of preservative-free alternatives, tear lubricants, possible short course of topical steroids.
Glaucoma Medications
Multiple medications and doses are applied daily for chronic conditions.
Chronic, long-term use can span decades, making cessation challenging due to severe consequences of untreated conditions.
Persistent use can lead to long-term effects on lid margins, cornea, and conjunctiva, resulting in ocular surface disease and permanent dry eye.
Non-preserved unit dose options available include Lumigan PF and Ganfort PF, while less cytotoxic preservatives in formulations like Travatan, Duotrav (Polyquad), and Alphagan P (Purite) can help but remain pro-inflammatory/cytotoxic.
Compliance with medication can be affected by severe symptoms resulting from treatments for an otherwise asymptomatic condition in mild to moderate cases.
Ocular Surface Impact from Glaucoma Medications
Associated signs include:
Abnormal Schirmer test
Abnormal tear osmolarity
Meibomian gland dysfunction
Lid margin vascularization
Corneal/conjunctival staining
limbnal or bulbar hyperaemia
punctate epithelial erosions
Prevalence of ocular surface disease (OSD) in patients on various topical therapies is significant.
Safety Concerns with Unpreserved Preparations
Unpreserved fluorescein: Pseudomonas can use fluorescein as an energy source, now supplied on dry filter paper
Unpreserved saline: touching the tip introduces bacteria
Unit doses and minims: no need to contain preservatives, eliminates tear and cornea toxicity, expensive
Tap water: Contains acanthamoeba, free living protozoan which causes severe corneal ulceration, don’t rinse contact lenses in tap water or use dirty lens cases
Contamination Risks in Diagnostic Minims
Study outcomes highlight microbial contamination risks in reused minims.
Cost analysis shows significant financial implications in reducing contamination risks.
Contamination of Autologous Serum
Risk of bacterial and fungal contamination in autologous serum eye drops is low, but monitoring is critical for safety.
not preserved