Unit 3: Intermolecular Forces and Properties (copy)
Polarity
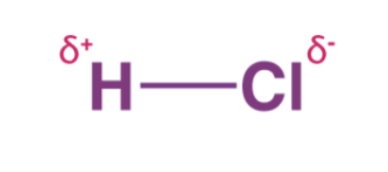
When a covalent bond is formed and electrons are shared, they are shared unequally. The more electronegative element in the bond has the stronger pull on electrons. The unequal share of electrons is called a polar covalent bond which also causes a slight partial charge on each atom.
When there's a slight charge, dipoles are created. Dipoles are opposite charges separated by some distance. Partial charges are indicated using the lowercase Greek letter delta.
When there's a covalent bond of equal or similar electronegativity, there is no dipole and it is a nonpolar covalent bond.
Molecular Polarity
Based on the type of molecular geometry and the dipoles of bonds, molecules can have polarity.
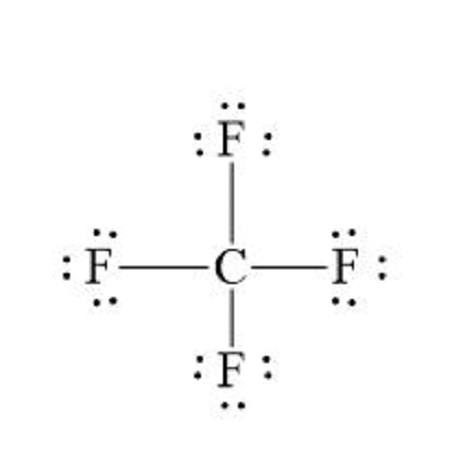
In carbon tetrafloride, the individual carbon-florine bonds are polar but because there are four C-F bonds and they are geometrically organized in a way that they cancel out, the molecule is nonpolar.
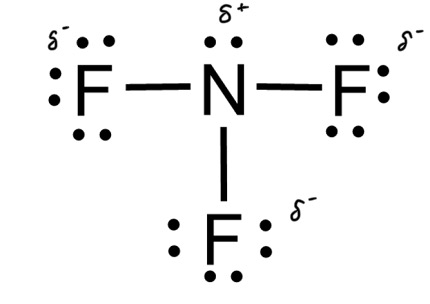
In NF3, only three of the four bonding locations has a fluorine, with the last being unfilled by a lone pair that belongs to nitrogen. fluorine is a more electronegative element than nitrogen, so it gets a negative charge.
Typically, if the central atom has a lone pair and different than the bonded atoms, it is asymmetrical and therefore polar. If the central atom has no lone pairs and all of the bonded atoms are the same, it is symmetrical and nonpolar.
- lone pairs on the central atom making a molecule polar has a few exceptions: trigonal planar with three lone pairs or octahedral with two lone pairs.
Because in Lewis dot structures the least electronegative atom is in the center, polar molecules usually have a central positive charge. However, when hydrogen is a terminal atom, it's low electronegativity makes the central atom negative.
Intermolecular Forces
- Intermolecular forces (IMFs) are the forces in between covalent bonds. in order for a phase change to occur, these forces must be broken.
- when ionic substances change phases, the bonds between individual ions are actually broken.
- when covalent substances change phase, the bonds between individual atoms remain but the bonds holding molecule to molecule together break apart.
