Chapter 12: Alcohols, Ethers, Aldehydes, and Ketones
12.1: Alcohols, Phenols, Thiols, and Ethers
Alcohol (C₂H₆O)
- The functional group known as a hydroxyl group replaces a hydrogen atom in a hydrocarbon.
- In the IUPAC system, alcohol is named by replacing the e of the corresponding alkane name with –ol. The common name of simple alcohol uses the name of the alkyl group followed by alcohol.
- Methanol (CH3OH): The simplest alcohol and is found in many solvents and paint removers.
- Ethanol (C2H5OH): It has been known as an intoxicating product formed by the fermentation of grains, sugars, and starches.
- Glycerol (C3H8O3): A trihydroxy alcohol and a viscous liquid obtained from oils and fats during the production of soaps.
- Ethylene Glycol (C₂H₆O₂): It is used as an antifreeze in heating and cooling systems. It is also a solvent for paints, inks, and plastics, and it is used in the production of synthetic fibers.
- Bisphenol A (C15H16O2): It is used to make polycarbonate, a clear plastic that is used to manufacture beverage bottles, including baby bottles.
- Thiols (R-SH): These contain a sulfur atom, shown in yellow-green in the ball-and-stick model, which makes a thiol similar to alcohol except that —OH is replaced by an —SH group.
Phenol (C6H6O)
- The hydroxyl group replaces a hydrogen atom attached to a benzene ring.
- These are found in several of the essential oils of plants, which produce the odor or flavor of the plant.
- The term phenol is the IUPAC name for a benzene ring bonded to a hydroxyl group, which is used in the name of the family of organic compounds derived from phenol.
- Eugenol (C10H12O2): It is found in cloves, vanillins, isoeugenol, and thymol.
- Thymol (C10H14O): It has a pleasant, minty taste and is used in mouthwashes and by dentists to disinfect a cavity before adding a filling compound.
Ether (C2H5)2O
- The functional group consists of an oxygen atom, which is attached to two carbon atoms.
- They have a bent structure like water and alcohols except both hydrogen atoms are replaced by carbon groups.
- The name of each alkyl or aromatic group attached to the oxygen atom is written in alphabetical order, followed by the word ether.
- Example: Methyl Propyl Ether
- Ethers have been associated with anesthesia because diethyl ether was the most widely used anesthetic for more than a hundred years.
12.2: Properties of Alcohols
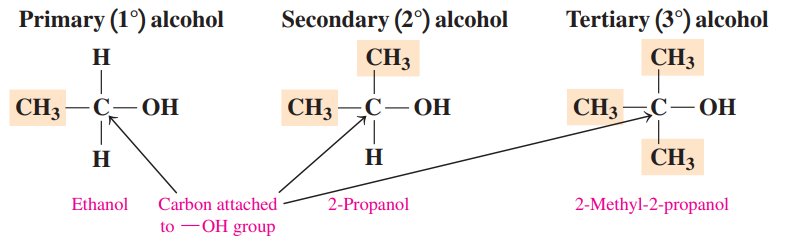
Primary (1°) Alcohol: It has one alkyl group attached to the carbon atom bonded to the –OH group.
Secondary (2°) Alcohol: It has two alkyl groups attached to the carbon atom bonded to the –OH group.
Tertiary (3°) Alcohol: It has three alkyl groups attached to the carbon atom bonded to the –OH group.
Solubility of Alcohols in Water
- Alcohols with one to three carbon atoms are miscible in water, which means any amount of the alcohol is completely soluble in water.
- Alcohols with four carbon atoms are slightly soluble in water, and alcohols with five or more carbon atoms are insoluble.
Solubility of Phenols
- Phenol is slightly soluble in water because the —OH group can form hydrogen bonds with water molecules.
- Phenol is very corrosive and highly irritating to the skin; it can cause severe burns, and ingestion can be fatal.
- Joseph Lister: He is considered a pioneer in antiseptic surgery and was the first to sterilize surgical instruments and dressings with phenol, which was initially named carbolic acid.
- Antiseptic: A substance applied to the skin to kill microorganisms that cause infection.
12.3: Aldehydes and Ketones
Aldehyde (C2H4O)
- The carbon of the carbonyl group is bonded to at least one hydrogen atom.
- That carbon may also be bonded to another hydrogen atom, a carbon of an alkyl group, or an aromatic ring.
- The aldehyde group may be written as separate atoms or as —CHO, with the double bond understood.
- In the IUPAC system, an aldehyde is named by replacing the e of the corresponding alkane name with –al.
- Example: Ethanal; Propanal; Butanal.
- The aldehydes with carbon chains of one to four carbons are often referred to by their common names, which end in aldehyde.
- Example: Formaldehyde; Acetaldehyde; Propionaldehyde
Ketones (CnH2nO)
- The carbonyl group is bonded to two alkyl groups or aromatic rings.
- The keto group (C = O) can sometimes be written as CO.
- In the IUPAC system, the name of a ketone is obtained by replacing the e in the corresponding alkane name with –one.
- Example: Propanone; Butanone; 3-Pentanone
- In the common names, the alkyl groups bonded to the carbonyl group are named as substituents and are listed alphabetically, followed by ketone.
- Example: Dimethyl ketone; ethyl methyl ketone
12.4: Reactions of Alcohols, Thiols, Aldehydes, and Ketones
In a dehydration reaction, alcohols lose a water molecule when they are heated with an acid catalyst such as H2SO4.
- The components H— and —OH are removed from adjacent carbon atoms of the same alcohol to produce a water molecule.
