Chapter 3 - reactions of metals
Metallic Bonding and Properties of Metals
Metallic Bonding
Metals are extracted from ores found in the Earth’s crust.
Metals possess low ionization energies, requiring minimal energy to remove valence electrons.
Metal atoms lose valence electrons to form cations.
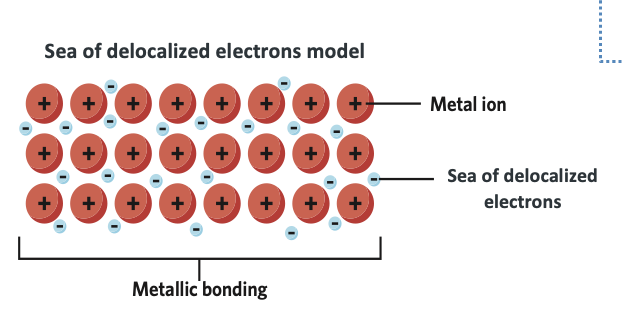
Metallic bonding involves the electrostatic attraction between delocalized electrons and cations in a metallic lattice structure.
Delocalized electrons move freely between metal cations.
Metallic bonding is non-directional; electrostatic forces act in all directions.
The strength of metallic bonding arises from the electrostatic attraction between delocalized electrons and the cation lattice.
Limitations of the metallic bonding model:
Does not explain different melting and boiling points of metals.
Does not explain differences in electrical conductivity of metals.
Does not explain magnetic properties of iron, nickel, and cobalt.
Metallic Bonding Model
Metal atoms lose valence electrons, forming a sea of delocalized electrons.
These delocalized electrons are electrostatically attracted to the cation lattice.

Properties of Metals
Malleability: Metals can be shaped without breaking due to delocalized electrons moving alongside the cation lattice.
Ductility: Metals can be drawn into thin wires because delocalized electrons are free to move towards a positive electrode.
Electrical Conductivity: Metals allow the flow of charge because electrons are attracted to a positive electrode in an electric circuit.
Heat Conductivity: When metal absorbs heat, the kinetic energy of delocalized electrons and cations increases. Cations vibrate rapidly, and delocalized electrons transfer kinetic energy, creating a chain reaction.
High Melting and Boiling Points: Metals can withstand high temperatures.
Lustre: Metals are shiny and reflective due to delocalized electrons at the surface reflecting light.
Lustre Explanation
Delocalized electrons constantly move across the cation lattice.
Light rays falling on delocalized electrons are reflected, giving a lustrous appearance.
Reactions of Metals
Reactions with Acids
Metals react with acids to varying degrees due to their lower ionization energies.
Loss of electrons during the reaction is called oxidation.
General reaction: acid + reactive metal → ionic salt + hydrogen gas
H_2 gas can be detected using the ‘hydrogen pop test’.
Ionic compounds (salts) contain one or more cations and anions.
Qualitative signs of a chemical reaction (COBALT): Colour, Odour, Bubbles, Appearance/disappearance of solid, Light/sound, Temperature change
Example Reaction
zinc + nitric acid → zinc nitrate + hydrogen gas
Zn(s) + 2HNO3(aq) → Zn(NO3)2(aq) + H2(g)
Reactions with Water
Group 1 and Group 2 metals react with water.
General reaction: water + reactive metal → metal hydroxide + hydrogen gas
Phenolphthalein indicates the presence of hydroxide ions.
Group 1 metals react explosively with water.
Going down a group lowers ionization energy.
Example Reaction
lithium + water → lithium hydroxide + hydrogen gas
2Li(s) + 2H2O(l) → 2LiOH(aq) + H2(g)
Reactions with Oxygen
Many metals react with oxygen.
General reaction: metal + oxygen → metal oxide
The loss of electrons is called oxidation; corrosion of iron is called rusting.
Example Reaction
magnesium and oxygen: 2Mg(s) + O_2(g) → 2MgO(s)
*Example showing aluminium forming a protective layer with oxygen.
aluminium + oxygen → aluminium oxide
4Al(s) + 3O2(g) → 2Al2O_3(s)
Reactivity Series
Metals vary in their ability to react with oxygen. Some do not react under normal conditions, while others react to form oxides.
Metal Recycling and Circular Economy
Circular Economy
Transition from a linear economy (take-make-dispose) to a circular economy (continuous cycle of resource use and re-use).
Circular economy focuses on optimal use and reuse of resources, from raw material extraction to repurposing waste materials.
Green Chemistry Principles
Four principles related to a circular economy:
Atom economy: maximize incorporation of reactant materials into the final product.
Design for energy efficiency: minimize negative environmental and economic impacts.
Prevention of wastes: prevent waste rather than treating it later.
Use of renewable feedstocks: use renewable materials instead of fossil fuels.
Metal Recycling Steps
Mining: extract natural resources.
Refining: prepare metal for use.
Made into a product: manufacturing.
Used: product consumption.
Disposed of via recycling: waste management.
Reprocessed as the same original product: reuse materials.
Repurposed as a new product: create new products from waste.
Alloys
An alloy is a mixture of elements, mainly metals.
bronze: copper, tin
brass: copper, zinc
steel: iron, carbon, chromium
Benefits of Recycling
Most metals can be recycled an unlimited number of times
Example metal energy savings: aluminium (95%), nickel (90%), copper (84%).