2.2 Proteins and membranes
Cells and Tissues
Protein Structure and Function
Part 1
Learning Outcomes
Summarise the four levels of protein structure
Define the different types of proteins according to their structure and/or function
Describe the structure and list the functions of the cell membrane
Explain the role of the cell membrane in regulating the transport of materials into and out of the cell
Describe the active and passive processes that transport substances across the cell membrane
Core Concepts
Genome and Proteins
Genome is composed of chromosomes, which contain genes that provide instructions for protein synthesis
Proteins perform various cellular functions, either independently or as part of complexes
The Human Protein Atlas
Identifies proteins present in various human tissues including:
Brain, adrenal gland, thyroid gland, lung, bone marrow, heart, skeletal muscle, gastrointestinal tract, liver, kidneys, and more.
Proteins
Essential for health and cellular function
Involved in:
Cell shape and organization
Signal reception and responding to signals
Structural integrity, transportation, immunity, and communication
polymers of amino acids
amino acid sequence assembled according to DNA sequence
Peptides < 50 amino acids
Proteins > 50 amino acids
A peptide bond is a covalent bond that forms between the carboxyl group of one amino acid and the amino group of another amino acid, releasing a molecule of water (a process known as dehydration synthesis). This bond is fundamental in linking amino acids together to form peptides and proteins, and is characterized by its strong stability, which is essential for maintaining the protein's structure.
Polymers of Amino Acids
Proteins are polymers formed by amino acids based on the DNA sequence
Peptides consist of less than 50 amino acids, while proteins have more than 50.