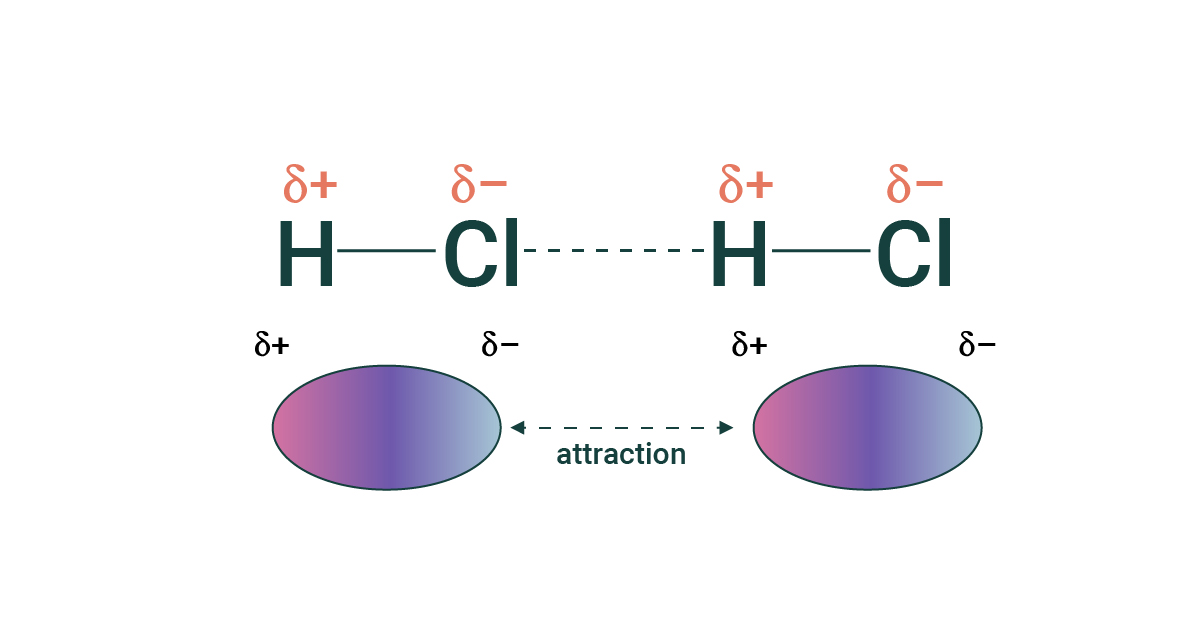
Dipole-Dipole Forces
Dipole-dipole forces occur when the positive center of one polar molecule is attracted to the negative end of another polar molecule.
Molecules with greater polarity, and therefore greater dipole-dipole forces, have high melting and boiling points. However, dipole-dipole forces are weak and most substances held together by these forces are gases or liquids at room temperature.
Hydrogen Bonds
- Hydrogen bonds are a type of dipole-dipole force where the hydrogen (positive) end of a molecule is attracted to ONLY oxygen, nitrogen, or fluorine (negative end).
- Hydrogen bonds are stronger than basic dipole-dipole forces because when hydrogen gives up it's electron, the nucleus in exposed. Water and ammonia have higher melting and boiling points than other molecules held together by non-hydrogen bonds.
London Dispersion Forces
- London Dispersion forces (LDFs) occur between all molecules and are very weak attractions caused by random electron movements.
- Because LDFs depend on random electron movement, a molecule with more electrons has stronger LDFs. Weak LDFs result in low boiling and melting points, ever lower and weaker than dipole-dipole forces. They are typically gases and liquids at room temperature.
- even though LDFs are weak, it's hard to compare molecules with lots of electrons to polar substances with few electrons. LDFs may be equal to hydrogen bonds if there's enough electrons.
IMF Strength
- To break the lattice structure of ionic solids and turn them into liquids, the bonds holding the lattice together must be broken.
- Covalent substances are liquid at room temperature and will boil when their IMFs are broken. For similar size molecules, their IMF relative strength can be ranked.
- hydrogen bonds are the strongest
- nonhydrogen bond permanent dipoles
- LDFs (more electrons = more/stronger (LDFs)
- Covalent bonds are always weaker than ionic bonds
- Metallic bonds tend to be very strong with high melting points, especially transition metals. Network covalent bonds are the strongest and are very hard to melt
Bonding and Phases
- The stronger the IMF of a substance, the tighter the atoms are packed together. Solids have very tight-knit molecules and have the strongest IMFs. Gases have the weakest IMFs and have the most spread apart atoms.
- substances with weak IMFs are gases at room temperature, such as nitrogen gas. Substances with hydrogen bonds are liquid at room temperature, such as water.
- Ionic substances do not experience IMFs and have their phase determined by the ions in the bond. Ionic substances are usually solids at room temperature.
Vapor Pressure
- IMF's can predict vapor pressure, the more movement there is in a liquid, the more likely it is to become a gas.The more kinetic energy, the higher likelihood of overcoming IMFs and changing phases.
- Boiling is different than vaporization because boiling adds heat (energy) to cause the IMFs to break. Vaporization requires no excess heat to be added. However, the higher temperature of the liquid, the more likely the molecules are to break free of the IMF's
- Stronger IMF's = lower vaporization.
Solution Separation
- Using the different IMFs and Coulombic attractions of molecules, substances can be separated.
Solutes and Solvents
- Like dissolves like.
- polar substances will dissolve in polar solvents (salt in water). Nonpolar substances will dissolve in non polar solvents (oil). The dissociation, or breaking up, of ions can conduct electricity because they become electrolytes.
Paper Chromatography
- Through chromatography, a mixture can be separated by passing it through a medium and each substance in the mixture reacts differently. A common form of chromatography is paper chromatography. in paper chromatography, paper is suspended with one end submerged in the solvent that slows climbs up the paper and pulls apart the solvent as it goes up.
