
Methods of purification and separation
Paper Chromatography - Used to separate and identify mixtures
A pencil line is drawn at about 1cm from the bottom of the paper, and a sample of the mixture is dropped on this line.
The chromatography paper is dipped into a solvent (ethanol or water). The pencil line must be above the surface of the solvent.
The solvent moves up the paper through capillary action, and the mixture is drawn up with the solvent, travelling up (it won’t move if it is insoluble in the solvent)
The speeds of the travel of each compound in the mixtures is different, and therefore they reach different heights.
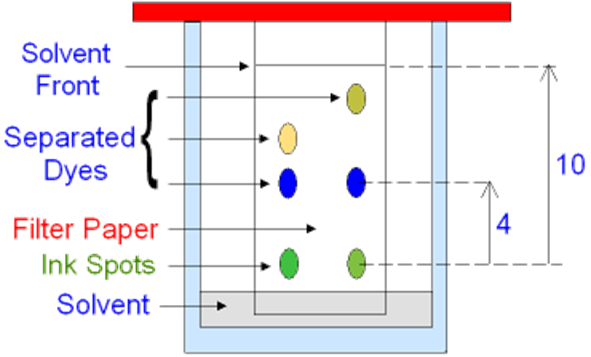
Then, using a factor called the “retention factor” or the Rf, the compounds are identified.
We can calculate the retention factor using the following formula:
Rf = distance moved by the compound ÷ distance moved by the solvent
Every compound has a unique retention factor, and this allows us to identify the compound, by comparing the experimental Rf with a known chart of Rf.
We can use locating agents (e.g. ninhydrin) to help identify colourless compounds.
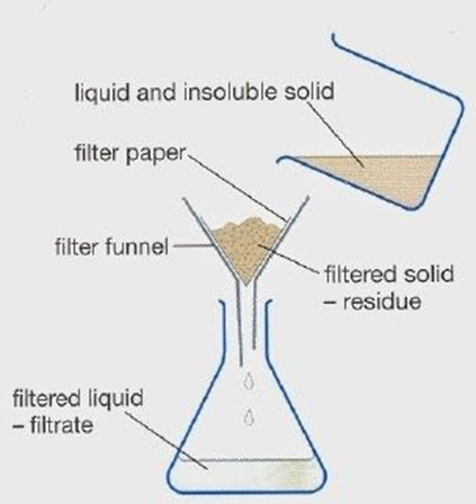
Filtration - used to separate insoluble solids from a mixture
The mixture is passed through filter paper in a filter funnel, catching the insoluble solid. The solid left in the funnel is called the residue. The liquid filters through to the beaker underneath. The filtered liquid collected at the bottom is called the filtrate.
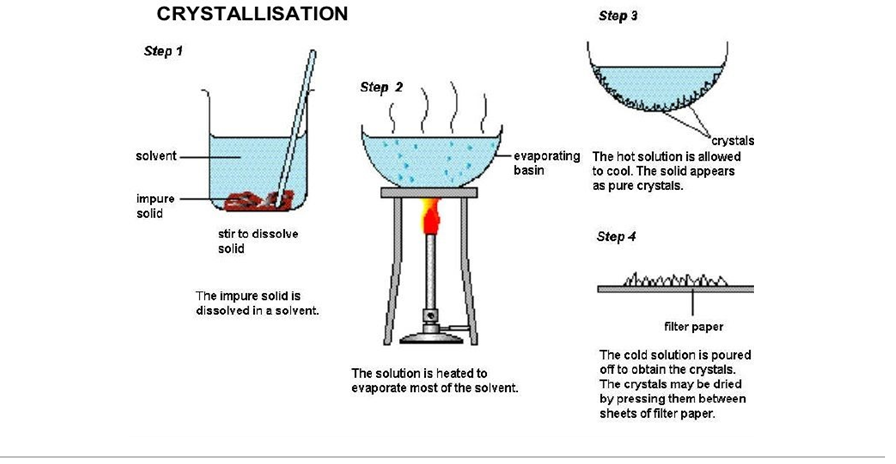
Crystallisation - used to separate a dissolved solid from a solution
A solution is left to evaporate over a bunsen burner. This means that the liquid content it lost.
This will leave the dissolved solid behind, which will crystallise into a solid crystal.
The purer the solution, the nicer the crystal. All crystals should be washed, filtered and dried to ensure a higher purity level.
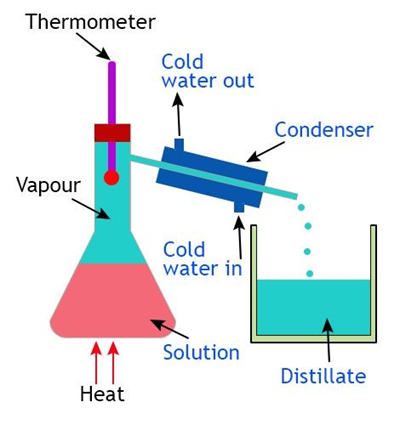
Distillation - used to separate a liquid from a solution of dissolved solid and the desired liquid
Heat the solution until the liquid is boiled away.
The vapour is collected using the apparatus and then cooled (condensed) back to a liquid using a Liebig condenser.
The desired liquid is collected into a new container.
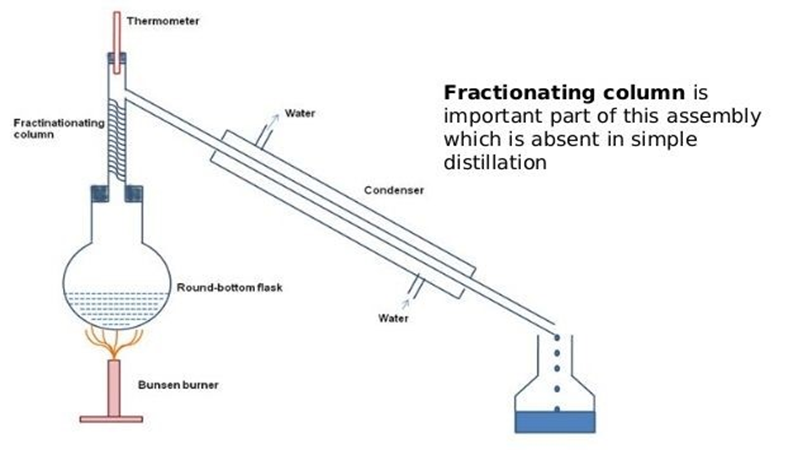
Fractional Distillation - used to separate one liquid from another
To be able to separate these liquids they need to have different boiling points.
If we want to separate a mixture of ethanol (b.p 78°C) and water (b.p. 100°C), the flask needs to be heated to a temperature between 78°C and 100°C (e.g. 82°C)
The ethanol will boil, turning into gas, but the water will not.
The ethanol vapour will be collected in the condenser, and turned back into a liquid.
The water will remain in the original flask.
Methods of purification and separation
Paper Chromatography - Used to separate and identify mixtures
A pencil line is drawn at about 1cm from the bottom of the paper, and a sample of the mixture is dropped on this line.
The chromatography paper is dipped into a solvent (ethanol or water). The pencil line must be above the surface of the solvent.
The solvent moves up the paper through capillary action, and the mixture is drawn up with the solvent, travelling up (it won’t move if it is insoluble in the solvent)
The speeds of the travel of each compound in the mixtures is different, and therefore they reach different heights.