Four Levels of Protein Structure
Primary Structure
Sequence of amino acids from amino to carboxyl terminus
Hydrophobic/hydrophilic composition impacts structure
Begins at the a Amino terminus and ends in the Carboxyl terminus
Secondary Structure
First folding level stabilized by hydrogen bonds forming alpha-helices or beta-sheets
Alpha-helix
starts with an Amino terminus and ends with a Carboxyl terminus
Hydrogen of a carbonyl group bonds with a hydrogen of an amine group
3.6 residues per turn
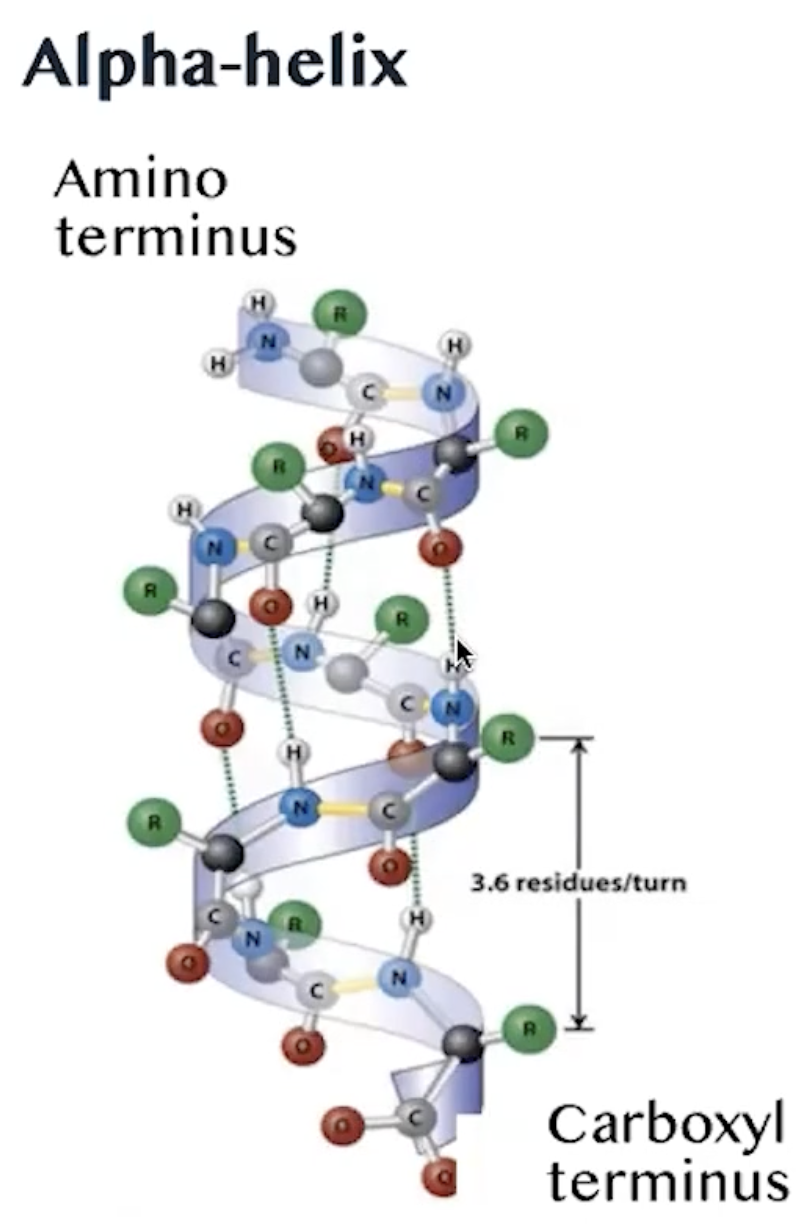
Resulting in a right-handed coil structure that is stabilized by these hydrogen bonds, contributing to the overall stability and functionality of the protein.
This helical structure is a common motif found in many proteins, allowing them to maintain their shape and perform biological functions effectively. Additionally, the presence of specific amino acid sequences can influence the propensity of a protein to adopt an alpha-helical conformation, which is critical for the protein's interaction with other molecules and its role in cellular processes.
The stability of alpha helices is further enhanced by the presence of hydrophobic interactions and van der Waals forces, which help to minimise the exposure of hydrophobic residues to the aqueous environment, thus promoting a more favorable energy state for the protein.
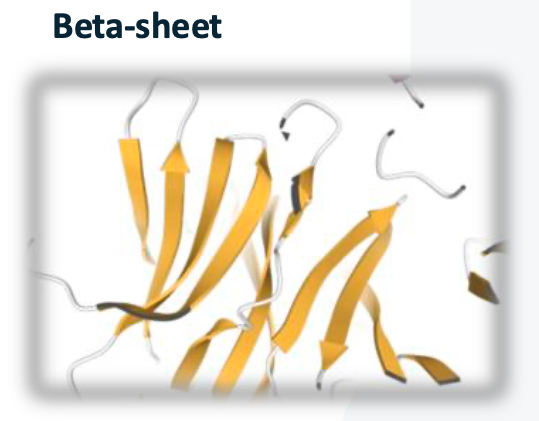
Beta-sheet
parallel peptide chains linked by hydrogen bonds
side chains exposed on both side of polypeptides create a distinctive zigzag pattern, contributing to the overall stability and structural integrity of the beta-sheet. In addition, the arrangement of beta-sheets can be classified into parallel and antiparallel configurations, which differ in the orientation of the peptide chains and can influence the mechanical properties and functionality of the protein.