Oxidation of Alcohols: In organic chemistry, it involves the addition of oxygen or a loss of hydrogen atoms. There is an increase in the number of carbon–oxygen bonds.
- The oxidation of primary alcohol produces an aldehyde.
- The oxidation of secondary alcohol produces ketones.
- Tertiary alcohols do not oxidize readily because there is no hydrogen atom on the carbon bonded to the —OH group.
Reduction of Alcohols: In organic chemistry, the product has fewer bonds between carbon and oxygen.
Thiols also undergo oxidation by the loss of hydrogen atoms from each of the two —SH groups. The oxidized product contains a disulfide bond.
Aldehydes oxidize further by the addition of another O to form a carboxylic acid, which has a carboxyl functional group.
Aldehydes and ketones are reduced by sodium borohydride or hydrogen.
- Aldehydes reduce to primary alcohols, and ketones reduce to secondary alcohols.
- A catalyst such as nickel, platinum, or palladium is needed for the addition of hydrogen to the carbonyl group.
Tollens’ Test: It uses a solution of Ag+ and ammonia, which oxidizes aldehydes but not ketones.
The silver ion is reduced and forms a “silver mirror” on the inside of the container.
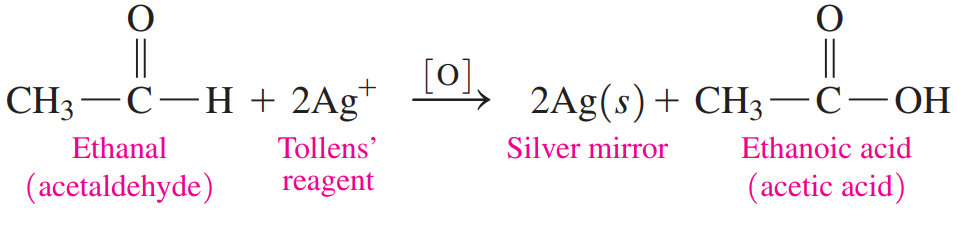
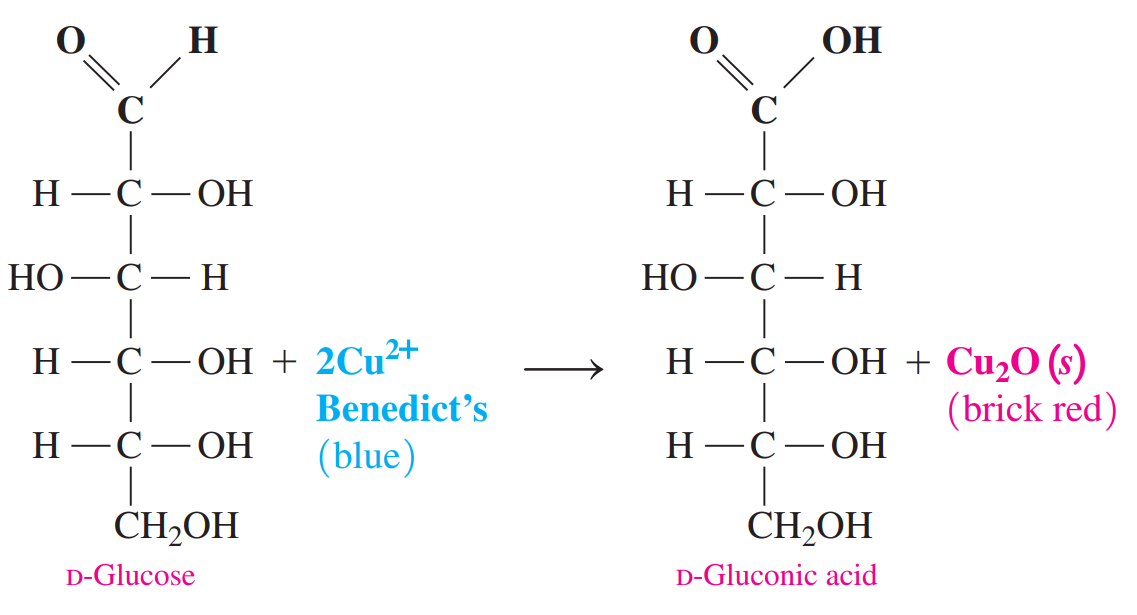
Benedict’s Test: It gives a positive result with compounds that have an aldehyde functional group and an adjacent hydroxyl group.
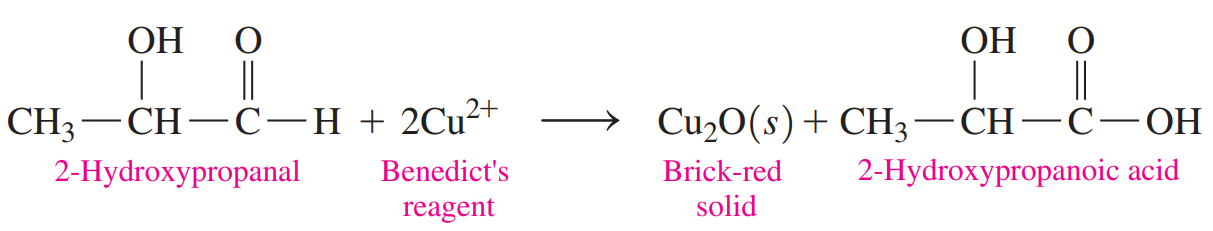
Because many sugars, such as glucose, contain this type of aldehyde grouping, Benedict’s reagent can be used to determine the presence of glucose in blood or urine.