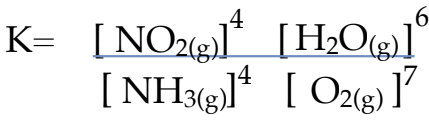Equilibrium Law & Equilibrium Constant
Equilibrium Constant Expression
it is the mathematical description of a chemical system at equilibrium using a balanced chemical equation at constant temperature
if we represented an equation as:
aA + + bB ←→cC dD
The equilibrium constant expression would be:
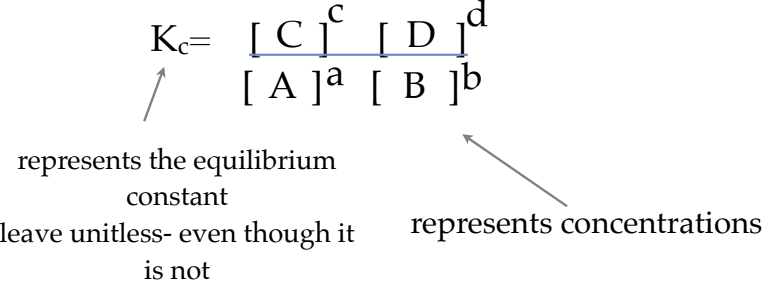
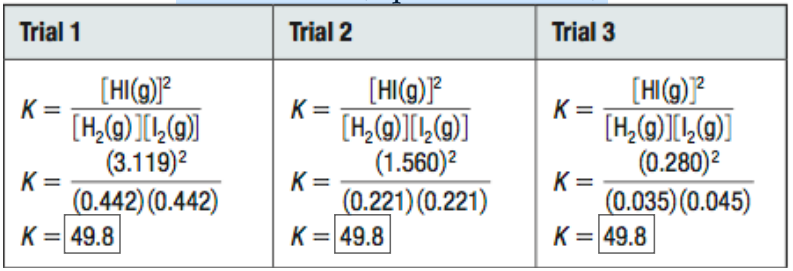
Kc ONLY changes with TEMPERATURE. If all other variables remain constant, Kc will remain unchanged for that reaction (equilibrium law)!
Writing equilibrium laws & calculating k
Write the equilibrium equation for the reaction described below
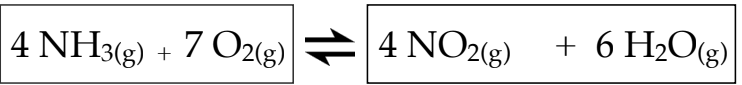
Identify reactants, products and their coefficients.
Apply the equilibrium law equation