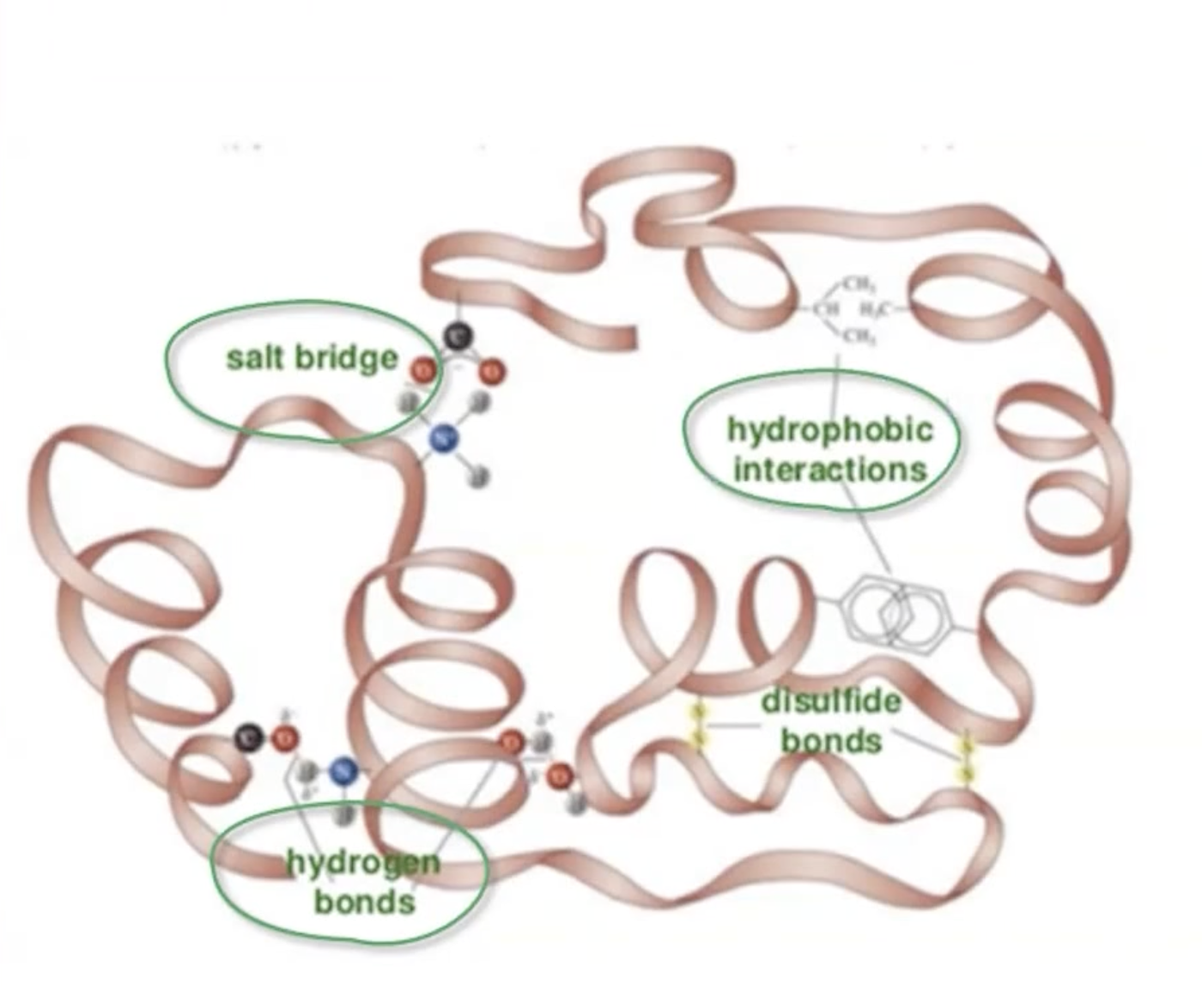
Tertiary Structure
Three-dimensional arrangement, involving hydrogen bonding, ionic bonds, hydrophobic interactions, and disulfide bonds
hydrogen bonds
side chains with partial positive and negative charges
ionic bonds
opposite charged side chains
hydrophobic interactions
non-polar side chains
disulphide bonds
cysteine residues
Quaternary Structure
Formation of total protein complex
multiple polypeptides