- Each substance has a different polarity and therefore a different attraction to the solvent. When black ink is separated via paper chromatography, the different colors in the ink are pulled apart. By determining which part moved the most with the solvent, you it can be determined which pert was the most polar or non polar (depending on whether or not the solvent is polar).
- The distance of the ink travelled is measured by the retention factor (Rf) where Rf = (distance travelled by solute) /(distance travelled by solvent front).
- Stronger attraction between solute and solvent front = stronger Rf value.
Column Chromatography
- Two liquids pass through stationary substances and the distance travelled is determined by polarity.
Distillation
- Two liquids with different boiling points are heated to the lowest temperature boiling point. The gaseous substance is collected and cooled to a liquid, which causes the two mixtures to be collected separately
Kinetic Molecular Theory
- Ideal gases have certain rules:
- The kinetic energy (KE) is directly proportional to the temperature. Higher temperature = higher KE.
- Kinetic energy of a single gas molecule = 0.5 (mv)^2
- m = mass of molecule in kg. v= speed of molecule in m/s. Ke measured in joules (j).
- If different gases are present at the same temperature, they will have the same KE
- The volume of a single ideal gas is negligible when compared to the volume the entire is contained in.
- There are no forces of attraction between a gas molecules and an ideal gas.
- Gas molecules are in constant motion, collides with the walls of the container and other gas molecules, and doesn't lose any energy.
Maxwell-Boltzmann Diagrams
A maxwell-boltzmann diagram shows the range of velocities for molecules of a gas. Even though KE is the same for gases at the same temperature, this cannot be said for velocity because gas molecules have different masses.
The first type of diagram involves plotting the velocity of molecules for one gas at different temperatures. The higher the temperature, the higher variation of velocity in particles.
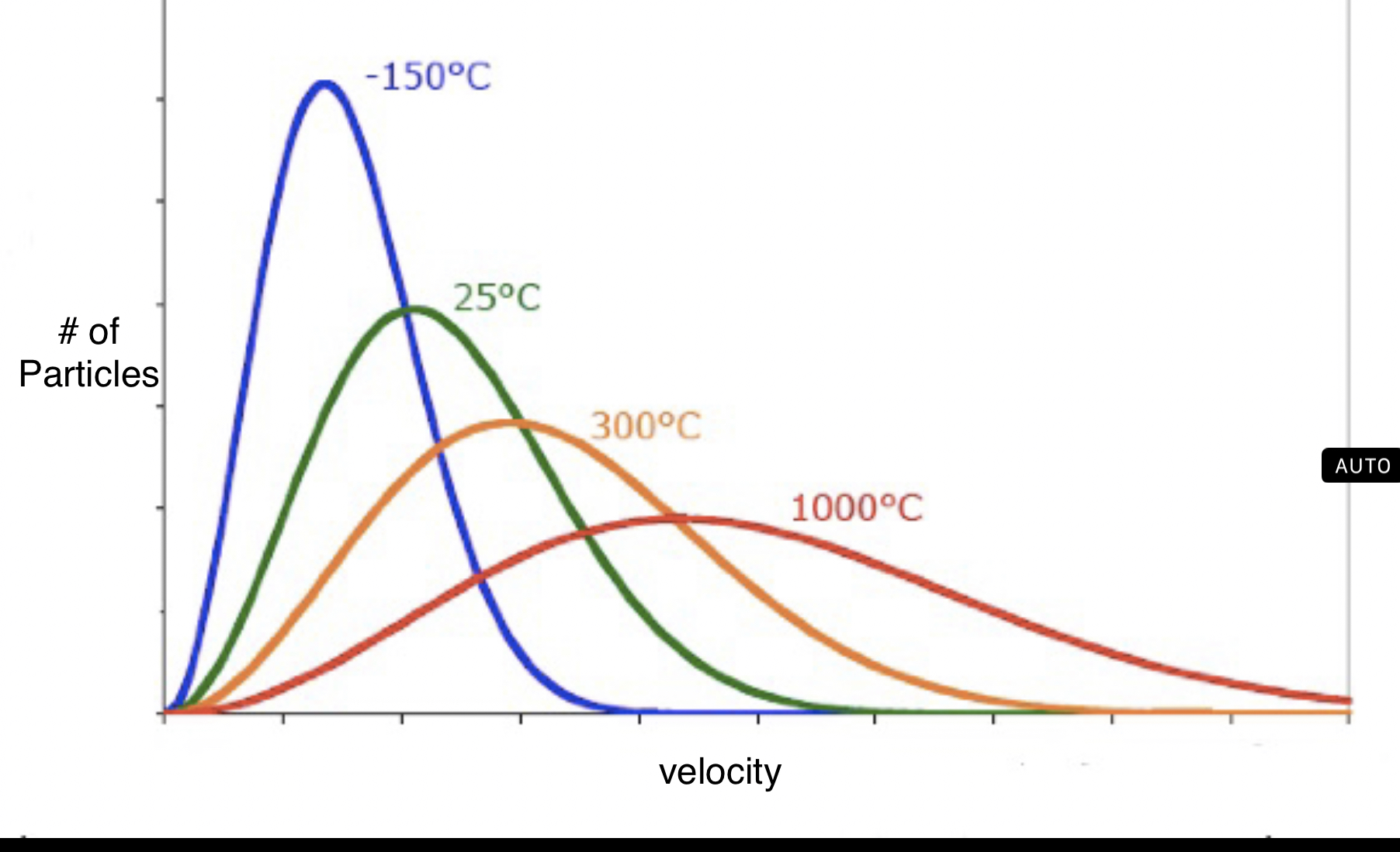
It can also show different gas velocities at the same temperature. Because oxygen gas weighs the most, it hasthe lowest velocity, but equal KE to hydrogen gas, which ways the least and has the highest velocity.
