AN SCI 320 FINAL
Defining Health and Disease - 01/25/24
Health: a state of complete physical, mental, and social well-being, not merely the absence of disease or infirmity.
DIsease: condition of living animal or plant body or of one its parts that impairs normal function, and is typically manifested by distinguishing signs and symptoms.
Defining “disease” is pretty hard
Disease is relative to what people consider “normal”
Can be subjective: “one person's diarrhea is another person normal day
Some believe that our use of “health” and “disease” reflect value judgments
The definition can change with time as our knowledge evolves
Illness: a person's subjective experience of their symptoms. What the patient brings to their health care provider
Disease: underlying pathology: biologically defined: the healthcare provider’s perspectives
Sickness: social and cultural conception of this condition: cultural beliefs and reactions such as fear or rejection. These affect how the patient reacts.
Naturalism
Most prominent philosophical approach to defining health and disease
Reference Class: a natural class of organisms of uniform functional design: specifically, an age group or a sex of species
Normal Function: part or process within members of the reference class is a statistically typical contribution by it to their individual survival and reproduction
Disease: type of internal state which is either an impairment of normal functional ability
Health: the absence of disease
Criticisms:
Neglects the role values play in determining healthy or diseased
Provide definitions that rely exclusively on info from the biological sciences, but, lacks a basis in biological theory
Normativism
Disease is deviancy from some alternative state of affairs which is considered more desirable
Suggest that we both la people and medical professionals, should use health and disease in ways that reflect our values
Physiological or psychological states that we desire are called healthy and those we want to avoid are labeled diseases
Criticisms
Cases where we agree that a state is undesirable but we disagree over whether it is a diseased state (ex. Overweightness, PMS)
Values can change
Hybrid Theories
A condition is a disorder if and only if (a) the condition causes some harm or deprivation of benefit to the person as judged by the standards of the person’s culture (the value criterion), and (b) the condition results in the inability of some internal mechanism to perform its natural function, wherein natural function is an effect that is part of the evolutionary explanation of the existence and structure of the mechanism (the explanatory criterion)”
The term disease should only apply to dis-valued states with the proper biological etiology
Criticism
A state where there is no evolutionary dysfunction yet we disvalue that state
What influences the balance between health and disease?
Is aging a disease?
Colonization vs Infection - 01.30.24
Colonization (normal flora)
Colonizing cells = 39 trillion
Normal Flora
Most areas of the body in contact with the outside environment harbor resident microbes
Microorganisms that normally reside at a given site and under normal circumstances do not cause disease
Normal flora is essential for health: (a) create an environment that may prevent infections and (b) enhance host immune defenses
Internal organs, tissues and fluids are microbe-free (relatively)
Transient flora
Occupy the body for only short periods
Usually picked up during daily activities (hand-shake, touch a door-knob, kissing)
Often eliminated easily (hand-washing)
Resident flora
Are permanently established (or for long periods of time)
Types of Relationships with Microbiome
Mutualism
Both the host and the microbe benefit
Examples: ruminants and their gut microorganisms (2) E. coli-microbe receives nutrients, but produces vitamins K and B- complex
Commensalism
One partner benefits, and the other neither benefits or is harmed
Parasitism
One organism benefits at the expense of the host
Cost to the host can vary from slight to fatal
An external parasite (flea, ectoparasite) is said to cause infestation
An internal parasite (endoparasite, tapeworm) is said to cause infection
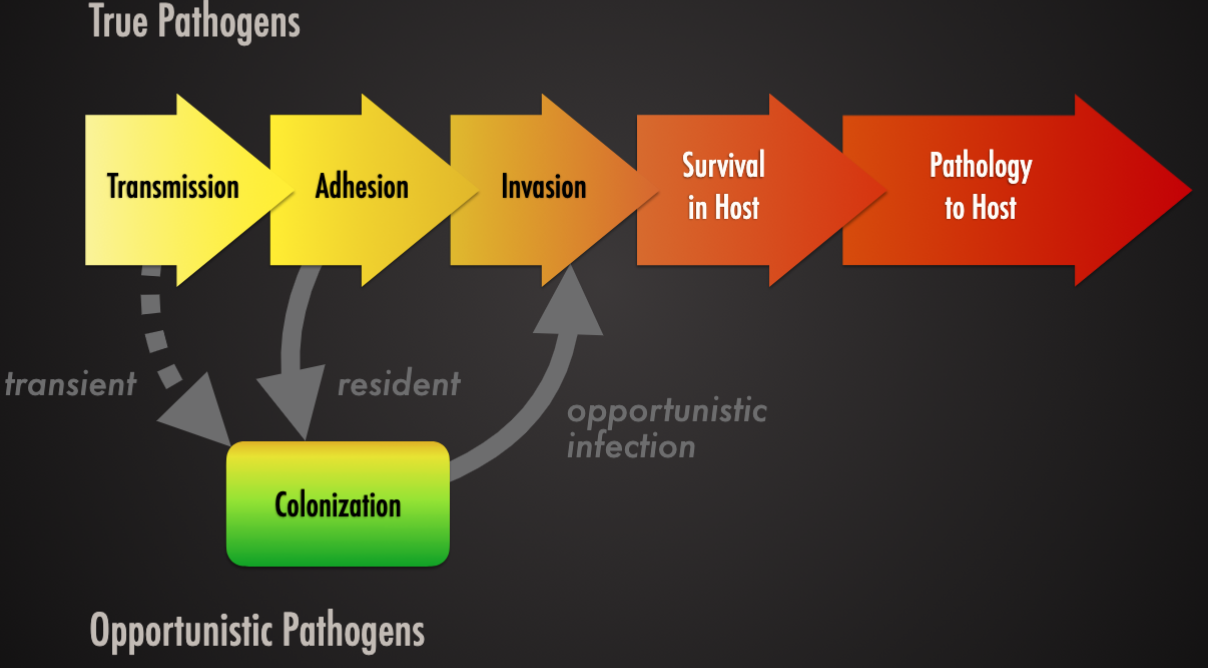
Pathogenic
Organism causes damage to the hose during infection
Distribution and Diversity of Colonization
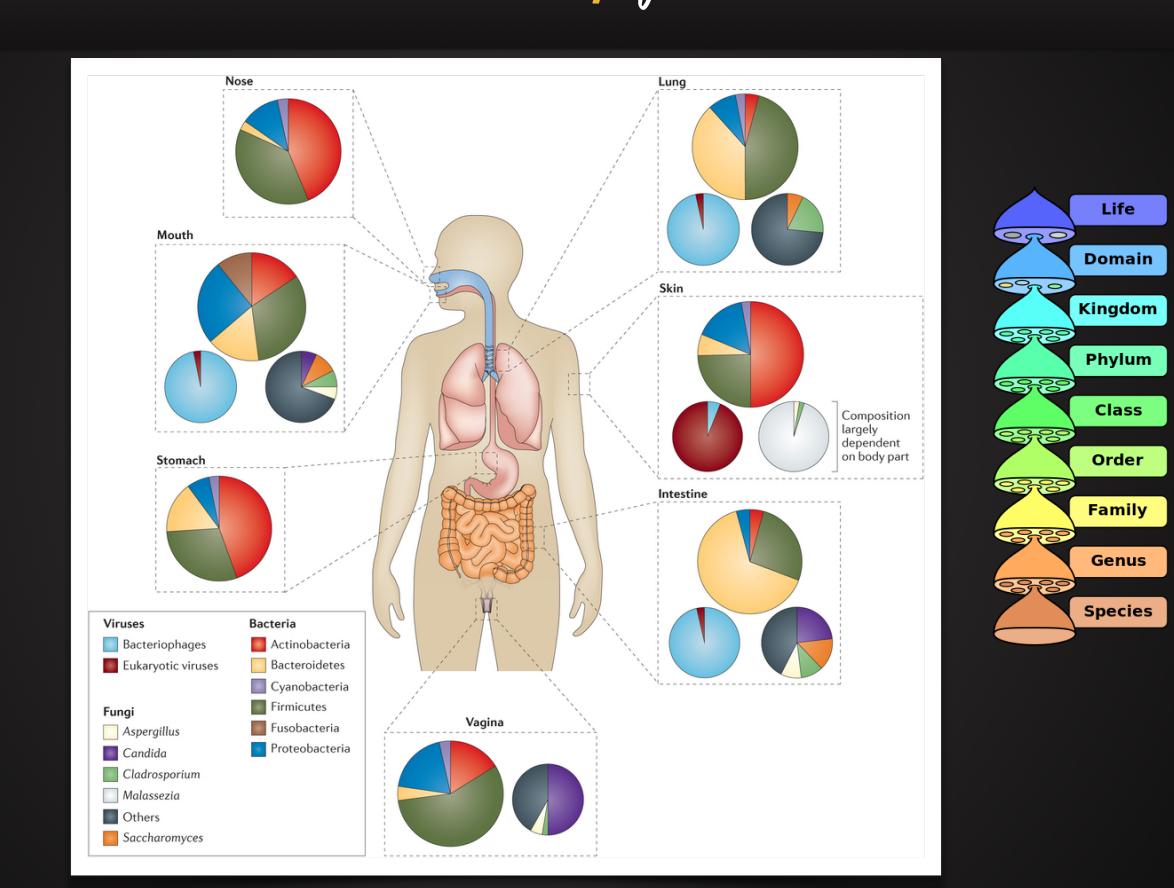
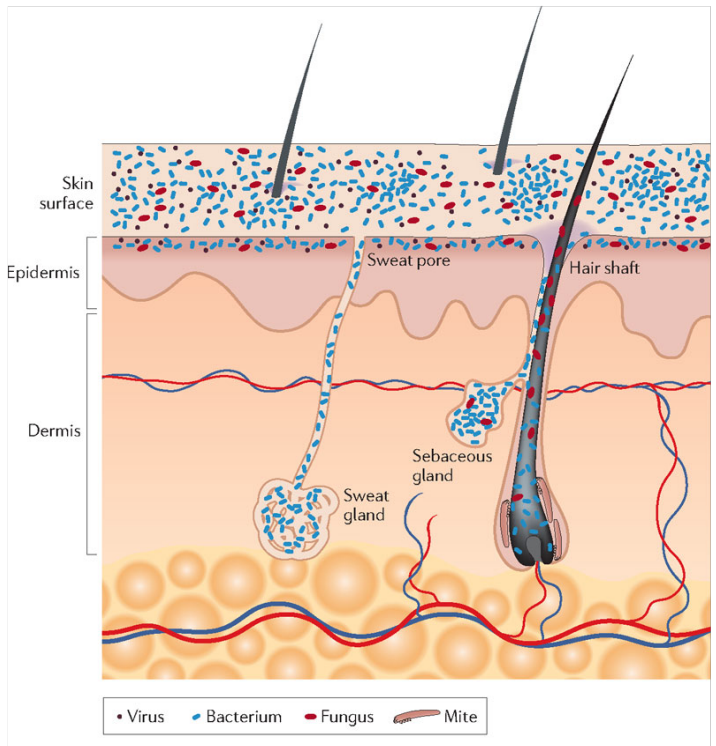
Microbial Colonization of the skin
Initial Colonization
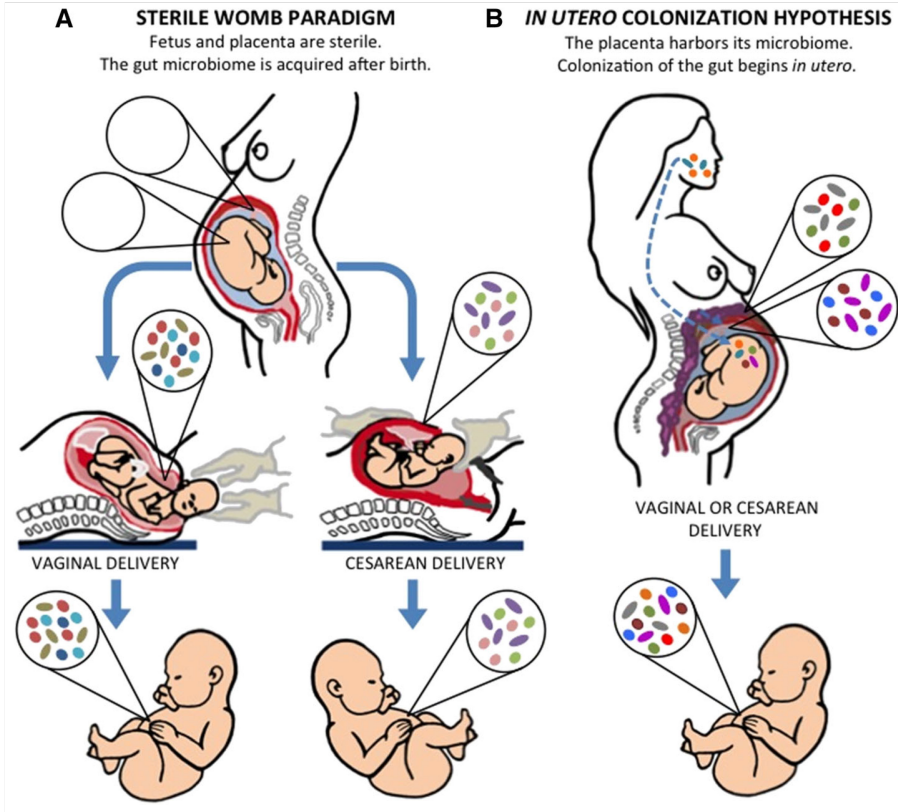
Prevailing paradigm
Uterus and contents are normally sterile and remain so until just before birth
Breaking of fetal membrane exposes the infant: all subsequent handling and feeding continue to introduce what will be normal flora
Is this paradigm entirely correct? → currently a controversial topic
Factors that influence Initial Colonization
Maternal factors
Gut microbiota
Vaginal health
Periodontal disease (other infections)
Genetics, diet, antibiotics
Birth
Vaginal vs. Cesarean delivery
Postnatal Factors
Genetics
Breastfeeding vs formula milk
Medications and antibiotics
Diet
Environment
Defining Infection
Infectious agent: viruses, bacteria, fungi, protozoa, worms and prions
Infection: condition in which infectious agent penetrates host defenses
Infectious disease: an infection that causes damage or disruption to tissues and organs and/or physiological homeostasis
Endogenous infections
Occurs when normal flora is introduced to a site that was previously sterile (epidermis infection of wound)
Exogenous infections
Caused by organisms that are normally present in the body, but have gained entrance from the environment (influenza virus infection and respiratory tract)
Types of pathogen
True pathogen (influenza virus): infectious agent that causes disease in virtually any susceptible host
Opportunistic pathogen (pseudomonas, candida albicans): normally harmless: causes disease when the normal flora is disrupted (by antibiotics) or when the host is immunocompromised ( by drugs or other illnesses ).
Patterns of Infection
Localized infection: infectious agent enter the body and remains confined to a specific issue
Systemic infection: infection spreads to several sites and tissue fluids usually the bloodstream
Focal infection: infectious agent breaks loose for a local infection and is carried to other tissues
Mixed infection: several microbes grow simultaneously at the infection site (poly microbial)
Primary infection: refers to the first time you are exposed to (and infected by) a specific pathogen (UTI)
Secondary infection: another infection by a different microbe succeeding a primary infectionAcute Infection: comes rapidly, with severe but short-lived effects
Chronic (persistent) infection: progresses and persists over a long period of time
Infections that go unnoticed
Asymptomatic (subclinical) infections: although infected, the host does not show any signs of disease
Inapparent infection, so the individual does not seek medical attention
Ex. typhoid, chlamydia, HIV-1, epstein-Barr virus, Gonorrhoea
Mary Mallon, “Typhoid Mary” (1869 – 1938)
First person in the United States identified as an asymptomatic carrier of Salmonella Typhi (typhoid fever).
She was presumed to have infected 51 people, three of whom died, over the course of her career as a cook.
She was twice forcibly isolated by public health authorities and died after a total of nearly three decades in isolation
Acquisitions and Transmission of infectious agent
Communicable infection
Infected host an transmit the infectious agent to another host
Highly communicable infection is contagious
Non-communicable infection
Infection does not arise through transmission from host to host
Occurs primarily when a composed person is invaded by his/her own normal flora
Contracted organism from natural, non-living reservoir
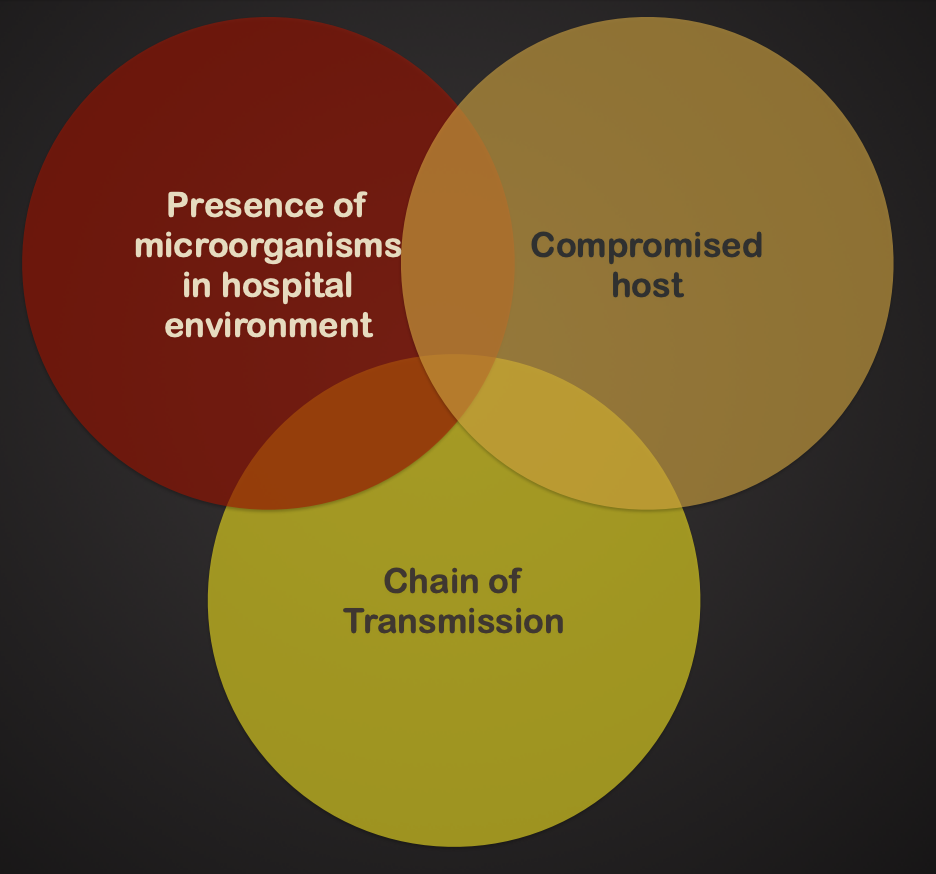
Nosocomial Infections
Infections acquired or developed during a hospital stay.