Then, using a factor called the “retention factor” or the Rf, the compounds are identified.
We can calculate the retention factor using the following formula:
Rf = distance moved by the compound ÷ distance moved by the solvent
Every compound has a unique retention factor, and this allows us to identify the compound, by comparing the experimental Rf with a known chart of Rf.
We can use locating agents (e.g. ninhydrin) to help identify colourless compounds.
Filtration - used to separate insoluble solids from a mixture
The mixture is passed through filter paper in a filter funnel, catching the insoluble solid. The solid left in the funnel is called the residue. The liquid filters through to the beaker underneath. The filtered liquid collected at the bottom is called the filtrate.

Crystallisation - used to separate a dissolved solid from a solution
A solution is left to evaporate over a bunsen burner. This means that the liquid content it lost.
This will leave the dissolved solid behind, which will crystallise into a solid crystal.
The purer the solution, the nicer the crystal. All crystals should be washed, filtered and dried to ensure a higher purity level.

Distillation - used to separate a liquid from a solution of dissolved solid and the desired liquid
Heat the solution until the liquid is boiled away.
The vapour is collected using the apparatus and then cooled (condensed) back to a liquid using a Liebig condenser.
The desired liquid is collected into a new container.

Fractional Distillation - used to separate one liquid from another
To be able to separate these liquids they need to have different boiling points.
If we want to separate a mixture of ethanol (b.p 78°C) and water (b.p. 100°C), the flask needs to be heated to a temperature between 78°C and 100°C (e.g. 82°C)
The ethanol will boil, turning into gas, but the water will not.
The ethanol vapour will be collected in the condenser, and turned back into a liquid.
The water will remain in the original flask.

 Knowt
Knowt