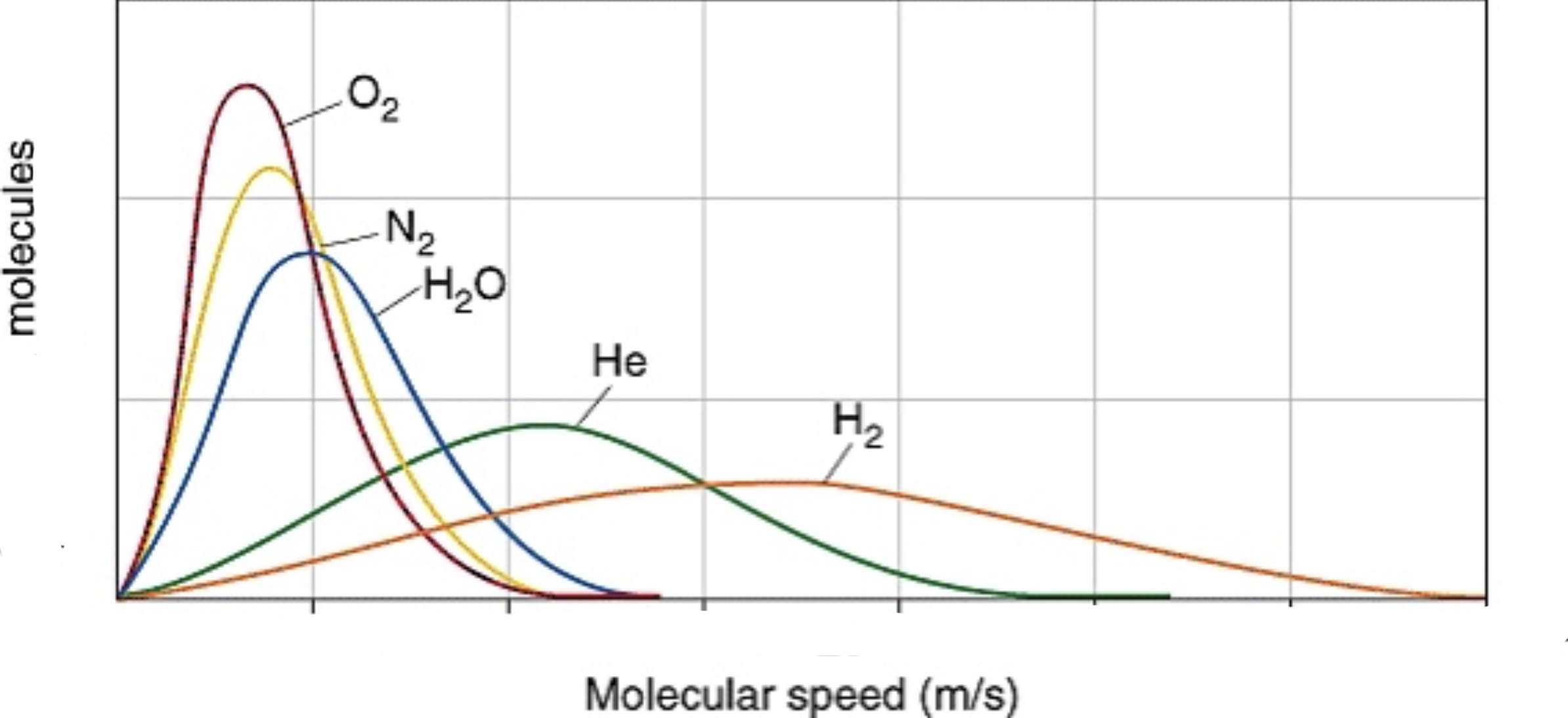
Effusion
- Effusion is the rate a gas will escape from a container with microscopic holes from high pressure to low pressure. A balloon has tiny holes which is why gas escapes slowly over time.