From surgical procedures, equipment, personnel, and exposure to drug-resistant microorganisms
2-4 million human cases/year in the US. 90,000 deaths. Impact in veterinary medicine not well studied.
What defines a particular disease?
Signs: (objective evidence)
Something that can be detected/measured by someone else (tachycardia, petechiae)
Symptoms: (subjective evidence)
Something that must be described by the one suffering the disease (anxiety, pain, fatigue)
Syndrome: the complete set of signs and symptoms associated with a specific disease (metabolic syndrome)
Introduction to Epidemiology 02.01.24
Roots of Epidemiology
Epidemiology is a fundamental science of public health.
Epidemiology has made major contributions to improving population health.
Epidemiology is essential to the process of identifying and mapping emerging diseases.
There is often a frustrating delay between acquiring epidemiological evidence and applying this evidence to health policy.
EPIDEMIOLOGY
The study and analysis of the patterns (frequency and distribution) causes and effects of disease and health -related factors in populations
Epi “on, upon, befall”
Demo “people”
-ology - study of
Epidemiology: the study of which befalls people
Cornerstone of public health: shapes policy decisions and evidence-based practice
Major areas of epidemiological study include
Disease etiology
Transmission
Outbreak investigation
Disease surveillance and screening
Forensic epidemiology and screening
Biomonitoring
Comparison of prevention/treatment outcomes
Epidemiologist rely on:
Scientific disciplines to better understand disease
Statistics for efficient use of data and draw appropriate conclusions
Social sciences to better understand proximate and distal causes
Other fields for exposure assessment
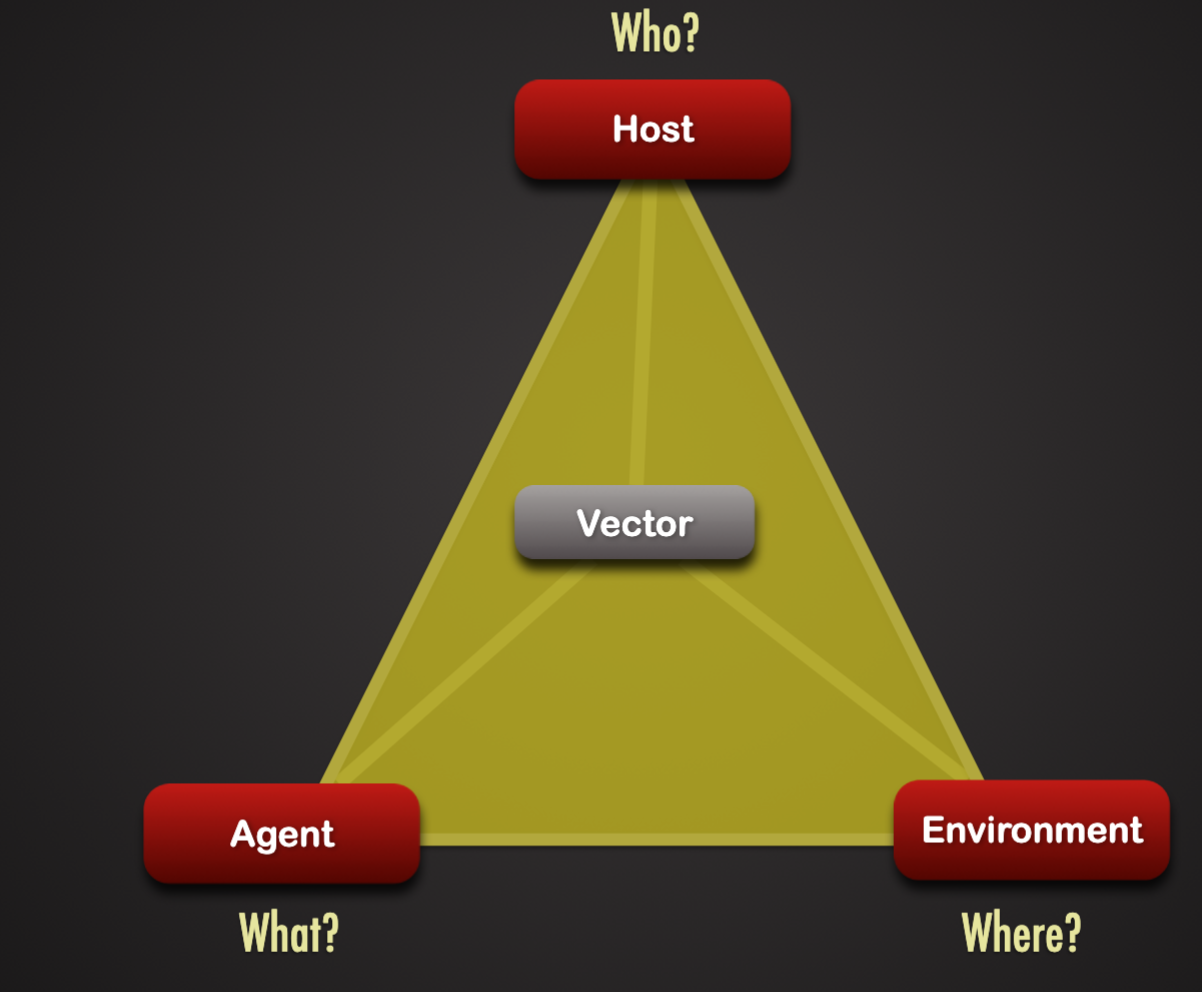
Host:
Immunity
General health
Age
Gender
Genetic background
religious/cultural practices
Agent
Pathogen traits
Virulence
Dose
Incubation period
Environment
Heat or cold stress
Food availability
Hygiene
Crowding
Cultural practices
Presence of vectors or reservoirs for pathogen
Pathogen Traits
Types:
Viruses, bacteria, fungi, protozoa, worms and prions
Other information (strain, serotypes, etc, ex. Salmonella enterica serovar Typhimurium)
Virulence
Ability to cause severe disease
Virulence factors: specific mechanisms that allow pathogen to adhere to or penetrate host cell, thwart immune defenses, damage host
Infectious dose:
Minimum number of pathogens required to cause illness
Incubation period:
Time it takes after first exposure for the pathogen to cause signs and symptoms: influences extent of spread
Virulence: Infectious Dose (ID)
Minimum number of microbes required to cause infection in the host
Smaller the ID, the greater virulence
If ID is not reached, infection will not occur
ID50 (median infectious dose): amount of pathogenic microorganism that will produce demonstrable infection in 50% of exposed hosts

Host Traits
Immunity to pathogen
Previous exposure, immunization
Antigenic variation of pathogen can overcome
General Health
malnutrition , overcrowding, fatigue
Developing wor;d more susceptible: crowding, poor nutrition poor sanitation
Age
Very young, elderly generally more susceptible
Immune system less developed in young: wanes in old
Elderly also less likely to update immunizations
Genetic background
Natural immunity varies widely
Specific receptors critical for infection may differ in individuals (african swine fever: domestic pigs s warthogs)
Sickle cell gene and resistance to malaria
Gender
Females more likely to develop urinary tract infections
Urethra is shorter: microbes more likely to ascend
Pregnant animals are at more risks
Pregnant animals can pass on some disease to offspring
Religious and cultural practices
Breastfeeding provides protective antibodies to infants
Consumption of raw fish can increase exposure (ex. Freshwater fish and tapeworm Diphyllobothrium latum).
Environmental Factors
Environmental factors may increase likelihood of disease transmission opportunities or lower the hosts resistance to infection
Heat or cold stress
Food availability
Hygiene
Crowding
Cultural practices
Presence of vectors or reservoirs for pathogen
Routes of Transmission
Direct contact: physical contact or fine aerosol droplets
Some pathogens cannot survive in environment
Hand washing considered single most important measure for preventing spread of infectious disease
Horizontal vs vertical transmission
Indirect contact: passes from infected host to intermediate conveyor and the to another host
Some pathogens can survive for a period outside of the host
Fomite: inanimate object that serves a role in disease transmission (pens, cups, doorknobs, clothing, boots, etc)
Vector: any agent (insect, animal, or microorganism) that carries a pathogen and transmits it (mechanical or biologically) to human or animal hosts
Vehicle: typically food, water, or air (droplet nuclei, aerosols) that transmits a pathogen to the host
Reservoir: the natural habitat (living or nonliving) in which a pathogen lives and reproduces that serves as a source of infection
Living reservoir may be symptomatic or asymptomatic
Asymptomatic:: harder to identify and control spread (up to 50% of women infected with Neiserria gonorrhoeae are asymptomatic).
Exclusively human reservoirs are easier to control (Smallpox)
Non-human reservoirs (arthropod, wild animal) challenging to control)
Environmental reservoir: difficult or impossible eliminate (Bacillus anthracis)
Portals of entry and exit
Skin: nicks, abrasions, punctures, incisions
Gastrointestinal tract: food, drink, and other ingested materials
Respiratory tract: oral and nasal cavities
Urogenital tract: sucal, displaces organisms
Transplacental
feces/urine
semen/ vaginal secretions
Sputum
Saliva
Blood
Pus and lesion exudates
Tears
Vomit
Cycle of transmission
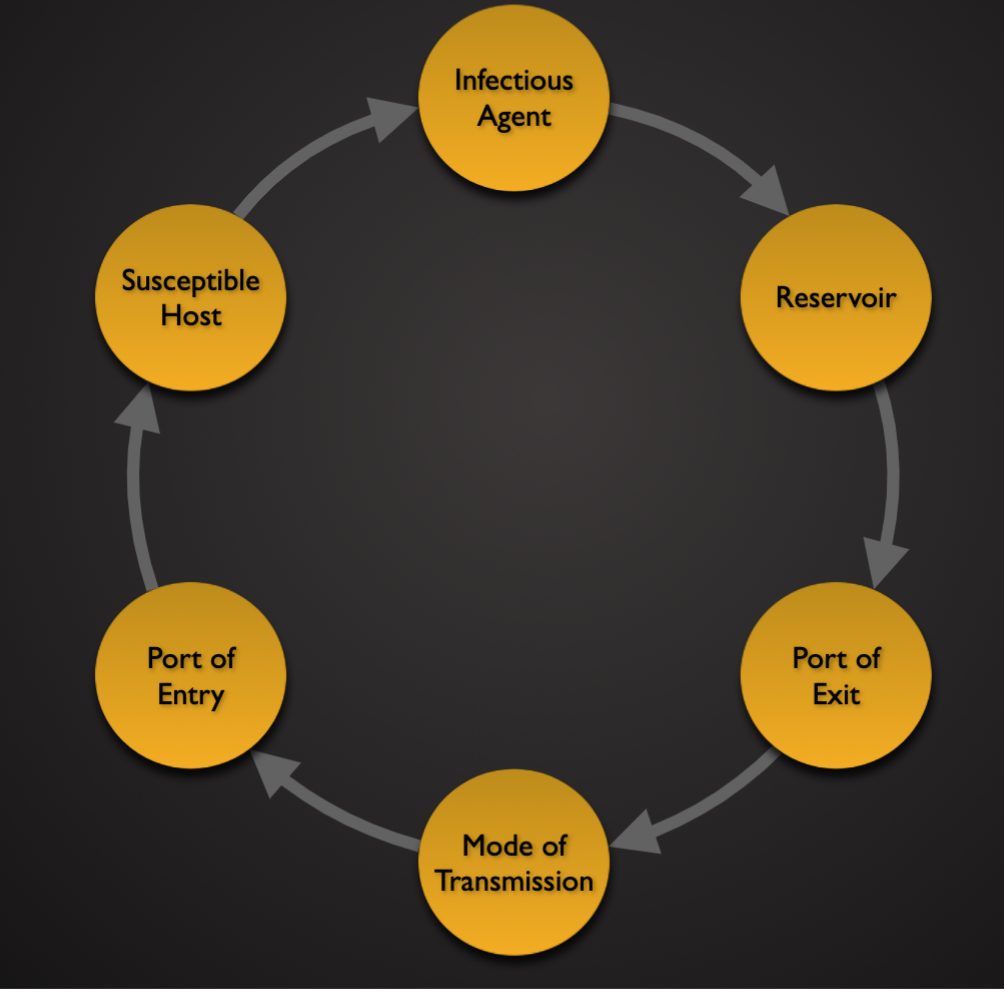
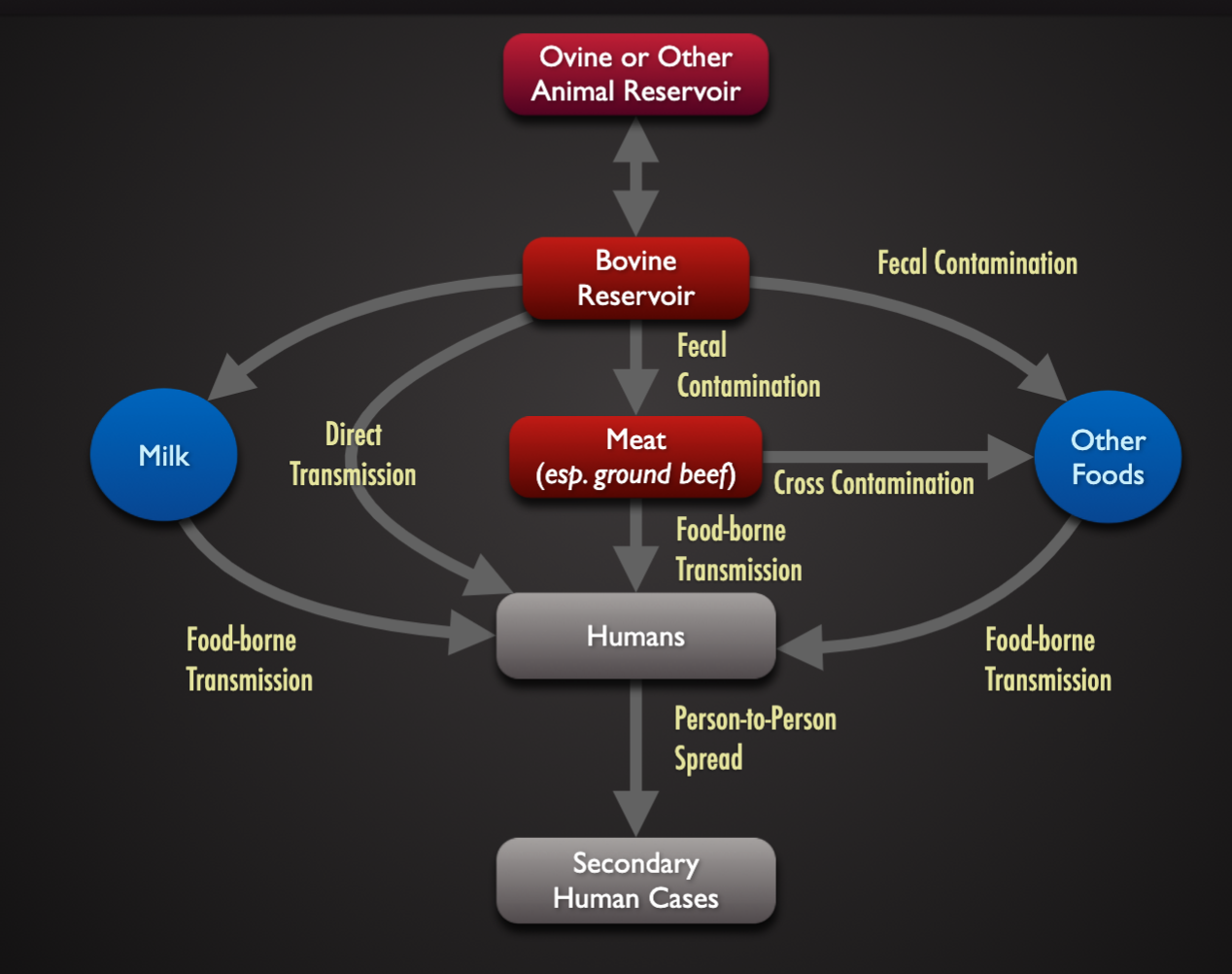
Reservoirs and Modes of Transmission
Epidemiology Part 2 - 02.06
Frequency of Cases
Prevalence: the total number or proportion of cases or events or conditions in a given population
Incidence: the number of new cases during a specified time period
Morbidity rate: number of people affected with certain diseases during a given period of time
Mortality rate: number of deaths in a population due to certain disease during a given period of time
Case-fatality rate: the percentage of people with a specific disease that dies from that disease
Attack rate: number of people affected by a disease divided by the number of people with a specific exposure
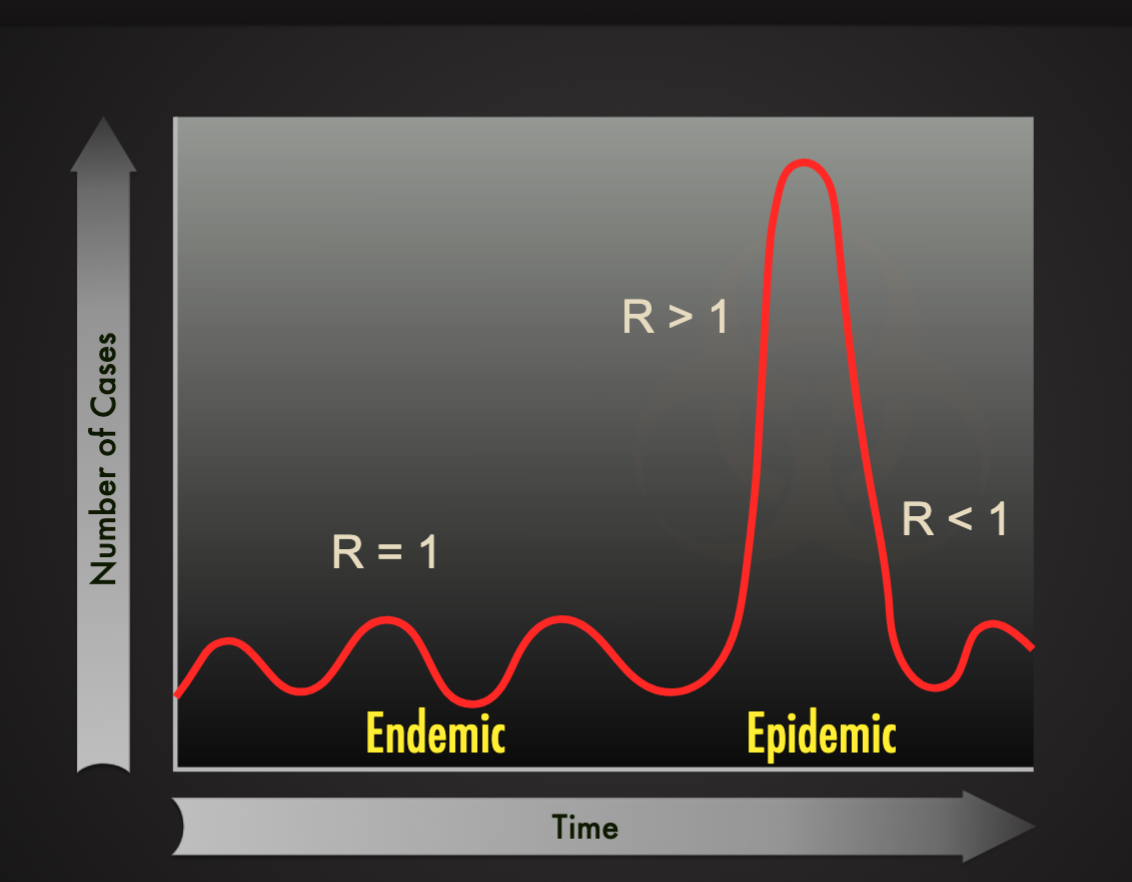
Disease Occurrence Patterns
Endemic: a relatively steady frequency over a long period of time in the particular geographic locale (ex. Common cold)
Sporadic: when occasional cases are reported at irregular intervals (ex. Rabies in the US)
Epidemic: increasing prevalence of a disease beyond what is expected (ex. Porcine epidemic diarrhea virus (PEDV) in the US in 2013)
Pandemic: epidemic across countries and continents (HIV/AID, COVID-19)
In an outbreak of aflatoxicosis among finishing pigs in a farm of 200 pigs: 100 pigs ate feed mixed using an old batch of corn. 20 pigs first began vomiting. A day later, 30 more pigs showed similar symptoms. Over the course of the week, 10 pigs died.