held together by
non-convalent bonds
hydrogen bonds and Van der Waals forces
subunits interact cooperatively
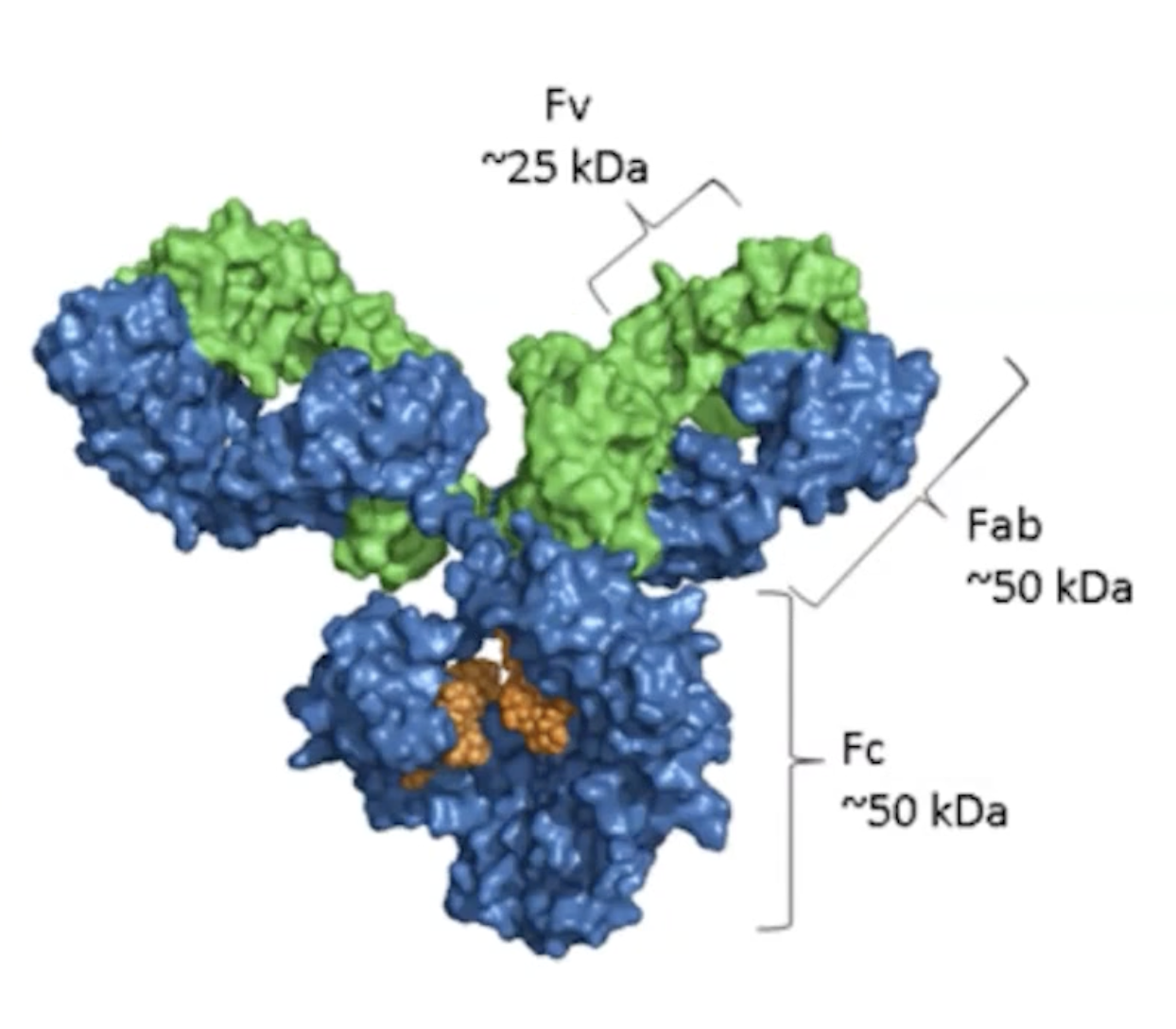
Fv (Fragment variable): This is the part of the antibody that contains the variable regions of both the heavy and light chains. It is responsible for the specific binding to the antigen. The Fv region is crucial for the diversity of antibodies, as it determines the specificity for different antigens.