- Effusion rate depends on the speed of the molecule, temperature, and molar mass. The faster a gas is moving, the more collisions there are with the sides of the container, which makes them more likely hit a hole and escape. If the temperature is high, the rate of effusion is higher. A gas particle with a low molar mass will effuse before another gas with a higher molar mass.
The Ideal Gas Equation
The ideal gas equation is PV=nRT where P is pressure in atm, V is volume of the gas (L), n is the number of moles a gas, R is the ideal gas constant (.0821), and T is temperature in kelvin.
- Kelvin can be determined by adding 273 to a temperature in Celsius.
The combined gas law is used when moles are held constant
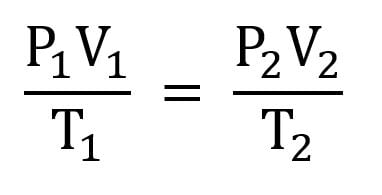
If the volume is constant: as pressure increases, temperature increases
If the temperature is constant: as pressure increases, volume decreases, according to Boyle's Law.
If the pressure is held constant: as temperature increases, volume increases, according to Charle‘s Law.
Dalton's Law
- Dalton's law states that the sum all of the partial pressures of individual gases in a container is equal to the total pressure of gases in the container.
- To find partial pressure of a gas in a container, divide the moles of that gas by the total moles of gas in the container and then multiply by the total pressure in the container. This is also called mole fraction.
Deviations From Ideal Behavior
- The kinetic molecular theory becomes invalid when the temperature is too low and/or the pressure is too high because the gases become too tightly packed.
- the volume of gases becomes significant because under high pressure the amount of volume of gas is taking up space that is significant when compared to the volume of the container.
- gas molecules start sticking together which affects pressure since there's less molecules bouncing around.
- Gases under normal conditions can deviate slightly if they have strong IMF's
Density
- Density of a gas can be calculated using D=m/V where D is density, m is mass of a gas in grams, and V is volume in liters.
- To find molar mass from density, use the equation MM=DRT/P
Electromagnetic Spectrum
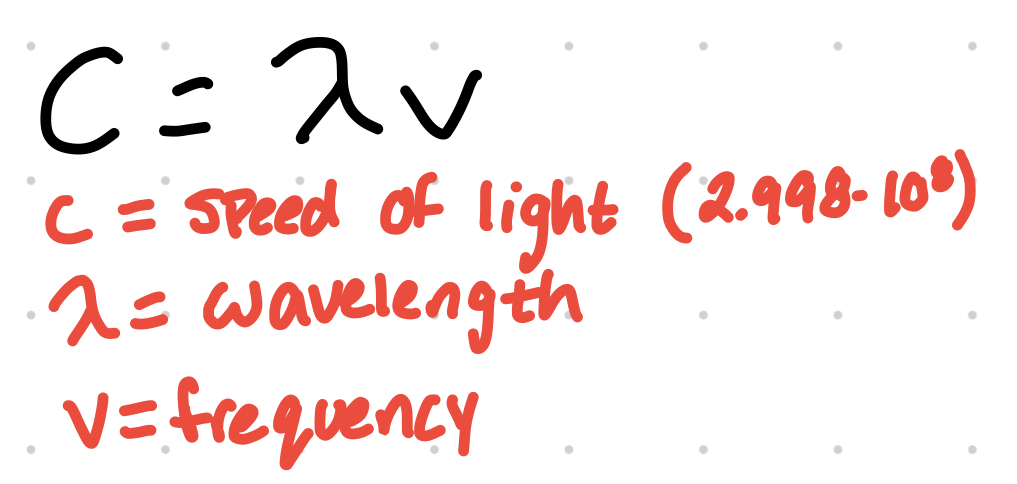
An electron changing energy levels and the amount of electromagnetic radiation absorbed is determined by E=hv where E is energy change (in joules), h is Planck's constant (6.626 x 10^-34 j•s), and v is frequency (in s^-1).
wavelength can be determined from frequency
Beer’s Law
- To measure the change of the concentration of a solution overtime, a spectrometer is used and can be calculated.
- A=abc where A is absorbance, a is molar absorptivity of the solution, b is pathlength (how for light is traveling in the solution), and c is concentration.