What is the prevalence of aflatoxicosis? 25%
What is the case fatality rate? 20%
What is the attack rate? PB: 50%
Basic Reproductive Number (R0)
The average number of new infectious generated by one infection in a completely susceptible population
Measure of the intrinsic potential of an infectious agent to spread
R0 = C x P x D
C = contact rate (contact/time)
The average rate of contact between susceptible and infected individuals
P = transmissibility (infection/contact)
The probability of infection given contact between a susceptible and infected individuals
D = duration of infectiousness (time/infection)
Effective Reproductive Number ( R )
A population will rarely be totally susceptible to an infection in the real world. The effective reproductive rate ( R ) estimates the average number of secondary cases per infectious case in a population made up of both susceptible and non-susceptible hosts.
R= R0 x S
R0 = Basic reproductive number
S = fraction of the host population that is susceptible
Endemic vs. Epidemic
Newyrok of Infection
Rate of spread of an infection depends on R0 and serial interval
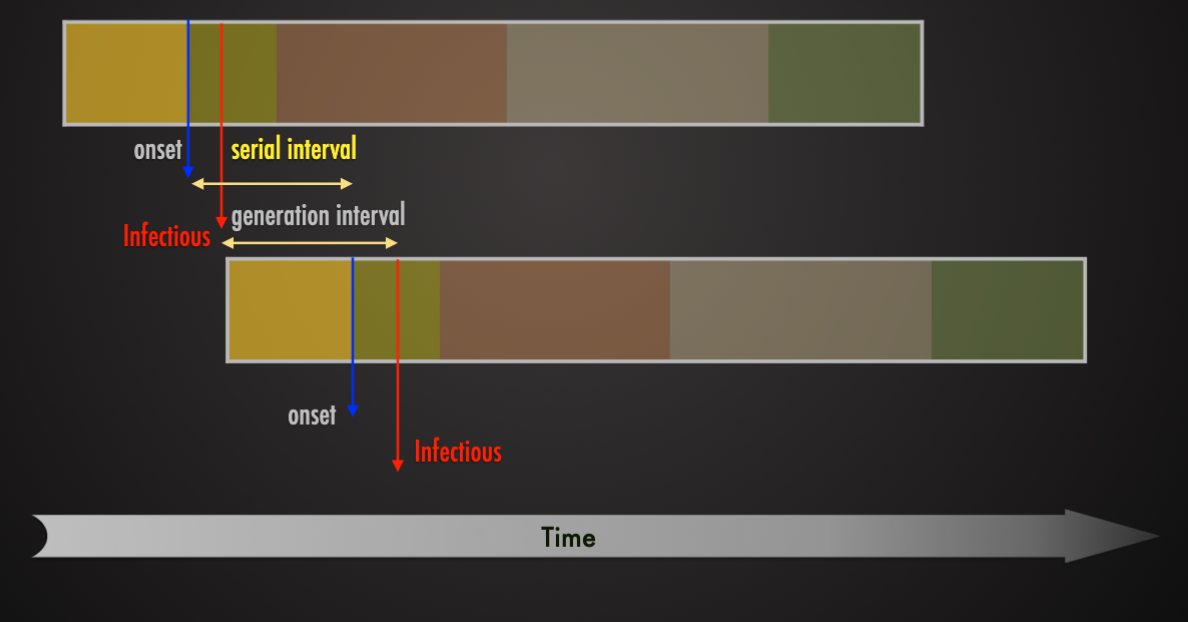
Serial Interval
The time between the same stage of illness in successive clinical cases in a chain of transmissions
SARS (Severe acute respiratory syndrome)
Outbreak: November 2022 - July 2033
Causes by SARS coronaviru (SARS-CoV)
Spread across 37 countries and regions
Infected 8,098 people (reported cases)
Killed 774 people (killed 1 in 10 people infected)
Spatial Heterogeneity
Rural Areas
Urban Areas
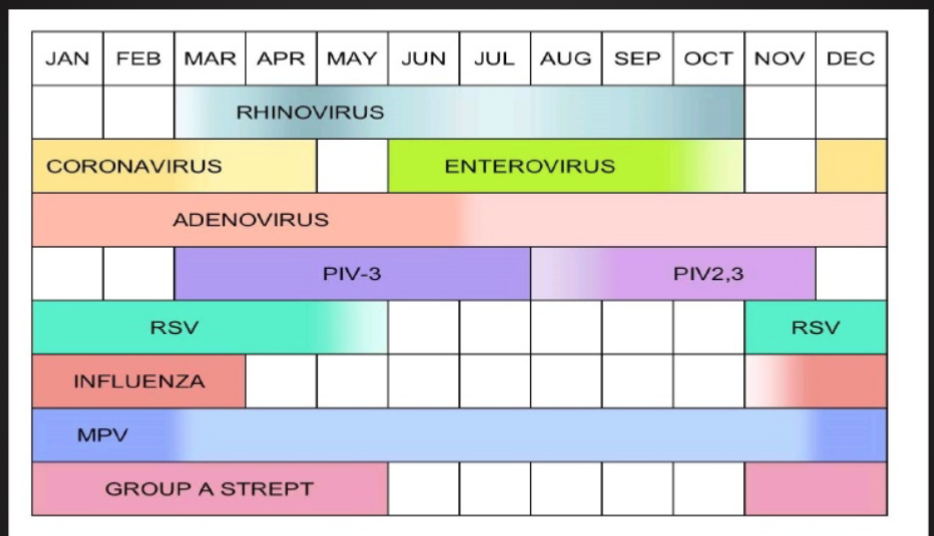
Seasonality of Infections
Why? What causes seasonality?
Climate, temperature, moisture levels, allergies
Impact of Movement and Modern Transport
Pathogenesis of Infections 02.08.24
Germ Theory
Robert koch
Louis pasteur
Koch’s Postulates
The microorganism must be found in abundance in all organism suffering from the disease, but should not be found in healthy organisms
The microorganisms must be isolated from a diseased organism and grown in pure culture
The cultured microorganisms should cause disease when introduced into a health organism
The microorganism must be reisolated from inoculated, diseased experimental jost and identified as being identical to the original specific causative agent
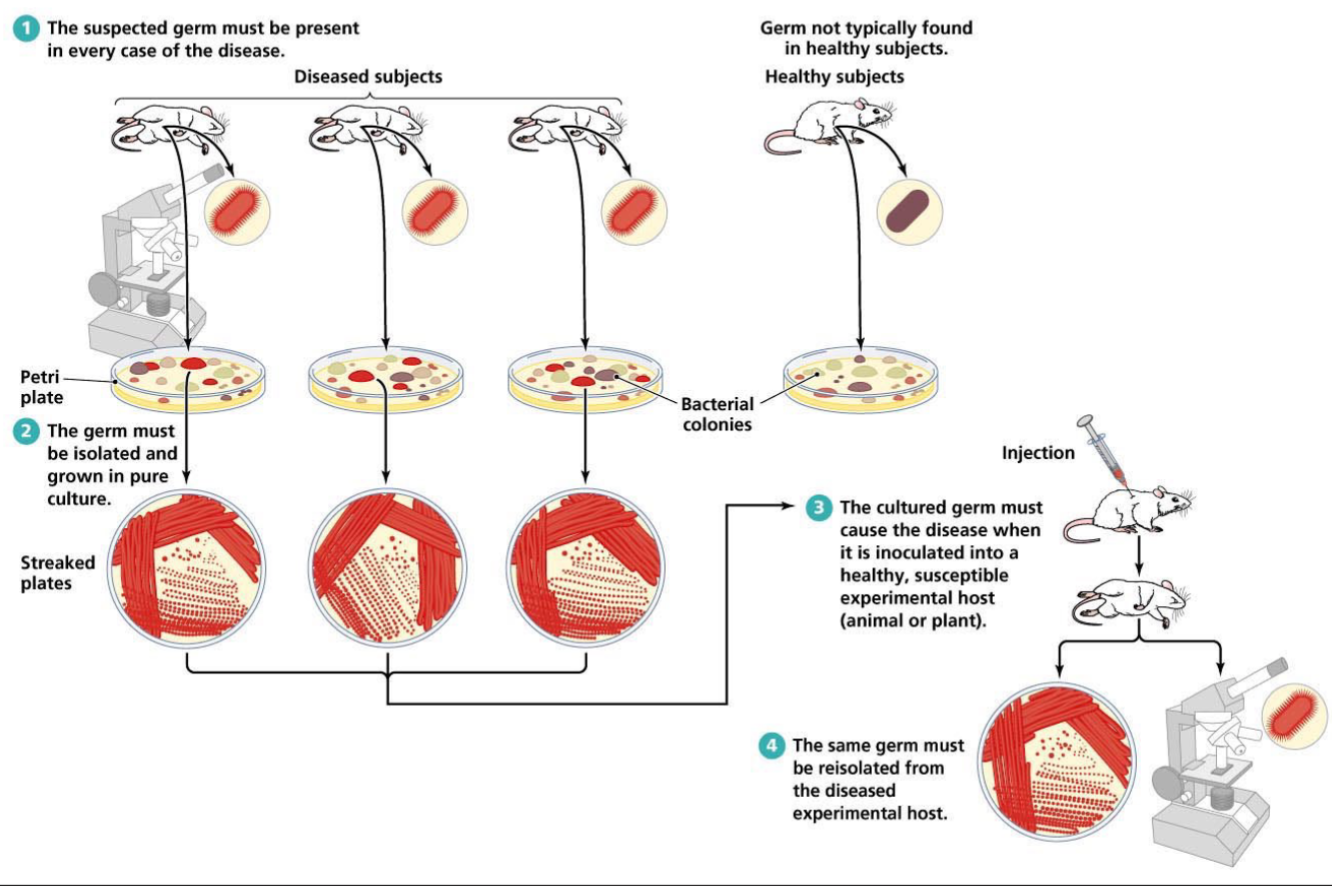
The suspect germ must be present in every case of the disease
The germ must be isolated and grown in pure culture
The cultured germ must cause the disease when it is inoculated into health, susceptible experimental host (animal or plant)
The same germ must be reisolated from the diseased experimental host
Infectious Agents
Bacteria
Viruses
Protozoa
Fungi
Parasitic worms (helminths)
Prions
Introduction to Bacteria
Bacterial taxonomy
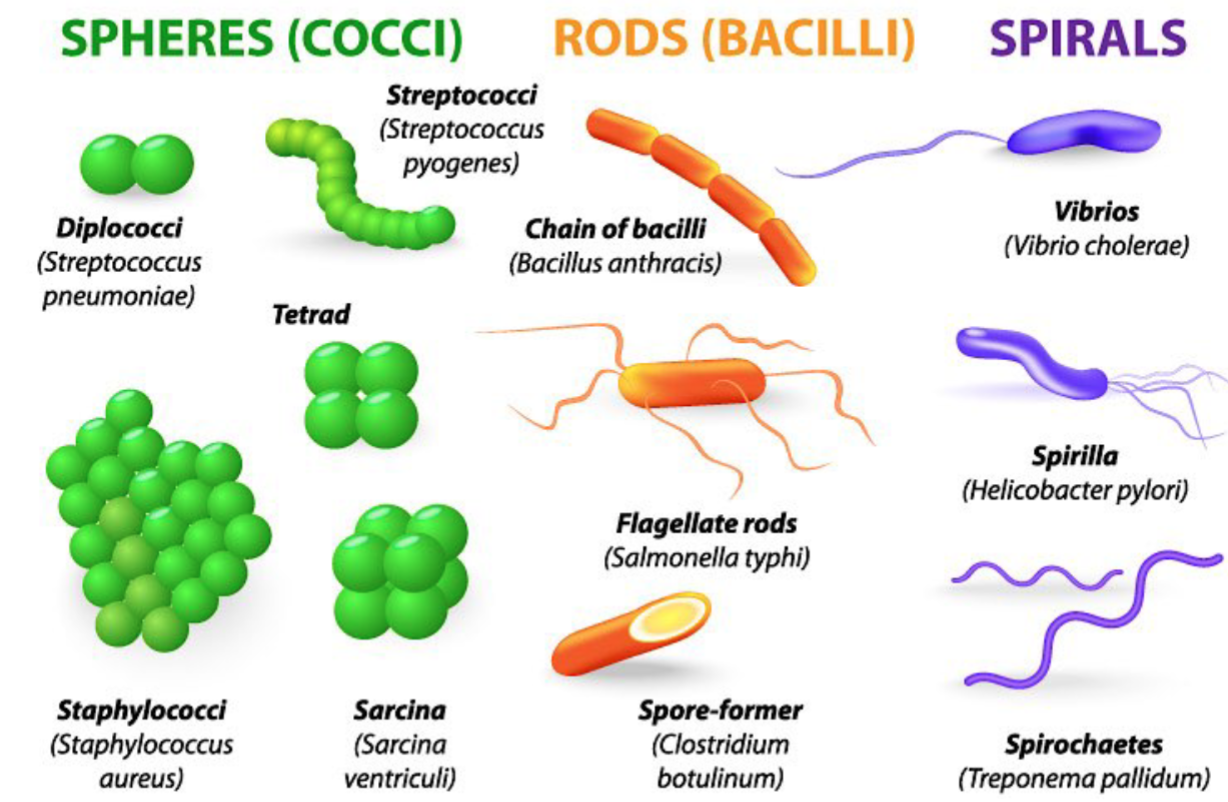
Bacterial Shapes
Coccus
Coccobacillus
Bacillus
Cocobacillus
vibrio
Spiral
Vibrio
Bacterial Shapes
Bacteria Cell Structure
Flagella
Presence is species.strain dependent
For motility
Number and arrangement vary
Pili/Fimbriae
Hair-like structures
Fimbriae shorter than pili
Adhere.attach to surfaces (key step in most infections
“F” or sex pilus: used for transfer of genetic material (plasmid) from one bacteria to another
Can provide resistance against engulfment by phagocytes
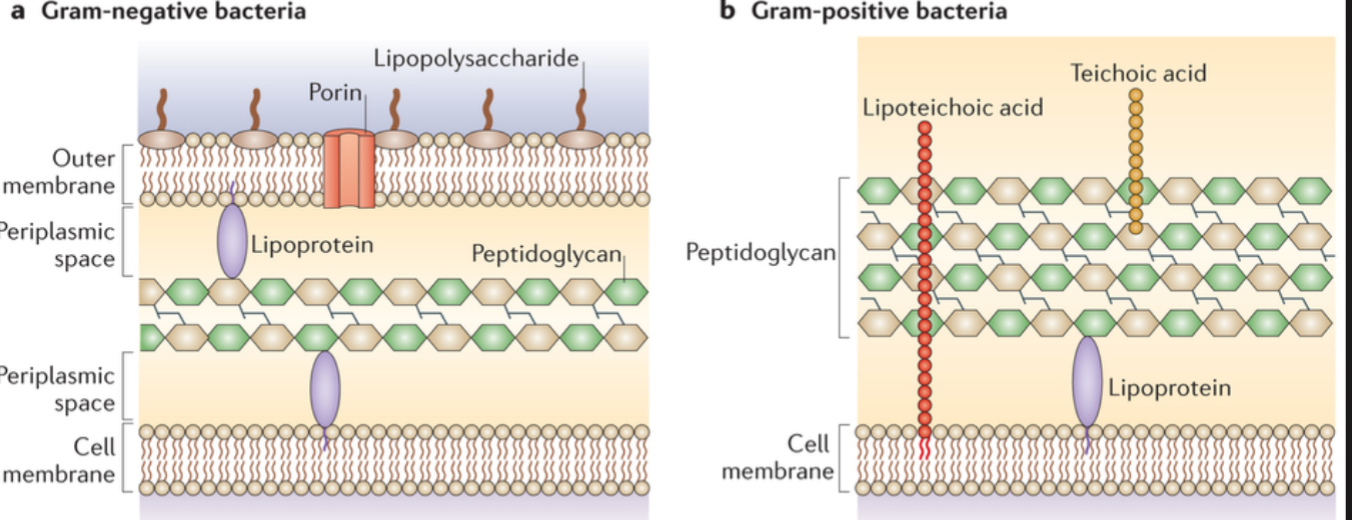
Bacterial Cell Wall Structure
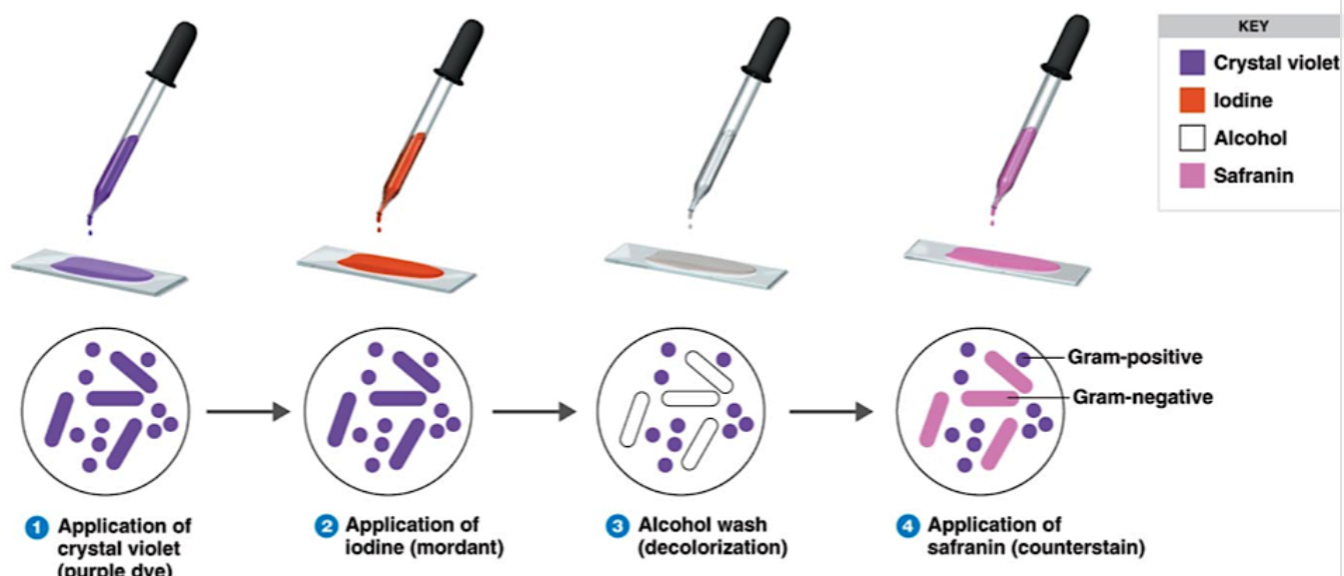
Gram Staining
Application of crystal violet
Application of iodine
Alcohol wash
Application of safranin
Bacterial Reproduction
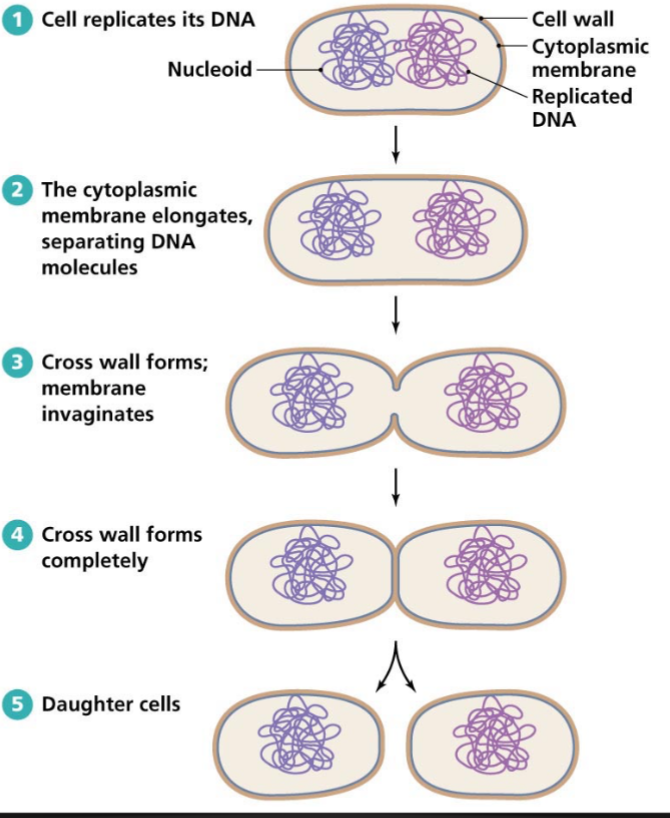
Cell replicate its dna
The cytoplasmic membrane elongates, separating DNA molecules
Cross wall forms completely
Daughter cells
Rapid Bacterial Growth
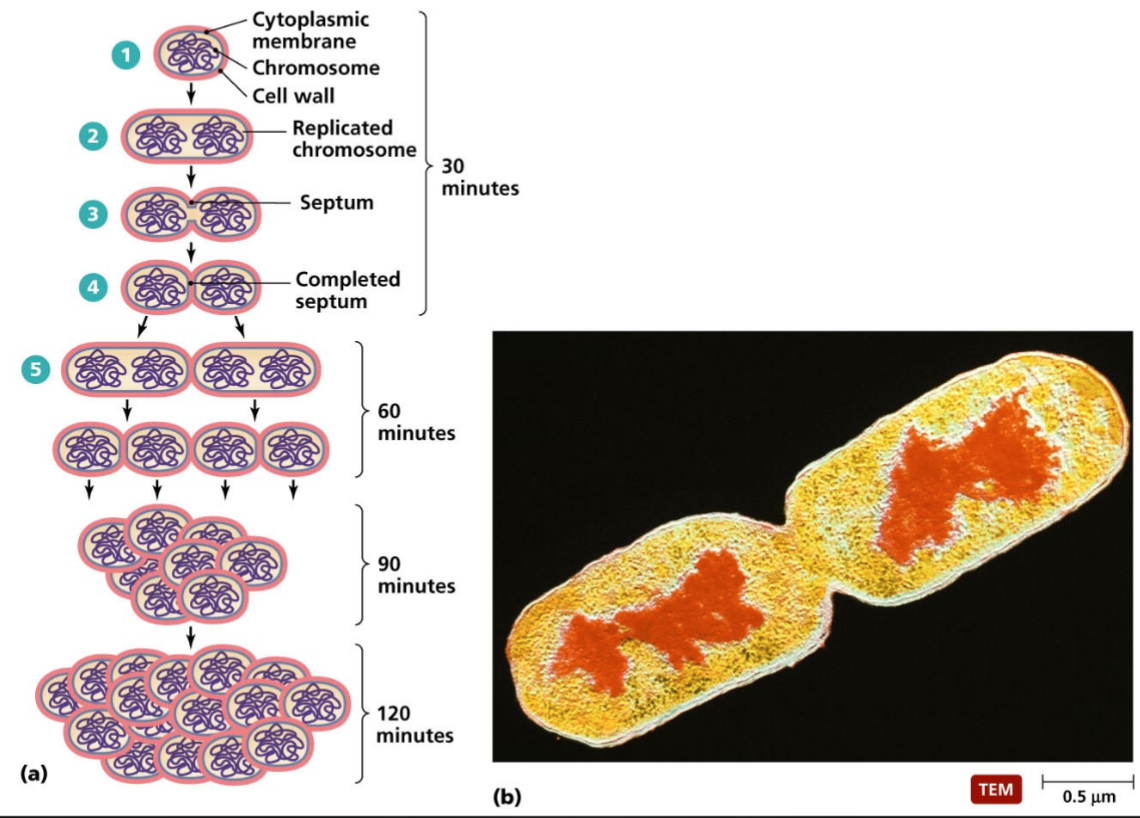
Cytoplasmic membrane
Replicated chromosome
Septum
Completed septum
60 mins – 90 mins – 120 mins
Bacterial Endospore Formation
Under stressful environments, certain gram positive bacteria are capable of forming endospores
Endospores can survive environmental assaults that would normally kill the bacteria. These stresses include high temperature, high uv irradiation, desiccation, chemical damage and enzymatic destruction
Endospores make them of particular importance because they are not readily killed by manu antimicrobial treatments
Types of Bacterial Pathogen
True pathogen (enterotoxigenic escherichia coli): infectious agent that causes disease in virtually any susceptible host
Opportunistic pathogen (pseudomonas, staphylococcus): normally harmless, causes disease when the normal flora is disrupted (by antibiotics) or when the host is immunocompromised (by drugs or other illnesses).
Bacterial Adhesion
Necessary to avoid innate host defense mechanisms ( peristalsis in the hugy and the flushing action of mucus, saliva and urine).
Adhesion is often an essential preliminary step to colonization and then penetration through tissues
At the molecular level, adhesion involves surface interactions between specific receptors on host cell membrane and ligands on the bacterial surface
Nonspecific surface properties of the bacterium. Including surface charge and hydrophobicity, also contribute to the initial stages of the adhesion process
Mechanism of Adherence to cell or tissue surfaces