Fab (Fragment antigen-binding): This includes the Fv region along with a portion of the constant region of the heavy chain. The Fab fragment is responsible for binding to antigens. Each antibody has two Fab fragments, allowing them to bind two identical antigens simultaneously.
Fc (Fragment crystallizable): This is the constant region of the antibody that is involved in mediating immune responses. The Fc region is recognized by immune cells and helps in various mechanisms of action, such as opsonization and activation of the complement system.
In summary, the Fv and Fab regions are involved in antigen binding, while the Fc region plays a role in the immune response.
Post-Translational Modifications
Various modifications enhance protein functionality:
Phosphorylation
adds a phosphate to serine, threonine or tyrosine, which can alter the protein's activity, localization, or interaction with other molecules.
glycosyation
attaches a sugar, usually to an '“N” or “O” in an amino acid side chain , which can affect protein folding, stability, and cell signaling pathways.
Ubiquitaination
adds ubiquitin to lysine reside of a target protein for degradation , which marks the protein for recognition by the proteasome, thus regulating protein levels and maintaining cellular homeostasis.
SUMOylation
adds a small protein SUMO (small ubiquitin-like modifier) to a target protein
Disulfide bond
Convalently links the “S” atoms of 2 different cysteine residues
Acetylation
adds an acetylene griup to an N'-terminus of a protein or at lysine residue
Lipidation
attaches a lipid, such as a fatty acid, to a protein chain
methylation
adds a methyl group, usually at lysine or arginine residues
hydroxylation
attaches a hydroxyl group (-OH) to a side chain of a protein
Structural Proteins
Muscle Proteins: Contractile proteins (actin/myosin) and regulatory proteins (tropomyosin, troponin).
Function
form thick and thin filaments
types
actin-myosin
Tropomyosin-troponin
Collagens: Fibrous proteins that constitute most of the extracellular matrix (ECM), provide strength and shape.
Function
provide strength, support and shape to tissues
Structure
3 polypeptide chains
Triple superhelix stabilised by hydrogen bonding
1000 different mutations associated with disease
osteogenesis imperfecta: A genetic disorder caused by mutations in the collagen genes, leading to brittle bones and increased fracture risk.
Cytoskeletal Proteins:
cytoskeleton, cilia and flagella
dynamic polymer structure
rapidly assembles and disassembles
Function
cell protection
motility
cytokinesis
transport
cell division
organelle organisation
Types
actin filaments
microtubules
intermediate filaments
DNA Associated Proteins
Histones: Basic proteins involved in DNA packaging.
Transcription Factors: Involved in RNA polymerase recruitment and gene expression regulation, mutations can impact diseases.
Immunity Proteins
Cytokines:
Manage immune responses and inflammation and haemopoisis
Haemopoiesis is the process of creating blood cells, mainly in the bone marrow. This includes red blood cells (which transport oxygen), white blood cells (which fight infections), and platelets (which help with blood clotting). It is important for replacing old or damaged blood cells and keeping the immune system healthy
Small proteins/peptides
produced by immune cells
Antibodies:
Structure defined by polypeptide chains, involved in pathogen clearance.
2 pairs of polypeptide chains
forms a “Y” shape
IgG, IgM, IgA, IgD, and IgE are the five main classes of immunoglobulins (antibodies) in the human body, each playing distinct roles in the immune response:
IgG (Immunoglobulin G): The most abundant antibody in blood and extracellular fluid, it plays a crucial role in the body's defense against infections and is the only class that can cross the placenta to provide passive immunity to the fetus.
IgM (Immunoglobulin M): The first antibody produced in response to an infection. It is primarily found in the blood and is effective at forming complexes for the elimination of pathogens.
IgA (Immunoglobulin A): Found mainly in secretions such as saliva, tears, and breast milk, IgA plays a vital role in mucosal immunity, protecting body surfaces exposed to foreign substances.
