Energy Changes in Chemical Reactions
Definitions
closed chemical system - closed system containing only reactants and products
chemical thermodynamics - study of energy changes in chemical systems
enthalpy - a measure of the internal energy of the system
activated complex - unstable transition state from reactants to products
reactants - a starting material in a chemical reaction
products - a substance formed in a chemical reaction
exothermic reaction - energy (in the form of heat and light) is released in a chemical reaction
endothermic - energy is absorbed in a chemical reaction
activation energy - minimum energy required to get a reaction to start
enthalpy change - amount of heat energy absorbed or released in a chemical reaction
Enthalpy
a chem. system holds energy in the energy of the bonds and kinetic energy of molecules and atoms
enthalpy change
energy absorbed or released in a chemical reaction
Exothermic reactions
negative resultant energy
gives off energy
Endothermic reactions
absorb energy
positive resultant energy
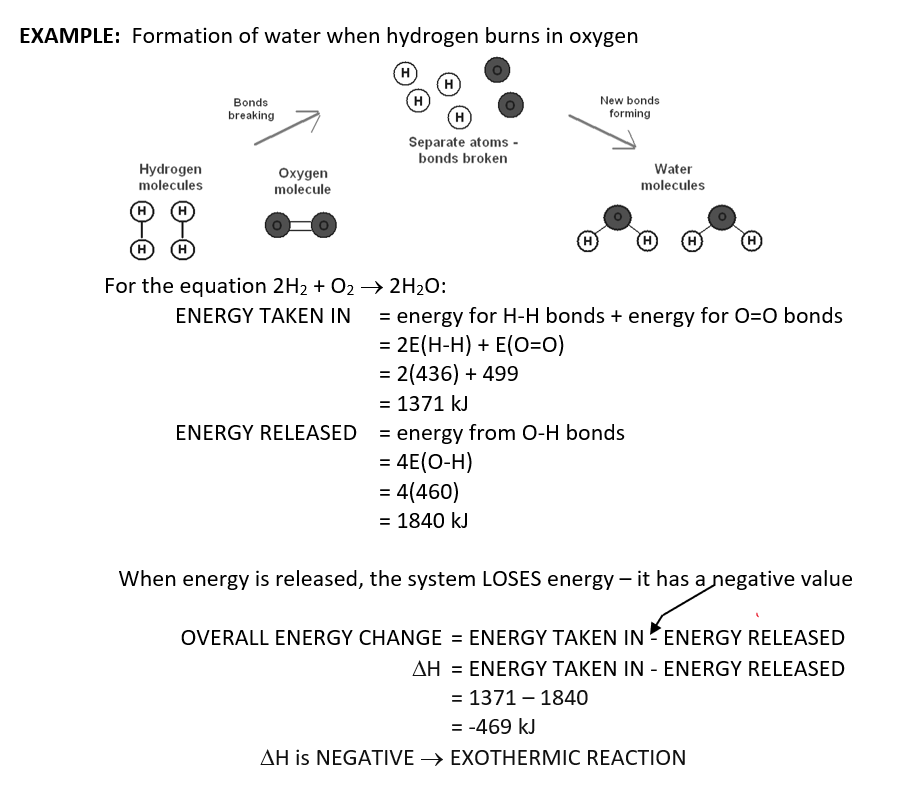
Chemical Bonds and Bond Energy
when a bond is formed → energy released to surroundings
when a bond is broken → energy taken in from surroundings
bond energy
energy taken in or given out when a mole of bonds broken
Activation Energy
minimum energy required for a reaction to take place
reactants → activated complex → product
very short amount of time, energy in system at maximum