Non-specific adherence: reversible attachment to the surface (dock)
Hydrophobic interactions
Electrostatic attractions
Atomic and molecular vibrations resulting from fluctuating dipoles of similar frequencies
Brownian movement
Recruitment and trapping by biofilm polymers interacting with bacterial glycocalyx (capsule)
Specific adherence: irreversible permanent attachment to the surface (anchoring)
Formation of many specific lock and key bonds between complementary molecules on each cell surface
Complementary receptor and adhesion molecules must be accessible and arranged in such a way that many bonds form over the area of contact between the two cells.
Once the bonds are formed, attachment under physiological conditions becomes virtually irreversible.
Specific adherence of bacteria to cell or tissues
Tissue tropism
Particular bacteria are known to have apartment preference for certain tissues over others
Ex s mutans is on dental plaque but not on tongue surfaces
Species specificity
Certain pathogenic bacteria infect only certain species of animal
Genetic specificity within a species
Certain strains or races within a species are genetically immune to a pathogen
Terminology: Adherence factors in host-pathogen interactions
Adhesion
A surface structure or macromolecule that binds a bacterium to a specific surface
Receptor
A complementary macromolecular binding site on a (eukaryotic) surface that binds specific adhesins or ligands
Lectin
Any protein that binds to a carbohydrate
Ligand
A surface molecule that exhibits specific binding to a receptor molecule on another surface
Mucous
The mucopolysaccharide layer of glycosaminoglycans covering animal cell mucosal her for the purpose of DNA transfer surfaces
Fimbriae
Filamentous proteins on surface of bacterial cells that often have adhesins for specific adherence
Common pili
Same as fimbriae
Sex Pilusx
A specialized pilus that binds mating prokaryotes together for the purpose of DNA transfer
Type 1 fimbriae
Fimbriae in enterobacteriaceae which bind specifically to mannose terminate glycoproteins on eukaryotic cell surfaces
Type 4 pili
Pili in certain gram - and gram + bacteria. In pseudomonas, thought to play a role in adherence and biofilm formation
S-layer
Proteins that form the outermost cell envelope component of broad spectrum of bacteria, enabling them to adhere to host cell membranes and environmental surfaces
Glycocalyx
A layer of exopolysaccharide fibers on the surface of bacterial cells which may be involved in adherence to a surface. Sometimes a general term for capsule
Capsule
A detectable layer of polysaccharide (rarely polypeptide) on the surface of a bacterial cell which may mediate specific or nonspecific attachment
Lipopolysaccharide (LPS)
A distinct cell wall component of the outer membrane of gram- negative bacteria with the potential structural diversity to mediate specific adherence. Probably functions as an adhesin
Bacterial Biofilm Formation
Aggregate of microorganisms in which cells that are frequently embedded within a self-produced matrix of extracellular polymeric substance adhere to each other and surfaces
Form on tissue surfaces (ex teeth, skin, mucosal membrane ) and inanimate surface (ex catheters kitchen counters, orthopedic implant)
Often impervious to detergents, antibiotics and immune system
Can act as reservoirs for repeated infections
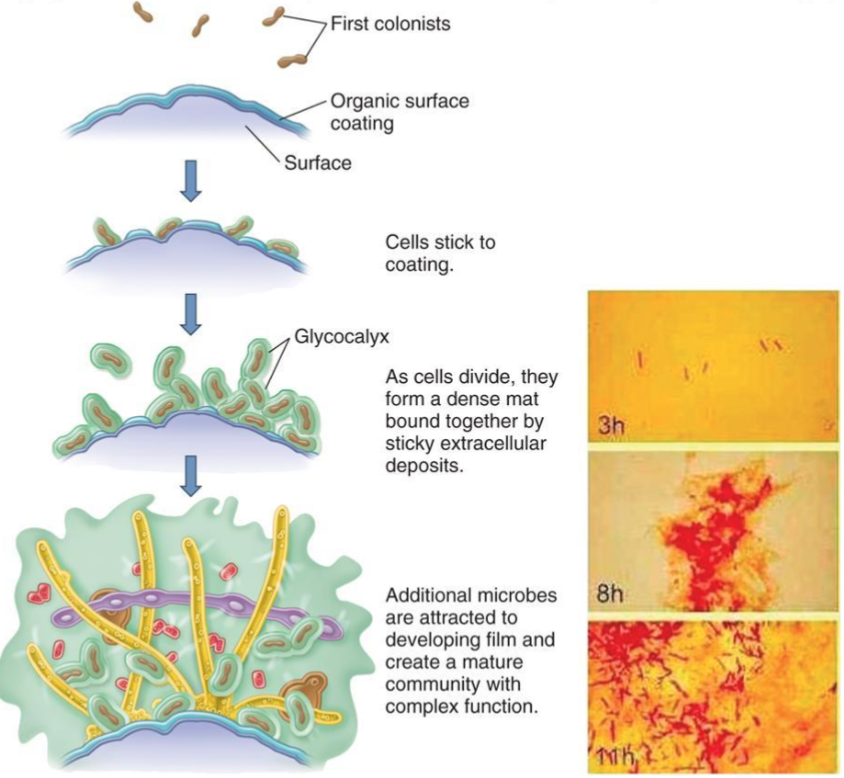
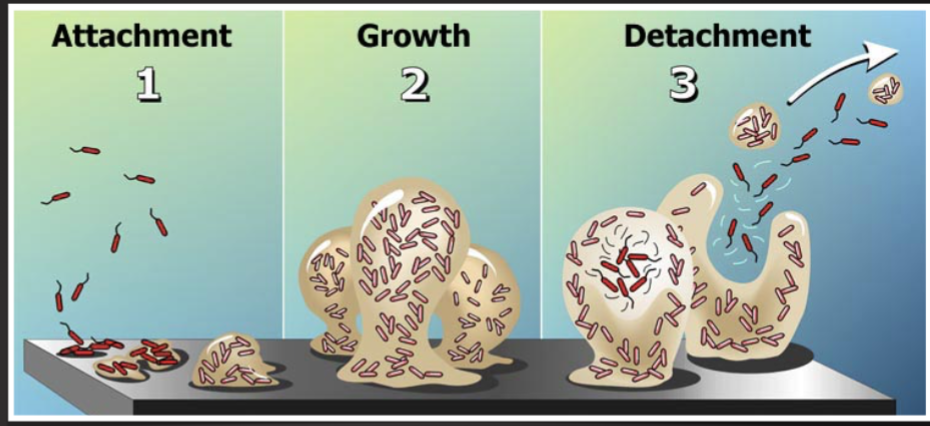
Know the definitions
Planktonic cells
biofilm -enmeshed cells
Persisters cells
Bacterial Pathogens
Facultative intracellular pathogen
Salmonella, shigella, yersinia
Obligate intracellular pathogen
Ex. rickettsia, chlamydia, coxiella
Extracellular pathogen
Ex. vibrio cholera, pseudomonas E. coli (ETEC)
Bacterial Pathogens
Facultative intracellular pathogen
Ex. salmonella, shigella, yersinia
Obligate intracellular pathogen
Ex. rickettsia, chlamydia, coxiella
Extracellular pathogen
Ex. Vibrio cholera, pseudomonas, E. coli (ETEC)
Bacterial Invasion
Penetration of host cells and tissues (beyond the skin and mucous surfaces)
Mediated by a complex array of molecules, often described an “invasins”
Invasins can be in the form of bacterial surfaces or secreted proteins which target host cell molecules (receptors)
Bacterial Invasins: Spreading Factors
Descriptive term for a family of bacterial enzymes that affect the physical properties of tissue matrices and intracellular spaces, thereby promoting the spread of the pathogen
Hyaluronidase:
Produced by streptococci. Staphylococci, and clostridia
Attacks the interstitial cement (“ground substance”) of connective tissue by depolymerizing hyaluronic acid
Collagenase
Produced by clostridium histolyticum and C. perfringens
Breaks down collagen, the framework of muscles, which facilitates has gangrene due to these organism
NeuraminidaseL
Produced by intestinal pathogens such as Vibrio cholerae and Shigella
Degrades neuraminic acid (also called sialic acid), an intercellular cement of the epithelial cells of the intestinal mucosa
Streptokinase and staphylokinase
Produced by streptococci and staphylococcus, respectively
Digests fibrin and prevents clotting of the blood
Relative absence of fibrin in spreading bacterial lesions allows more rapid diffusion of the infectious bacteria