IgD (Immunoglobulin D): Although its function is not fully understood, IgD is primarily found on the surface of immature B lymphocytes and is believed to play a role in the activation and regulation of B cells.
IgE (Immunoglobulin E): Involved in allergic reactions and responses to parasitic infections, IgE binds to allergens and triggers histamine release from mast cells and basophils, leading to allergic symptoms.
Complement:
over 30 proteins
involved in the innate immune system
clearing invading pathogens
form a membrane attack complec
cell lysis
Antigens
proteins, peptides or polysaccarides
Essential for antibody response
Epitope (specific part of an antigen that is recognized by the immune system, particularly by antibodies or T-cell receptors)
the surface of the polypeptide that binds the antibody
can complex with lipids and nucleic acids
Types of antigens
Exogenous
Exogenous refers to substances that originate from outside an organism.
Endogenous
Endogenous refers to substances that are produced within an organism, such as proteins generated by the body's own cells.
Autoantigens
Autoantigens are proteins produced by the body that can trigger an immune response against its own tissues, often leading to autoimmune diseases.
Tumor antigen
Tumor antigens are proteins expressed on the surface of cancer cells that are recognized by the immune system as foreign, prompting an immune response aimed at targeting and destroying the tumor.
Native antigen
Native antigens are substances that are extracted directly from their natural source, such as a virus or bacteria.
Coagulation Proteins
The process fo haemostasis
Haemostasis is the cessation of bleeding from a blood vesel
Blood clot formation
3 steps of haemostasis
Vasocontrsiction
temporarilry blockage of a plug
coagulation cascade, leading to the formation of a stable fibrin clot that seals the injury and prevents further blood loss.
Activation, adehsion and aggregation of platelets
Anticoagulation Proteins
Maintain homeostatic balance
natural occurring anticoagulants