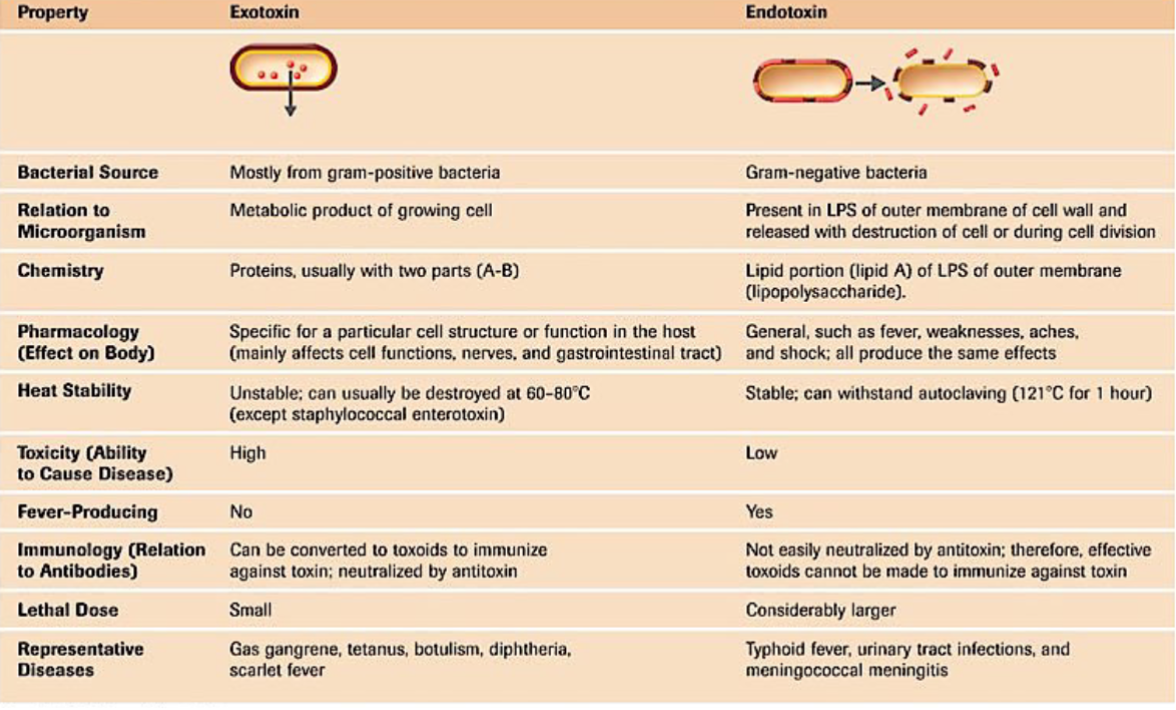
Bacterial Toxigenicity
exotoxins
Proteins produced inside pathogenic bacteria
gram-positive bacteria
Retain high activity at very high dilutions
Endotoxins
Lipid potions of LPS, outer membrane of cell wall
Gram-negative bacteria
Virulence Factors
Adhesion
Invasion
Competition for iron and nutrients
Resistance to host immunity
Secretion of toxins
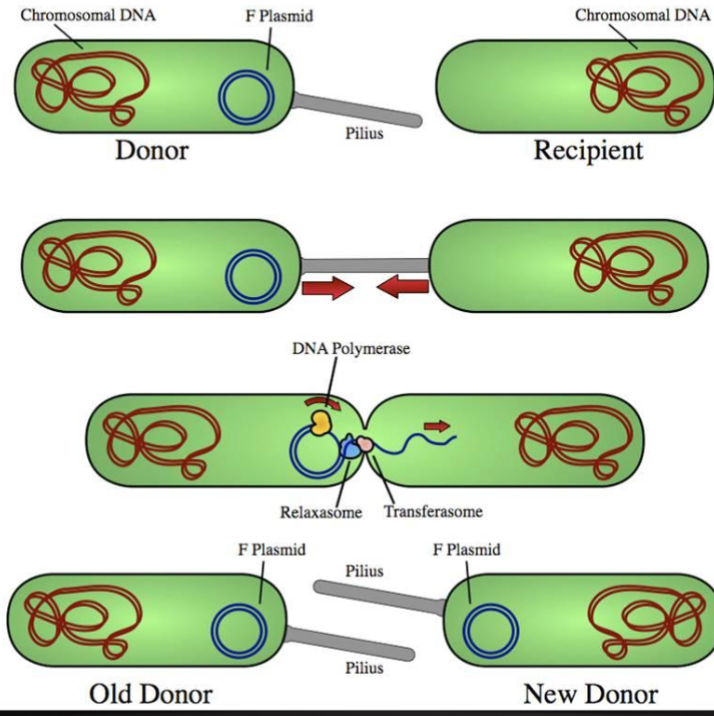
Bacterial Conjugation: Propagation of Virulence
Biofilms - 02.13.24
Biofilm
Highly structured microbial communities enmeshed in a self-produced slime matrix comprised of proteins, polysaccharides, eDNA, and lipids
Biofilms are highly complex
Stringy: only contains eDNA
Chunky: only contains proteins
Webby: contains everything
Biofilm Development Stages
colonization/adhesion
Non-specific attachment
Physio-chemical
Pili-mediated
flagella-mediate
Specific attachment
Receptor-mediated
host-ECM components
Aggregation
microlony/monolayer formation
Maturation
Dispersal
Biofilm EPS formation, Maturation (3-5)
Bacterial communication
Quorum sensing
Biofilm Dispersal (6)
Bacterial-mediated
Matrix degradative enzymes
Dissemination
Persistence
Why form biofilm
To protect from predators
Cooperation strategy for bacteria
Biofilms provide a nutritional advantage for the bacteria
To establish dominance in the environment
Biogenic Habitat-Forming Organisms
protection/safety
Shelter
Stress response
Predator evasion
Polymicrobial Nature
Biofilms are predominantly polymicrobial
Multiple bacterial species
Multi-organism communities
Bacterial-fungal and agal biofilms
High Bacterial Diversity = high survival
Biofilm = sponge
High diversity
Enhance EPS profile
High nutrition absorption
Enhance metabolic capacity
High ability to breakdown complex molecules
Antimicrobial/Antibiotic Resistance
Bacteria acquire/develop mutations that render the antibiotic/toxin ineffective
How?
Intrinsic resistance
Inherited physiological property
Cell wall (gram-, gram +)
Acquired resistance
Mutations
Gene acquisition
Horizontal gene transfer (HGT)
Antimicrobial/ Antibiotic Tolerance
Ability to survive transient exposure to a high concentration of an antibiotic, despite being susceptible
How
Intrinsic tolerance
Inherited physiological property
Spore formation
Biofilm formation
Acquired tolerance
Physiological property
Join a biofilm
Dormancy (metabolic shutdown)
Persister cells
Multi-Species Biofilm = Multi-functional biofilm
Why Biofilms
Environmental stress
Predator evasion
Nutrient acquisition
Antibiotic tolerance/ resistant
Horizontal gene transfer
Biofilm Infections
Approx. 34% of nosocomial infections
65% of all microbial infections
80% of chronic infection
Biogilm infection chronicity
Biofilm prolong/ prevent wound healing
Re-epithelialization
Granulation tissue formation
Manipulation of pro-inflammatory and anti-inflammatory responses
Anti-biofilm Strategies are difficult
Antibiotics are becoming ineffective
Metals such as silver and zinc can be toxic
Debridement procedures are aggressive and invasive
Preventative strategies are god but not enough
Wash hands
Cover coughs and sneezes
Clean surfaces
Adequate ventilation
Introduction to Viruses - 02.15
Virions/Viruses
Acellular and the virion consist of
DNA or RNA core
Single-stranded positive-sense RNA
Single-stranded negative-sense RNA
Double stranded RNA
Double stranded DNA
Protein coat (capsid)
Lipid envelope (on some viruses) and “spikes” (glycoproteins
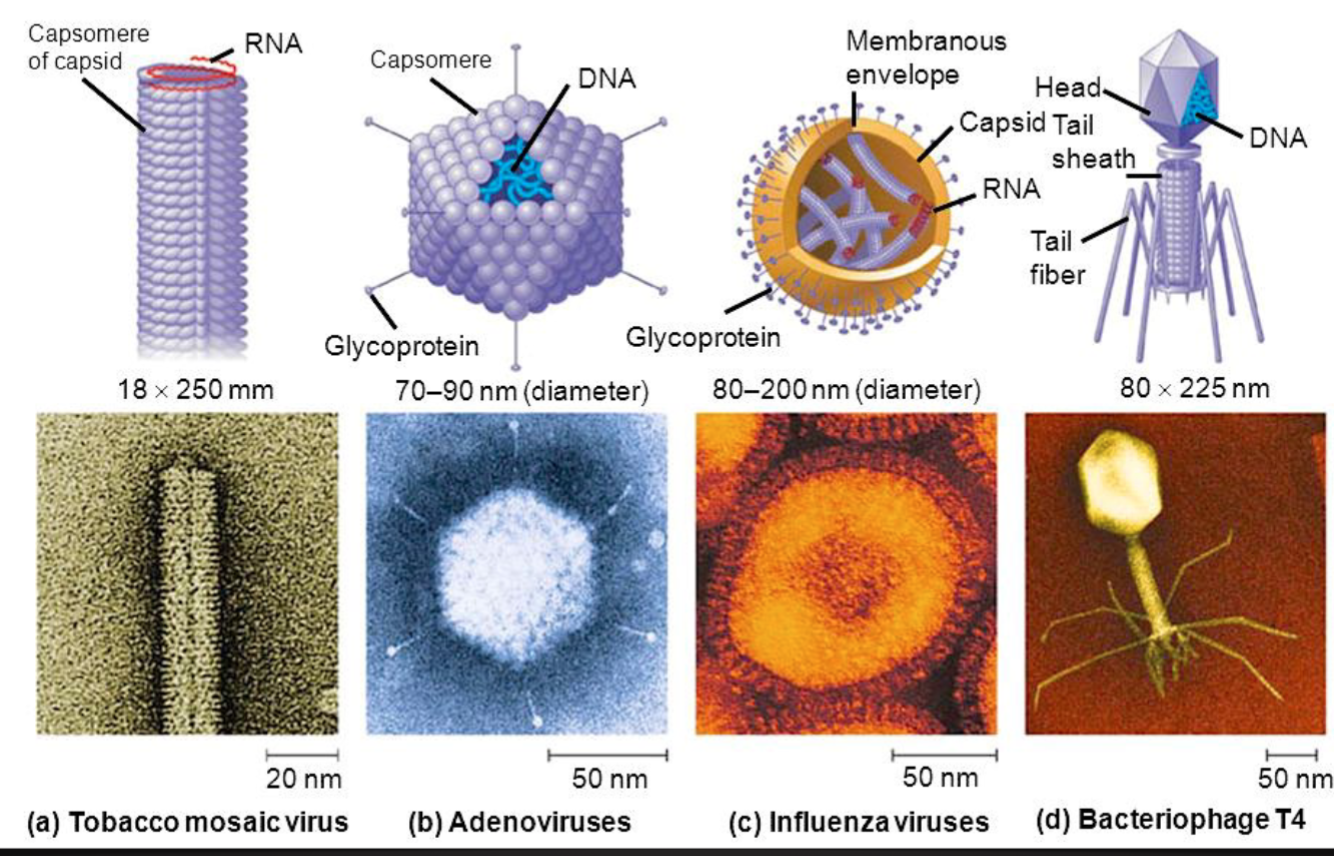
Viruses can infect all types of life forms, from animals and plants to microorganisms, including bacteria (“bacteriophage”) and archaea.
Can replicate only when within living host cell;
General rule: DNA viruses replicate within the nucleus while RNA viruses replicate within the cytoplasm
Viruses: Structure
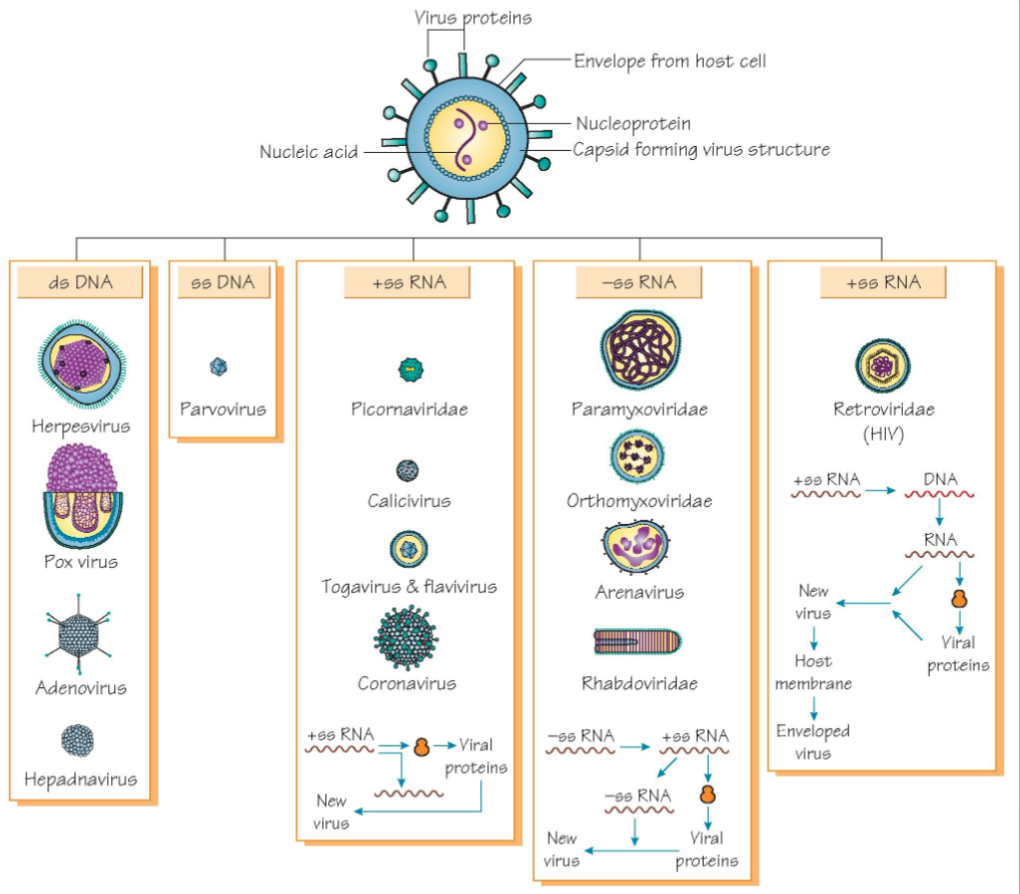
Structuve
Viral proteins
Envelop from host cell
Capsid forming virus structure
Nucleic acid
Nucleoprotein
Viruses: Classification
Two Systems of Classification
Hierarchical virus classification system
Four Main characteristic are used
Nature of nucleic acid: RNA or DNA
Symmetry of the capsid
Presence or absence of an envelope
Dimensions of the virion and capsid
The Baltimore Classification
Viruses can be classified into seven (arbitrary) groups
Double-stranded DNA
Single stranded (+) sense DNA
Double-stranded RNA
Single stranded (+) sense DNA
Single stranded (-) sense RNA
Single-stranded (+) sense with RNA with DNA intermediate in life-cycle
Double stranded DNA with RNA intermediate
Viral Pathogenesis
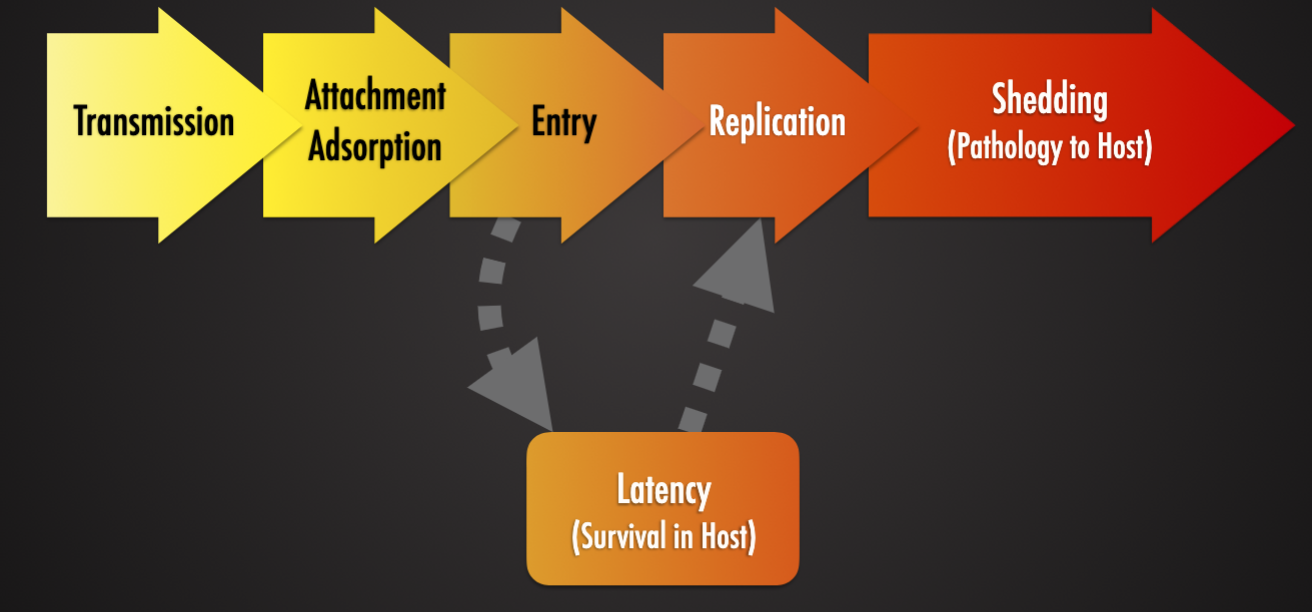
Viral Attachment and Entry
Direct penetration
Viral capsid or genome is inject into the host cell’s cytoplasm (poliovirus)
Membrane Fusion
The cell membrane is punctured and made to further connect with the unfolding viral envelope (influenza virus)
Endocytosis
The host cell engulfs and takes in the viral particle (HIV)
Viral Replication
Uncoating
Stripping off the viral protein coat, releasing the viral nucleic acid or genome
Transcription / mRNA production
Virus takes advantage of host cell structures to replicate itself
For some RNA viruses, the infection RNA produces messenger RNA (mRNA) for translation into protein (virus components).