Thrombomodulin, Protein C, Protein S, Antithrombin, Tissue factor pathway inhibitor
Usually given to patients who are at higher risk of getting clots: amd therefore more prone to getting stroke and heart attack
Transport Proteins
Carry substances across biological membranes
ions, sugars, proteins and messenger molecules
reside within cell membranes
form channels or a carriers
Carrier Proteins
transfers substance across the lipid bilayer
protein binds to the substrate
undergoes a revrsibe conformational change
moving the molecule to the interior of the call
usis adenonie tri-phosphate (ATP)
opens to only 1 part of the membrane, allowing selective transport of ions or molecules across the lipid bilayer.
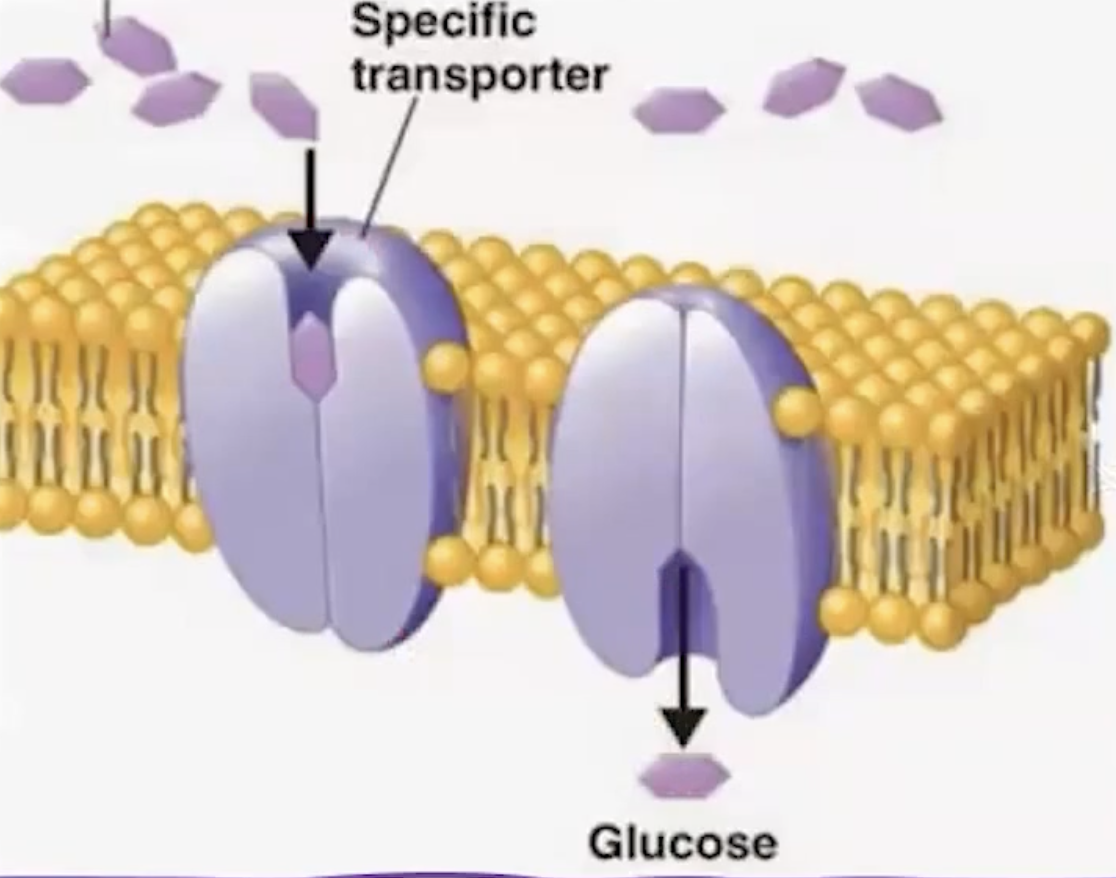
Channels
traversing through the cell membrane
hydrophilic channel through their core
open to intracellular and extracellular space
facilitate travel of polar molecules and ions
can be gated
Albumin
maintain oncotic pressure of the plasma
negatively changed and can bind to Cations and Hydrophobic molecules
Enzymes
catalyse reactions
contains a substrate binding site that perfectly fits the binding of substrate converting it into a product
function at specific temperatures and pH ranges
Types of Enzymes
Oxidoreductase (oxidation - Reduction reaction)
Transferases (Transfer of amino, carboxylate, acyl, carbonyl, methyl, phosphate
Hydrolases ( catalyze the hydrolysis of chemical bonds, breaking down molecules by the addition of water. )
Lyases ( Cleavage of carbon bonds)
Isomerases (Rearrangements of bonds)
Ligases (Formation of onds between Carbon, Oxygen, Sulphur and Nitrogen)
Signalling Proteins
Essential for cellular communication via ligands, promoting receptor-ligand interactions.
Neurotransmitters (e.g. GABA, serotonin) manage neurological functions.
Ligands
Extrcellular chemicals
secreted by a signaling cell
bind to a target cell
The Cell Membrane
Function: Protects cells, facilitates communication, and regulates chemical composition within the cell.