For others with negative stranded RNA and DNA, transcription occurs before translation
Synthesis of virus components:
Viral protein synthesis: virus mRNA is translated on host cell ribosomes into two types of viral protein
Viron assembly
Newly synthesized genome (nucleic acid), and proteins are assembled to form new virus particles (virions)
Can occur at the cell’s nucleus, cytoplasm or plasma membrane
Viral Latency
Ability of some viruses to remain dormant within the host dell (occult infection), sometimes for years
Viral genome can remain latent either as an episome or integrated in the host chromosome. The latter allows for replication of viral genome during host cell division
Latency is maintained by a few viral genes that keep viral genome silent and escape from host immune system
Latency can stop upon viral genome reactivation, often promoted by external stimuli (host stress cellular signals)
Viruses with the ability for latency have two options when infecting a cell:
Enter the latency or the lytic pathway. The decision is regulated by expression of regulatory proteins part of a genetic switch system, usually repressor(s) as well as proteins controlling the stability of the latter. The outcome depends on the ratio of these key regulators. The ratio may be determined by environmental factors such as the host cell type, its shape, or the nutrient availability.
Viral Shedding
Via Budding:
Most effective for viruses that require an envelope (HIV, smallpox)
Before budding, the viral receptor are placed on the host cell surface
Depletes host cell membrane → eventual host cell death
Via Apoptosis
Forced cell suicide released viral progeny.
As apoptotic host cells are phagocytosed by macrophages, virus has the opportunity to get into macrophages to infect or be transported to other tissues in the body
Via Exocytosis
Used by non-enveloped viruses
Host cell is not destroyed
Virus progeny are enclosed in vesicles and transported to cell membrane to be released
Protozoa 02/22/24
Pathogenesis of Infection: Protozoa 02/22/24
Paramecium
A protozoan
Abundant in freshwater, brackish, and marine environments (very abundant in stagnant basins and ponds)
Readily cumulative
Widely used in classrooms and labs to study biological processes
Dictyostelium Discoideum
Species of soli-living amoeba: referred to as “slime mold”
Social amoeba
Protozoan Parasites
Eukaryotic organism – “Proto-zoa” literally means “first animals”
About 32,000 living species (34,000 are extinct)
21,000 species occur as free-living organisms
11,000 species are parasitic in vertebrae and invertebrate hosts
Most protozoa are microscopic organism
Few grow to be seen by the naked eye, they are <50 um in size
Nutrition is holozoic (type of heterotrophic nutrition) as in higher animals
Via osmotrophy OR absorb nutrients through cell membrane
Via phagocytosis: engulf food particles with pseudopodia or take in food through a mouth-like aperture called a cytostome
Classified into four groups
Sarcodina (ameba)
Ex. Entamoeba
Mastigophora (flagellates)
ex. Giardia, leishmania
Ciliophora (ciliates)
ex. Balantidium
Sporozoa organism whose adult stage is not motile (can't move)
Ex. plasmodium, cryptosporidium
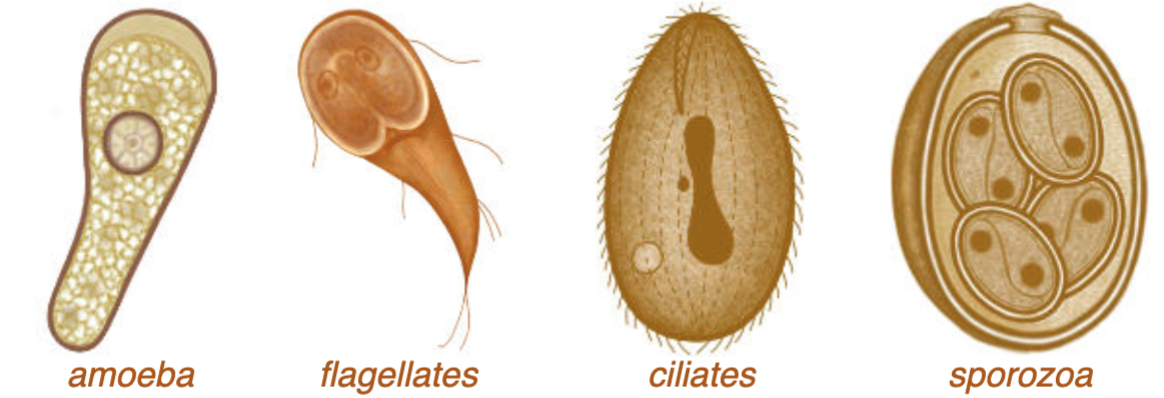
Protozoan Classes
Amoebae
Use pseudopodia to creep or crawl over solid substrates
Similar to human macrophages
Flagellates
Use elongate flagella: undulate to propel the cell through liquid environments
Flagella: whip-like extensions of the cell membrane with an inner core of microtubules arranged in a specific 2+9 configuration (similar to human spermatozoa)
Ciliates
Use numerous small cilia which undulate (move up and down) in waves allowing the ell to swim in fluids
Cilia: “hair-like” extensions of cell membrane similar to flagella but with interconnecting basal element facilitating synchronous movement (similar to human bronchial epithelial cells)
Sporozoa
“Spore-formers” form non-motile spores as transmission stages
Many pre-spore stages move using tiny undulating ridges or waves in the cell membrane imparting a forward gliding motion
Protozoan Life Cycles
Short generation times
Enormous reproductive potential → rapidly causes acute disease
Increased chances for mutation → changes in virulence, drug susceptibility and other characteris
Asexual or sexual reproduction
Asexual (in intermediate hosts)
Budding: outgrowth of a mature cell grows and becomes a new daughter cell
Binary fission: one nuclear division gives rise to two daughter cells (closest to mitosis)
Schizogony: “multiple fission” – nucleus divides repeatedly, allowing one cell to give rise to many daughter cells
Sexual (in definite hosts)
Conjugation: cells that java undergone a reduction division fuse, exchange haploid micronuclei, and separate – each gives ride to two daughter cells
Some protozoa have complex life cy;es requiring two different host species: others require only a single host to complete the life cycle
Protozoan Life Cycles
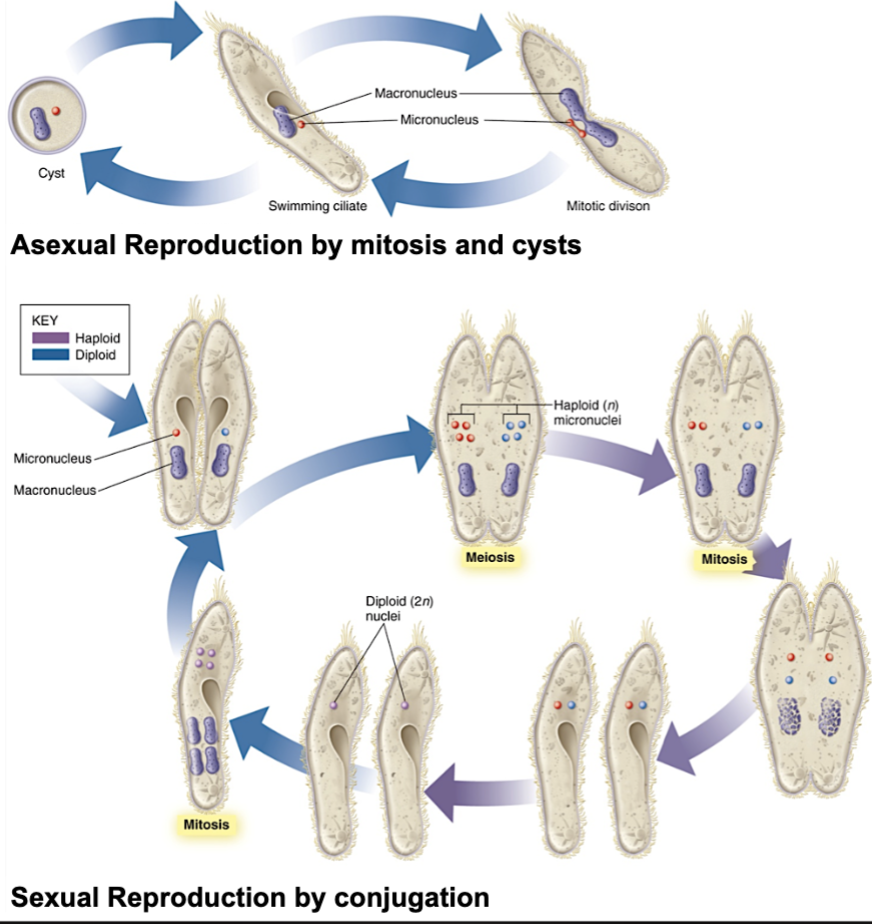
Protozoan Terminology
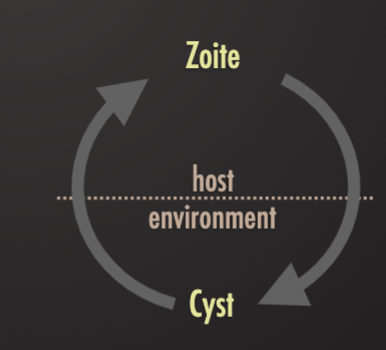
Trophozoite (“animals that feeds”)
Active, feeding, multiplying stage of most protozoa
Stage usually associated with pathogenesis
May be found intracellularly (within host cells) or extracellularly (in hollow organs, body fluids or interstitial spaces between cells)
Not very resistant to external environmental conditions and do not survive long outside of their hosts
Cyst (process: “encystment”)
Formed by only some protozoa
Can contain one or more infective forms
Multiplication can occurs in the cyst of some species, excystation releases more than one organism
Cyst released into the environment (stools) have a protective wall
Oocyst
A hardy, thick-walled stage of the life cycle of some protozoa (sporozoan): stage results from sexual reproduction
Oocysts of some protozoans (sporozoan) are passed in the feces of the host.
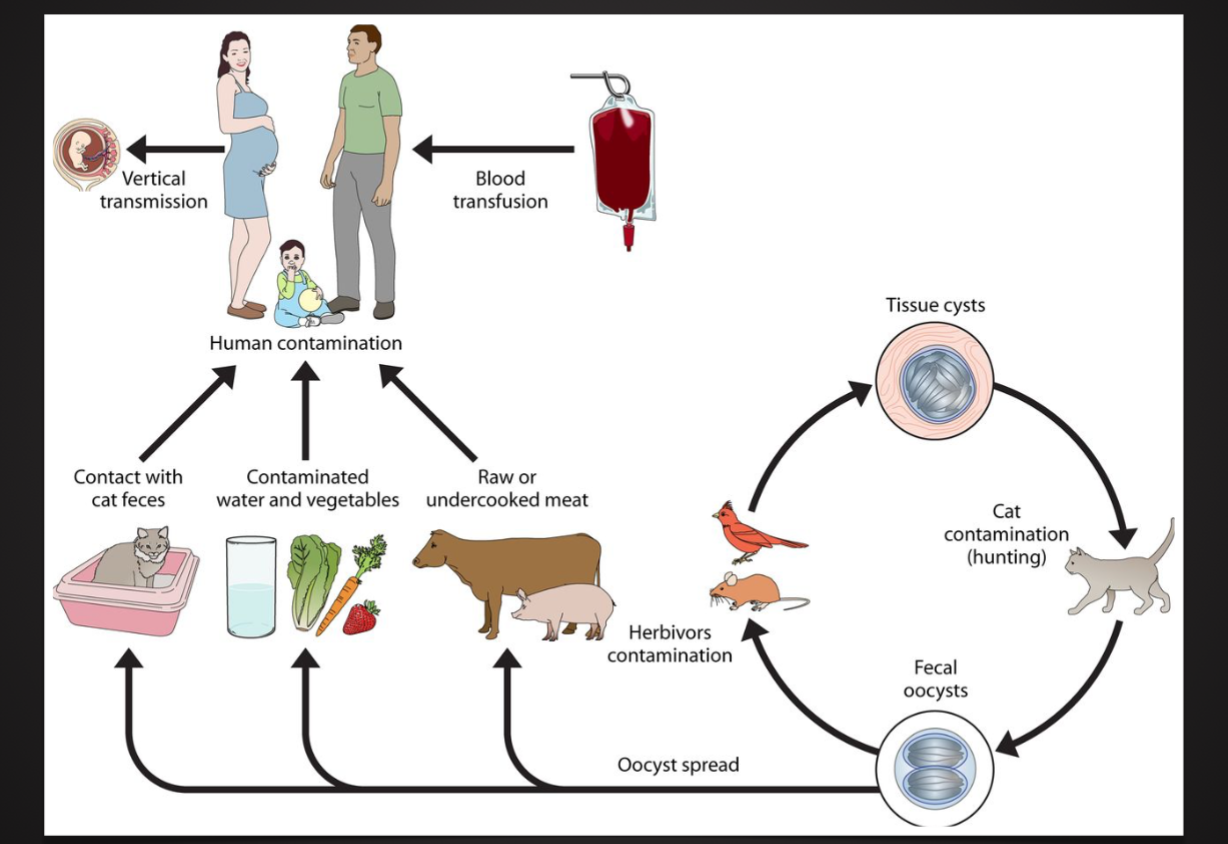
Oocysts of plasmodium (causative agent of malaria) developed in the body cavity of the mosquito vector
Protozoan Excystation
Ultrastructural morphology details of an oblong-shaped Giardia sp. Protozoan cyst, revealing the filamentous nature of the cyst wall.
The cyst is undergoing”excystation” with a flagellated trophozoite
Mode of Transmission
Direct Transmission
Of trophozoites through intimate body contact, such as sexual transmission
Ex. trichomonas spp. Flagellates: causes trichomoniasis → infertility in humans and cattle
Fecal-Oral transmission
Environmentally-resistant cyst stages passed in feces of one host and ingested with food/water by another
Ex. entamoeba histolytica, giardia duodenalis
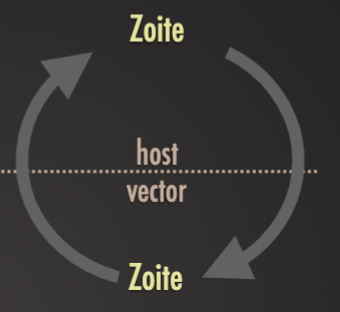
Vector-borne transmission
Trophozoites taken up by blood-sucking arthropods (insect or arachnid) and passed to new hosts when they next feed
Ex. Trypanosoma brucei by tsetse flies to humans (sleeping sickness), cattle, horses, and other livestock . Plasmodium spp. Haemosporidia by mosquitoes to humans (malaria)
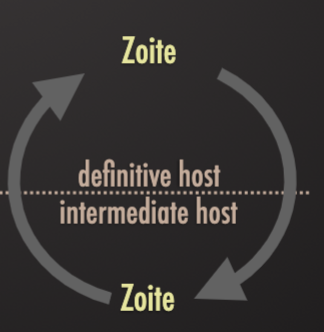
Predator-prey transmission
Ziotres encysted within the tissue of prey animal (herbivore) being eaten by a predator (carnivore) which subsequently sheds spores into the environment to be ingested by new pet animals
Ex. toxoplasma gondii ingested by cats
Protozoan Escape Mechanisms
Antigenic Masking (and Mimicry?)
Ability of a parasite to escape immune detection by covering itself with host antigens
Blocking of Serum Factors
Some parasites acquire a coating of antigen-antibody complexes or non cytotoxic antibodies that sterically blocks the binding of specific antibody or lymphocytes to a parasite surface antigens
Intracellular Location
The intracellular habitat of some protozoan parasites protects them from the direct effects of the host’s immune response.