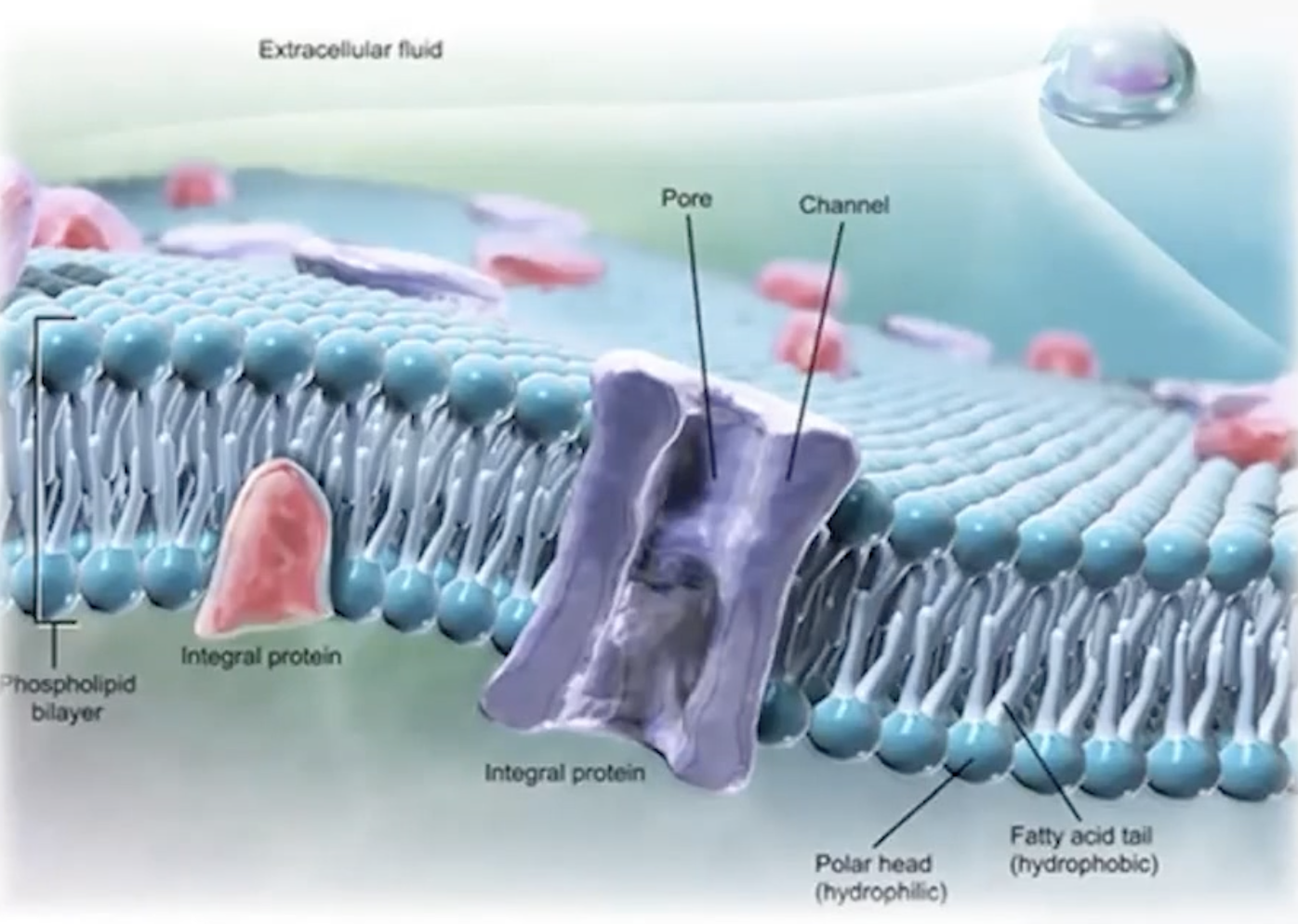
Membrane Structure
Consists of a phospholipid bilayer with hydrophilic heads and hydrophobic tails, retaining structural integrity.
Membrane Proteins
Include glycoproteins and glycolipids for recognition, integral proteins for transport, and peripheral proteins for regulation.
Transport Across the Plasma Membrane
Selective Permeability
Permits selective passage governed by substance characteristics: size, charge, and solubility.
Passive vs Active Transport
Passive Transport: Does not require ATP, includes simple diffusion and facilitated diffusion.
Active Transport: Requires ATP, moves substances against the gradient, includes vesicular transport (endocytosis/exocytosis).
Mechanisms of Transport
Simple diffusion: Movement of non-polar molecules (e.g., O2, CO2)
Facilitated diffusion: Assists charged molecules through channel proteins.
Active transport: Upholds concentration gradients through pumps and vesicles (e.g., endocytosis).