By concealing the parasites antigens, this strategy also delays detection by the immune system
Antigenic Variation
Some protozoa parasites change their surface antigens during the course of an infection
Parasites carrying the new antigens escape the immune response to the original antigens
Immunosuppression
Parasitic protozoan infections generally produce some degree of host immunosuppression
This reduced immune response may delay detection of antigenic variants
Can reduce the ability of the immune system to inhibit the growth of and/or to kill the parasites
Protozoan Pathogenesis
Cellular ,Tissue and Organ Damage
Extracellular or intracellular parasites that destroy cells while feeding can lead to organ dysfunction and serious or life-threatening consequences
Toxic protozoal products
Toxins associated with some protozoa (plasmodium) can cause fever and chills (can occur cyclically)
Interference with host function
Some parasites that inhabit the small intestine can significantly interfere with digestion and absorption and affect the nutritional status of the host
Delayed-type hypersensitivity
Pathology as a consequence of immune response mediated by antigen-specific effect T cells
Immunosuppression
Host is susceptible to secondary infection (by other pathogens)
Toxoplasma Gondii
Fungi 02/27/24
Pathogenesis of Infection: Fungi Tuesday 02/27/24
Some Terminology
Mycoses (singular = mycosis);
Diseases of warm-blooded animals caused by fungi
Medical Mycology
Study of fungi as animal and human pathogens
Fungal Pathogens
Eukaryotic organisms:
separate from plants and animals
Genetically more closely related to animals than plants
About 1.5 million different species exist
Only 300 species are pathogenic
Many pathogenic fungi are microorganisms
Most are multicellular (molds), but some are unicellular (yeasts)
Dimorphic fungi can exist as molds or yeasts
Heterotropic
Incapable of producing food
Fungi feed by extracellular digestion
Saprobic
Live of dead organic matter
Help to cycle nutrients (decomposition)
Mutualistic
Some are in symbiosis with plant roots
Parasitic
Live on living organism, cause disease of the host
Mostly asecual or secual reporduction and dispersion by spores
In contrast: yeast reproduce by budding or binary fission
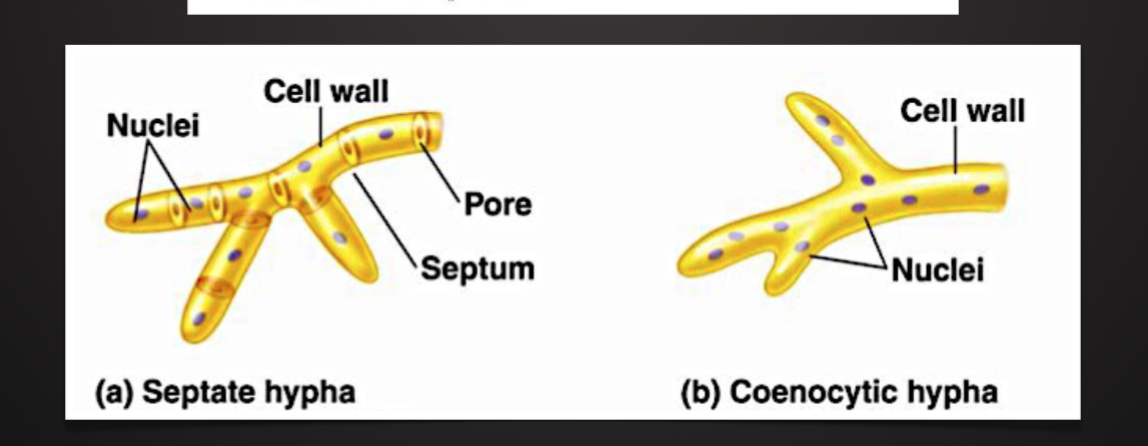
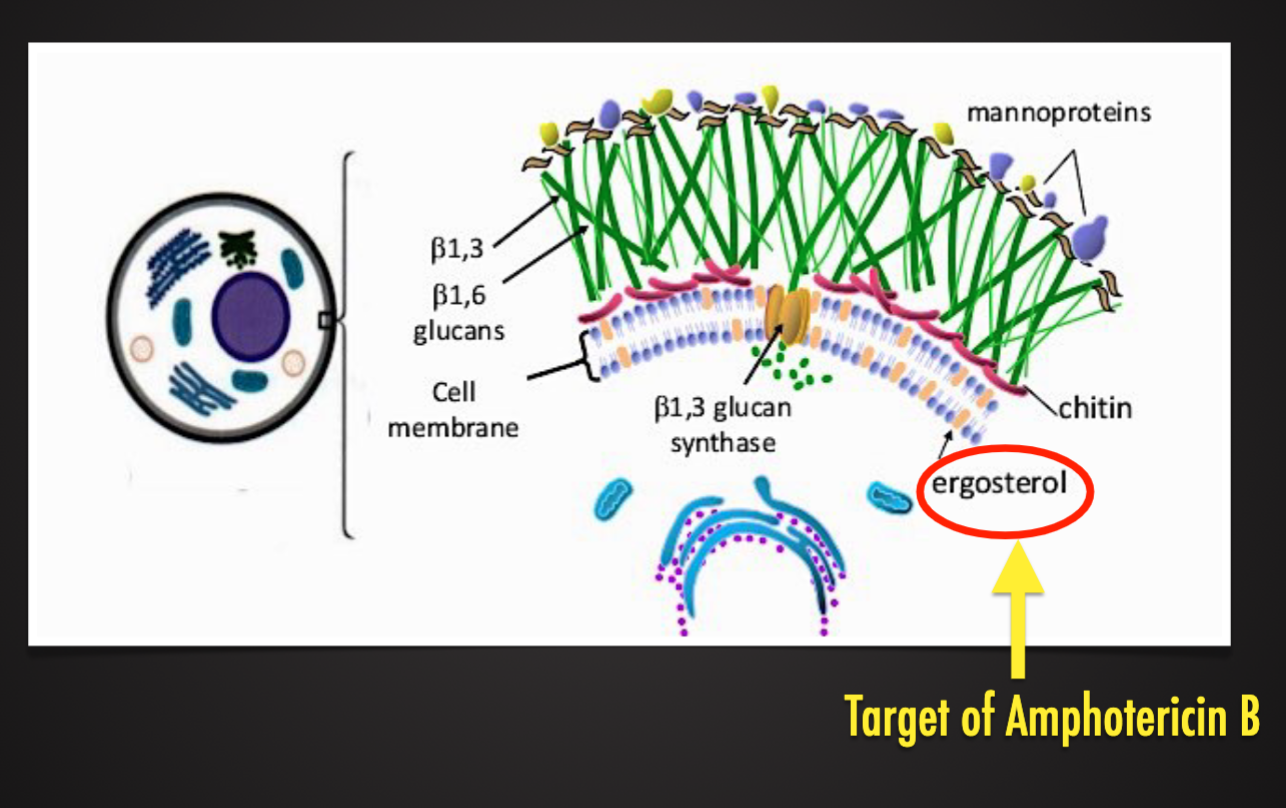
Have rigid cell wall (glucans and chitin)
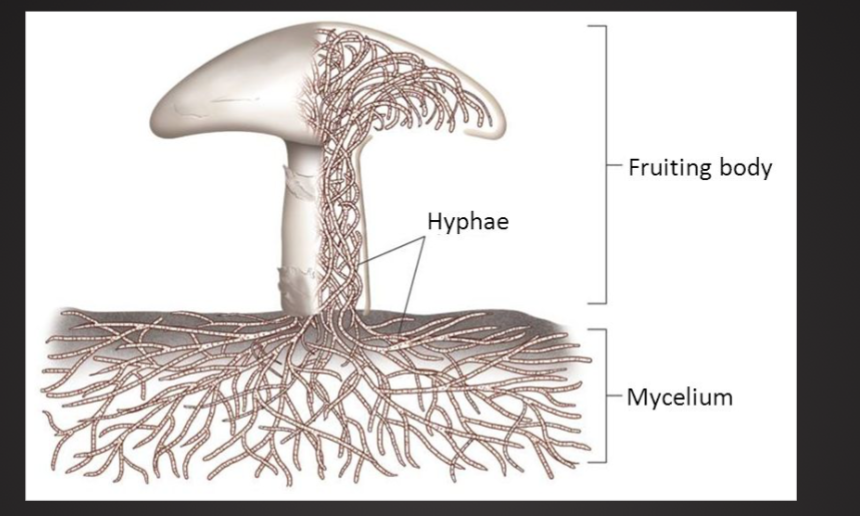
Basic Fungal Structure
Fungal Cell Wall
Yeast (Unicellular Fungi)
Yeast genus Candida
Most clinically relevant fungal group
Has multiple species which cause disease in humans and animals
Yeast genus Cryptococcus
Causes opportunistic infections (meningitis, pneumonia)
Has a few species which cause disease in humans and animals (most common in cats but also in dogs, cattle, horses, sheep, goats, birds and wild animals)
Molds (multicellular fungi)
Molds are extremely diverse group of organism, the vast majority of which are non-pathogenic
Has two major pathologic groups:
Genus aspergillus
Order mucorales
Molds and their pores are found in soil and decaying vegetation throughout the world
Both can cause rhino-sinustitis and various forms of pulmonary infections in immunocompromised humans and animals
Dimorphic Fungi
Can exist as either yeast or molds
Typically exist in environment as a mold but shen spores are inhaled they grow in the host as a yeast
Most commonly cause subacute pulmonary infections
Bacteria which Masquerade as Fungi
Nocardia and actinomycetes were originally believed to be fungi, but now are known to be bacteria
Develop filamentous, branching structures
Causes subacute and chronic infections of the lungs, CNS, and soft tissue: most commonly seen in immunocompromised humans and animals
Can be clinically indistinguishable from frugal disease
Streptomycin, actinomycin, and streptothricin and are all medically important antibiotics isolated from actinomycetes bacteria
Risk Factors for developing fungal infections
Use of antibiotics
Use of drugs that suppress the immune system
Cancer chemotherapy drugs
Corticosteroids
Anti-organ rejection drugs (ex. Azathioprine, methotrexate and cyclosporine)
Tumor necrosis factor inhibitors (treat rheumatoid arthritis and related disorder
Disorders
AIDS
Burns, if extensive
Diabetes
Hodgkin lymphoma ar other lymphomas, leukemia
Environmental Factors
Warm, moist areas of the body (mouth, vagina)
Sweaty clothes and shoes enhance fungus growth on the skin
Indwelling catheters
Mechanical ventilation
Genetic predisposition
Not well understood
General Features of Fungal Infection
Often seen in immunocompromised patients or animals
Exceptions: dimorphic fungi, dermatophytes
Clinical presentation is typically subacute to chronic
Exceptions: candidemia and mucormycosis
Person-toperson transmission doesnt usually occur
Exceptions: dermatophytes and mucocutaneous candida infections
There are no specific symptoms or signs which reliably distinguish fungal infections from bacterial ones
Typical antibiotics are ineffective
Exception: pneumocystis
Classifications of Fungal Infections
Superficial and Cutaneous Mycoses
Involve outermost (stratum corneum) or deeper (keratinized) layers of skin and its appendages (nails or hair)
“Tinea Infections” or “Ringworm”
Usually no inflammation (superficial) allergic or inflammatory response (when deeper)
Primarily caused by dermatophytes
Mucocutaneous Mycoses
Involved skin, eyes, sinuses, oropharynx, external ears, vagina
Candidal Infection
Manifests a superficial mucocutaneous disease to invasive disease with dissemination
Oral candidiasis (thrush) and esophageal candidiasis are characterized by white patches on the tongue or mucosal surfaces (can be removed by scraping).
Vulvovaginitis is seen in the settings or oral contraceptive use, diabetes mellitus, pregnancy , and antibiotic therapy, it manifests with vaginal discharge and vulvar edema and pruritus
Subcutaneous Mycoses
Often caused by saprophytes from soil or vegetation
Many are confined to tropical and subtropical regions
Involve subcutaneous tissues, muscles and fascia
Traumatic inoculation
Localized (often chronic) infection
Treatment usually involves use of antifungal agents and/or surgical excision
Treatment of some serious subcutaneous mycoses reamines unresolved
Systemic (Deep) Mycoses
Diseases that occur deep within the tissues, involving vital organs and/or the nervous system
Entry into the body is usually through inhalation of spores or open wounds
Causes by the true pathogenic fungi (usually dimorphic fungi) or opportunistic saprobes
Usually subclinical/ subacute presentation
May be fatal (can also be chronic)
Examples: blastomycosis, coccidioidomycosis, cryptococcosis and histoplasmosis
Symptoms: fever, cough, chest pain, weight loss, fatigue
Allergic Disorders
Mold exposure can trigger several allergic disorders, including:
Asthma
Allergic bronchopulmonary aspergillosis (ABPA)
Allergic fungal rhinitis
Hypersensitivity pneumonitis
No established safe limits for indoor mold
Visible mold growth in a home is not reliable measure of exposure
Mycotoxins
Types of Mycotoxins (Fungus poisons)
Mushrooms
50-100 toxic species
Identical to non-toxic ones
Many variety of toxins - different clinical manifestations and severity
Acute liver and renal failure: life-threatening
Aflatoxins
Produced by Aspergillus sp.
At least 14 types
Contaminate corn, soybeans, and peanuts
Acutely, can cause liver failure
Chronic exposure increases risk of hepatocellular carcinoma
Ergot Alkaloids
Produced by genus, Claviceps
Affected grains: tye, wheat, barely
Ergot alkaloids similar in structure to neurotransmitters, also have vasoconstriction properties.
Ergot Alkaloids
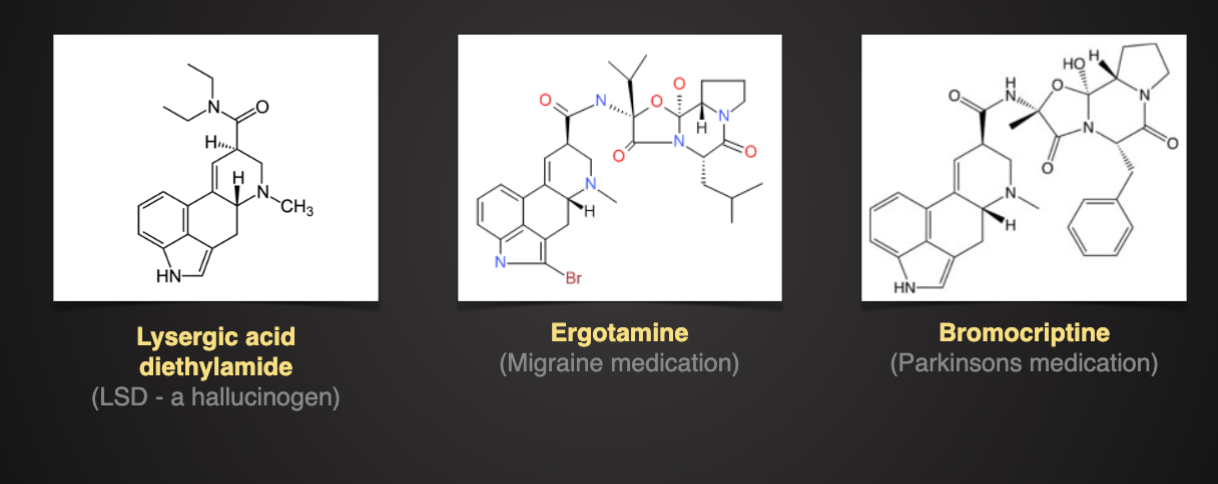
Two forms of ergotism
Acute: seizures, hallucination, mani, vomiting, diarrhea
Chronic- ischemia, and dry gangrene of extremities
Dracunculus Medinensis – 02.29.24
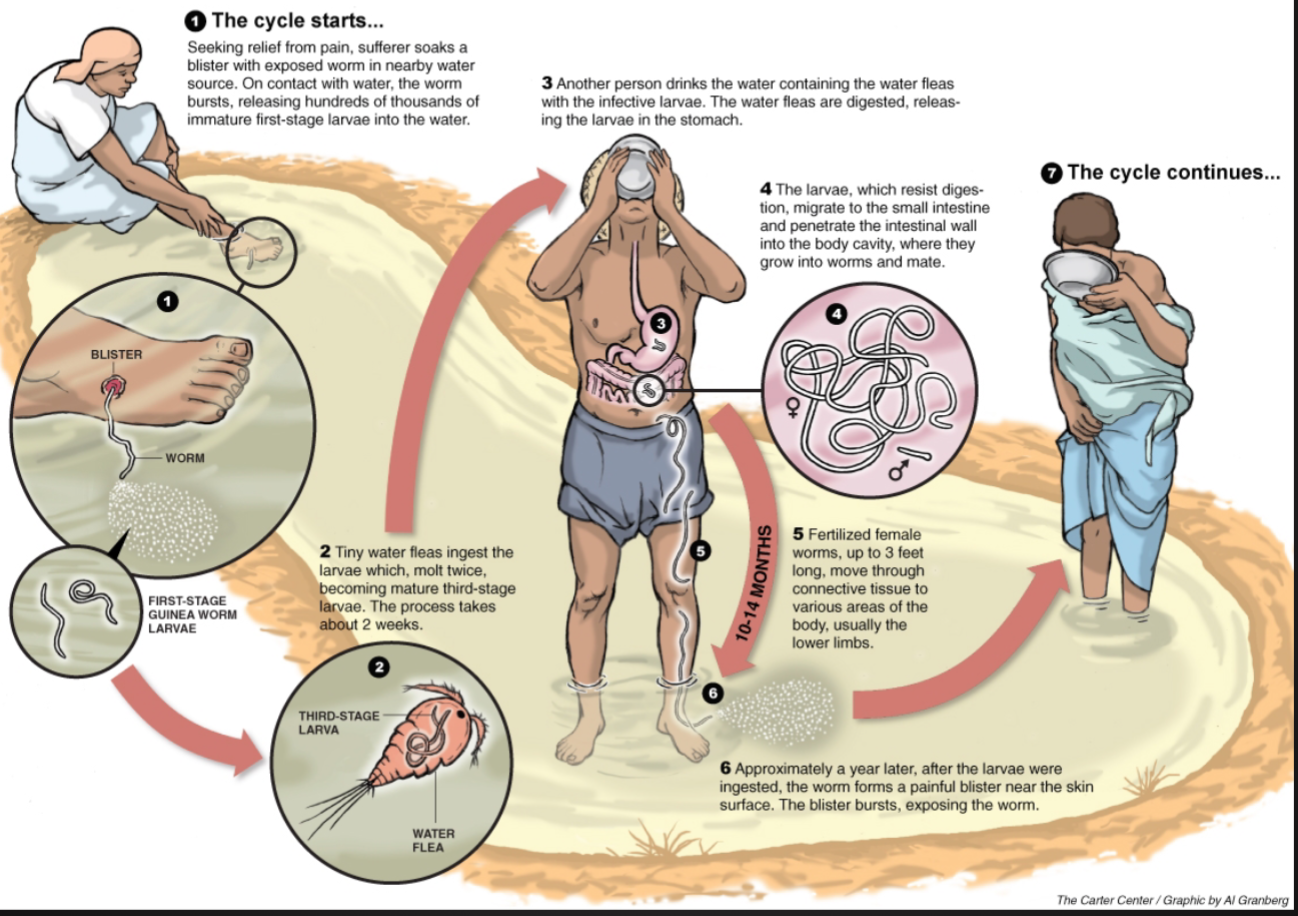
Dracunculus Medinensis (Guinea Worm)
Parasite: Dracunculus Medinensis - “Little Dragon from Medina”
A nematode: females up to 31” in length, males: ⅙ in length
Disease: Dracunculiasis: Guinea-worm disease (GWD)
One of the older diseases known to humankind
Mentioned in number of historical texts
Sanskrit greek, hebrew (15-16 BS)
Parasite found in egyptian mummies (3000 years old_
Remains endemic in 3 countries: Sudan, Mali, and Ethiopia
Infects humans and domestic animals
Transmitted by drinking water
The Life Cycle of the Guinea Worm
Rod of Asclepius (symbol of medicine)
Greek god Asclepius deity associated with healing and medicine
Symbol continues to be used in modern times, associated with medicine and health care
Historians believe that the extraction of Dracunculus Medinensis (“the fiery serpent”) on a stick led to the symbolism behind the Rod of Asclepius
Caduceus (Staff of Mercury and Hermes)
The caduceus is recognized symbol of commerce and negotiation
Mistakenly used as the symbol of medicine in the united states
Introduction to Helminths (Parasitic Worms)
Helminths (Parasitic Worms)
Eukaryotic organism, invertebrates
Relatively large (>1 mm long) some are very large (>1 m long)
Have well-developed organ systems and most are active feeder
The body is either flattened and covered with plasma membrane (flatworms) or cylindrical and covered with cuticle (roundworms)
In their adult form, helminths are unable to multiply in humans
Some helminths are hermaphrodites (monoecious) other have separate sexes (dioecious)
Worldwide distribution: infection is most common and serious in poor countries. The distributions by clime, hygiene, diet and explore to vectors
Many infections are asymptomatic, pathogenic manifestations depend on the size, activity, and metabolism of the worms. Immune and inflammatory responses also cause pathology
Production losses due to:
Competition for nutrients
Damage to body systems (gut, liver, can lead to death)
Animal welfare: companion animals - food animals
Public health: zoonotic infections
Taxonomy of Helminths 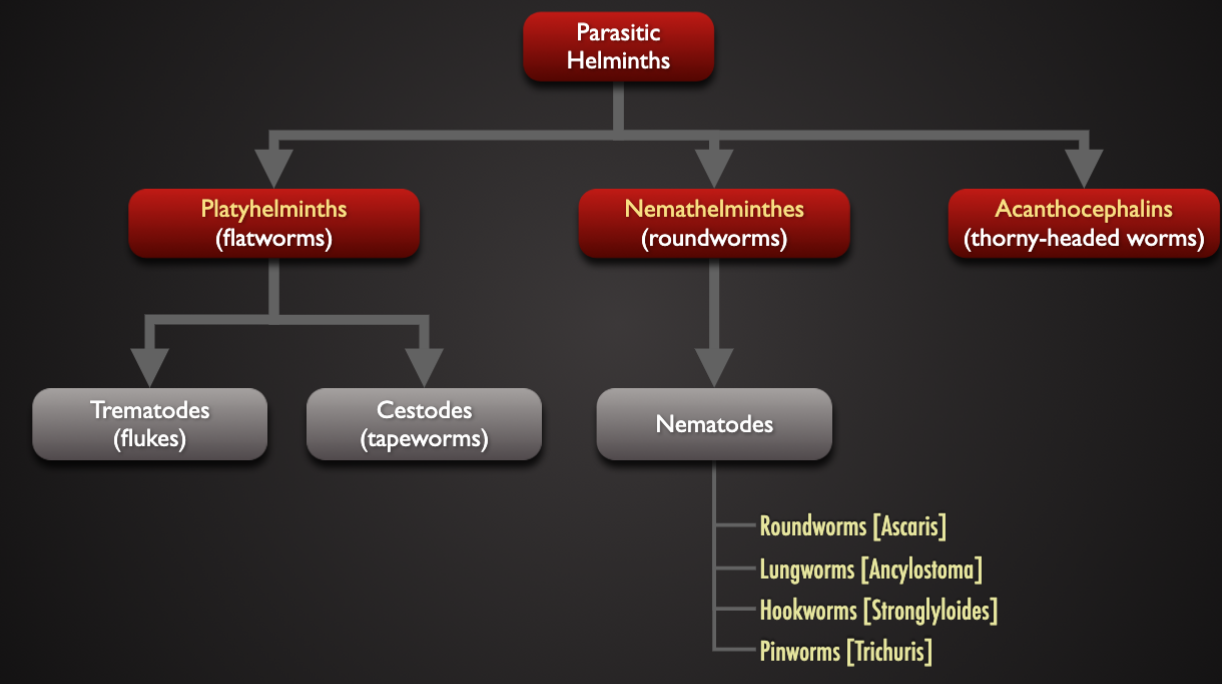
Characteristic of Helminths
Trematode (flukes) | Cestode (tapeworm) | Nematode (roundworm) | |
|---|---|---|---|
Appearance | leaf-life | tape-like | worm-like |
cross-section | Flattened | flattened | cylindrical |
Body cavity | absent | absent | fluid-filled |
gut | Blind sack | absent | true-gut |
Life cycle | indirect | indirect | Direct and indirect |
reproduction | monoecious | monoecious | dioecious |
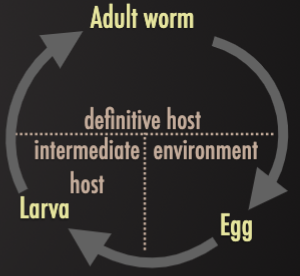
Host Types
Definitive Host
Host where adult stages develop
Intermediate Host
Host where immature stages develop to produce usually ineffective stages of it without reaching to maturity
Paratenic Host
Immature stage retained but no parasite development
Transmission Route
Fecal-Oral
Eggs or larvae passed in the feces of one host and ingested with food/water by another
Ex. ( Ingestion of Trichuris eggs leads directly to guy infections in humans.) (Ingestion of Ascaris eggs and Strongyloides larvae leads to a pulmonary migration phase before gut infection in humans)
Transdermal
Infective larvae in the soil (geo-helminths) actively penetrate the skin and migrating through the tissues to the guy where adults develop and produce eggs that are released in host feces
Ex. larval hookworms penetrate the skin, undergoing pulmonary migration and infecting the guy where they feed on blood causing iron-deficiency anemia in humans
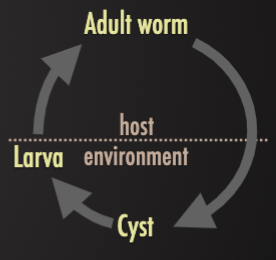
Vector-borne
Larval stages taken up by blood-sucking arthropods or undergoing amplification in aquatic molluscs
Ex. (1) Onchocerca microfilariae ingested by blackflies and injected into new human hosts, (2) Schistosoma eggs release miracidia to infect snails where they multiply and form cercariae which are released to infect new hosts. Cause of “river blindness”
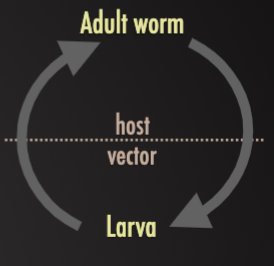
Predator-prey
Encysted larvae within prey animals (vertebrate or invertebrates) being eaten by predators where adult worms develop and produce eggs
Ex. (1) Taenia cysticerci in beef and pork being eaten by humans, (2) Echinococcus hydatid cysts in offal being eaten by dogs.
Survival Strategies
Morphological Adaptations
Degeneration of organs
Organs of locomotion
Tropic organs
Nervous system and sense organs
Attainment of new organs
Body shape (adapted to host environment and migration)
Developed of protective covering (cuticle is resistant to digestive enzymes)
Development of adhesive organs (sucks, hooks, jaws, secretory glands, acetabulum)
Physiological Adaptations
Secretion of anti-enzymes and mucous
Facultative anaerobic respiration
Osmotic pressure adaptability
Chemotaxis
Hypobiosis
Critical hatching conditions
Periparturient rise
Reproductive
Hermaphroditism
Development of cyst wall
Fecundity
Complexity of life cycle
Avoidance of Host Defence
Acquisition of host molecules to reduce antigenicity
Release substance the depress immune system (ex. Depress lymphocyte function, inactive macrophages, or digest antibodies )
Producing anti-complement factors (to protect their outer layers from lytic attack)
Release large amounts of antigenic material - antigen overload ( to divert immune responses, locally exhaust immune potential)
Induce a form of immune tolerance (infections acquired in life- before or shortly after birth)
Pathogenesis of Helminth Infections
Direction damage from worm activity
Block of internal organs
Ex. Gi transit, blood flow through organs or lymph flow affected by worm size, migration and granuloma formation
Pressure exerted by growing parasites
Ex. large fluid-like cyst in the liver, brain, lungs or body cavity
Physical Damage
Ex. tissue necrosis, feeding by worms, damage during migration
Chemical damage
Ex. release of excretory-secretory materials, release of enzymes and factors such as anticoagulants)
Nutritional impact in under-nutritioned individuals or animals
Infect Damage from Host Response
Hypersensitivity-base inflammatory changes
Ex. contribute to lymphatic blockage associated with filarial infections)
Local allergic responses
Eosinophilia, edema, and joint pain
Structural changes
Ex. villous atrophy, mucosa permeability changes
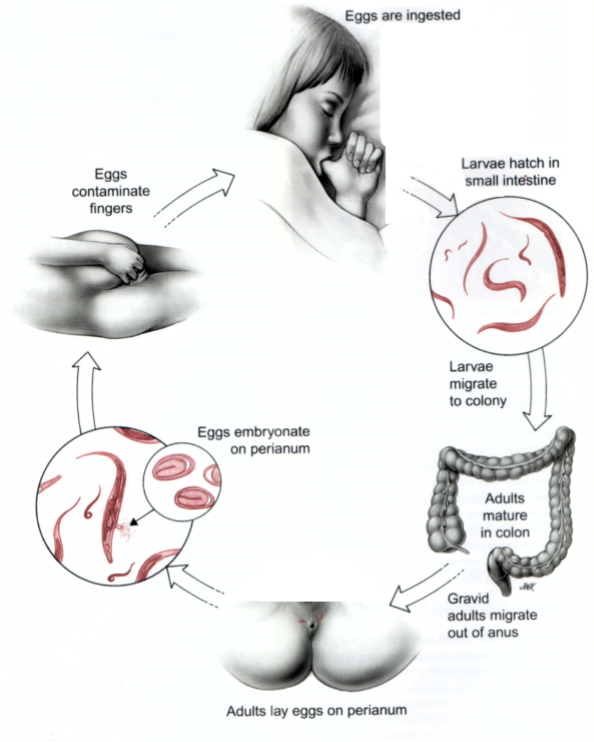
Simple (direct) Life Cycle: Enterobius Vermicularis (pinworm)
Complex Life Cycle: Paragonium westermani (Lung Fluke)
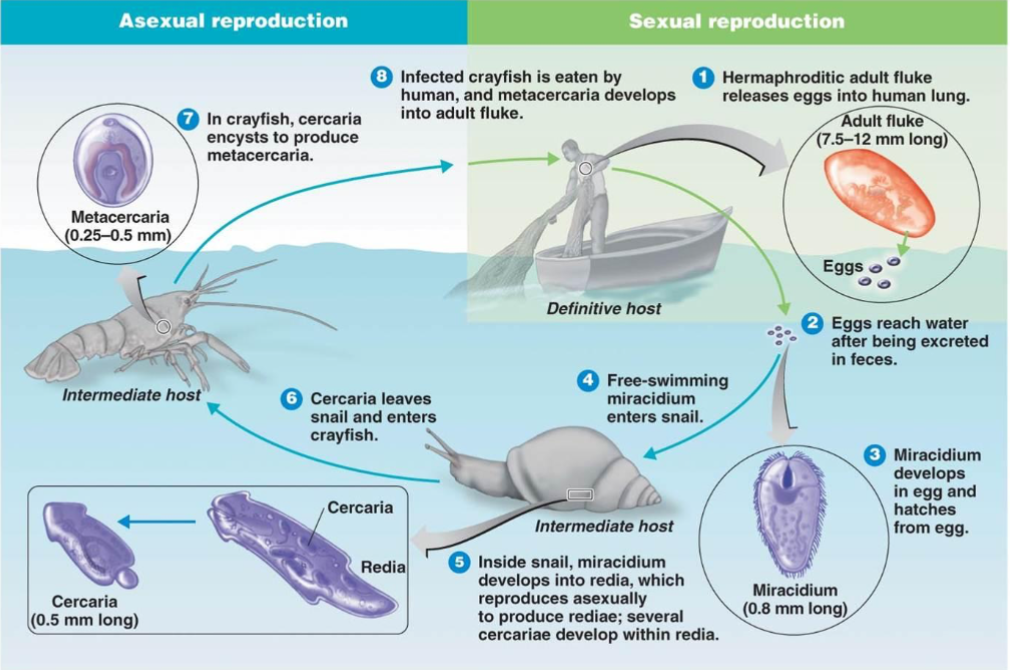
Complex Life Cycle: Fasciola Hepatica (Liver Fluker)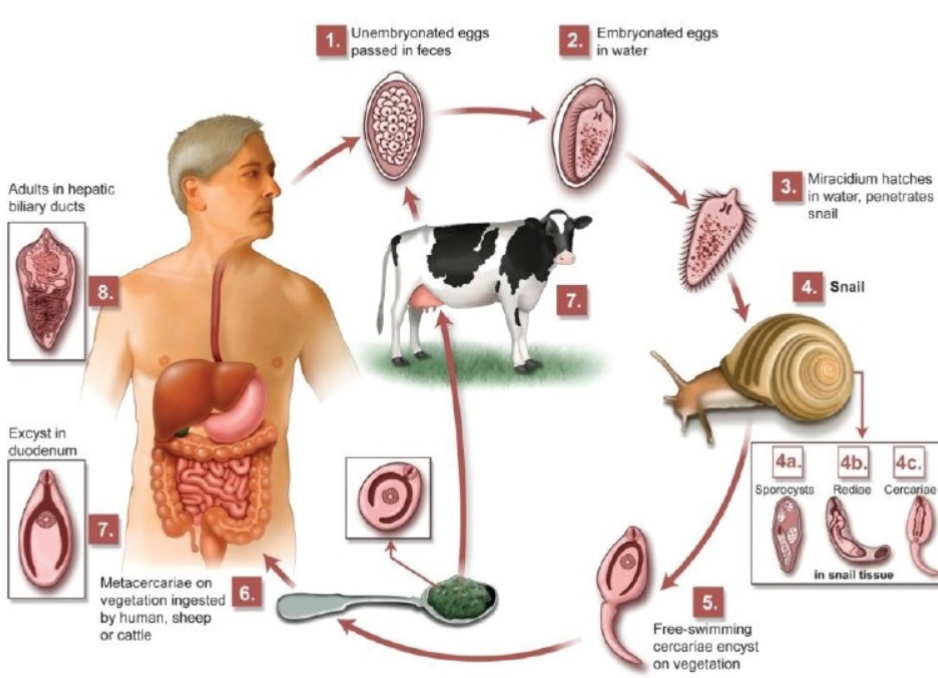
Prion Disease 03/05/24
Prion Diseases (transmissible spongiform encephalopathies (TSEs))
Humans
Kuru (“to shake”)
Creutzfeldt Jakob Disease (CJD)
Fatal Familial Insomnia (FF)
Gerstmann Straussler Syndrome (GSS)
Cattle
Bovine spongiform encephalopathy (Mad Cow Disease) (BSE)
Sheep/Goats
Scrapie
Deer/ELK
Chronic Wasting Disease (CDW)
Characteristics of TSEs (Prions Disease)
Spongiform (brain vacuolation
Neuronal loss, gliosis and astocytosis
Atrophy of brain tissue
Accumulation of misfolded prion plaques
Different Prions affect different parts of the brain
Cerebral cortex
Symptoms: loss of memory, mental acuity, visual impairment (CJD)
Thalamus
Damage leads to insomnia (FFI)
Cerebellum
Damage leads to body coordination movement problems and hard to walk (kuru, GSS)
Brain stem
Mad cow disease (BSE) – brain stem is affected
Characteristics of Infection
Encephalitis (inflammation of the brain)
widespread neuronal loss
Loss of motor control
Dementia
Paralysis
Kuru
Identified by epidemiology in Papua New Guinea based on anthropological research by Robert and Louise Glasse in 1950’s
1% of the Fore tribe was afflicted: most women, some children, few adult males
Symptoms: headache, joint,6-12 weeks later →difficult walking, death within 1-2 years
1910-1920
Evidence lead Glasse to suggest that endocannibalism was associated with disease
Carlton Gadjusek did research and proved Kuru killed patients
From Kuru to Scrapie
Animals needed to study TSEs
Scrape disease in sheep is similar to kuru with symptoms and etiology
Scrapie can be transmitted to hamster and mice, not kuru
Mice infected with scrape were the first good animal model
Infectious agent purified 5000 fold:
Nuclease resistant
UV and heat resistant
Sensitive to protease (only at high levels) and protein denaturants
Bovine Spongiform Encephalopathy (BSE) “mad cow disease”
1970: hydrocarbon-solvent extraction of meat and bone meal for cattle feed was abandoned in Britain
1986: 7000 infected cows. BSE became reportable, epidemiology suggested a prion ideas, and meat and bond meal use was abandoned
BSE incubation period is 5 years: 1 mill cows were infected
1989: human consumption of bovine CNS tissue (though to have highest prion concentration) banned based on fears of transmission to humans
1996: new type of CJD appeared in Britain and France on young patients and different neuropathology. Linked with consumption of BSE-contaminated beef
Introduction to Prions
“Pree-ons”
Shortened for “proteinaceous infectious particle”
Causes transmissible spongiform encephalopathies (TSEs) among other disease
No treatment available to halt progression of TSEs. Treatment in humans is aimed at alleviating symptoms and making patient comfortable
TSEs are ALWAYS FATAL
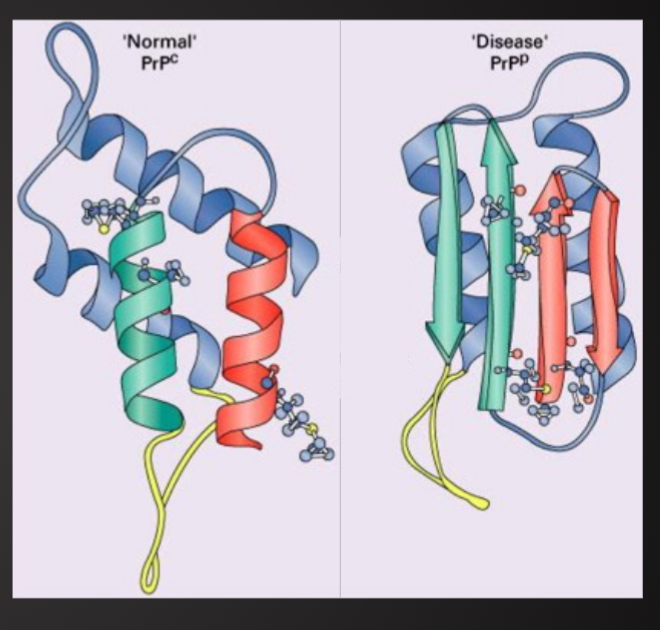
Basic Structure
Normal prions (PrPC ) – common in brain cells
Contains 200-250 amino acids twisted into coils(helices) with tails of more amino acids
The mutated and infectious form (PrPSC )
Built from same amino acids but take different shape
100x smaller than known virus
Types of Prion Diseases (TSEs)
Sporadic
Occur with no prion protein mutation
Most TSEs are sporadic
Cause unknown
Infected 1-2 mill people late in life
Infectious
Ex. Kuru,, BSE (mad cow disease), Scrapie
Consumption of infected material
Iatrogenic spread (organ transplant, esp. corneaL been operated with surgical instruments used on a CJD patient)
Familial / Hereditary
Due to autosomal dominant mutation of PrP
inherited : 10-15% of total human TSE cases
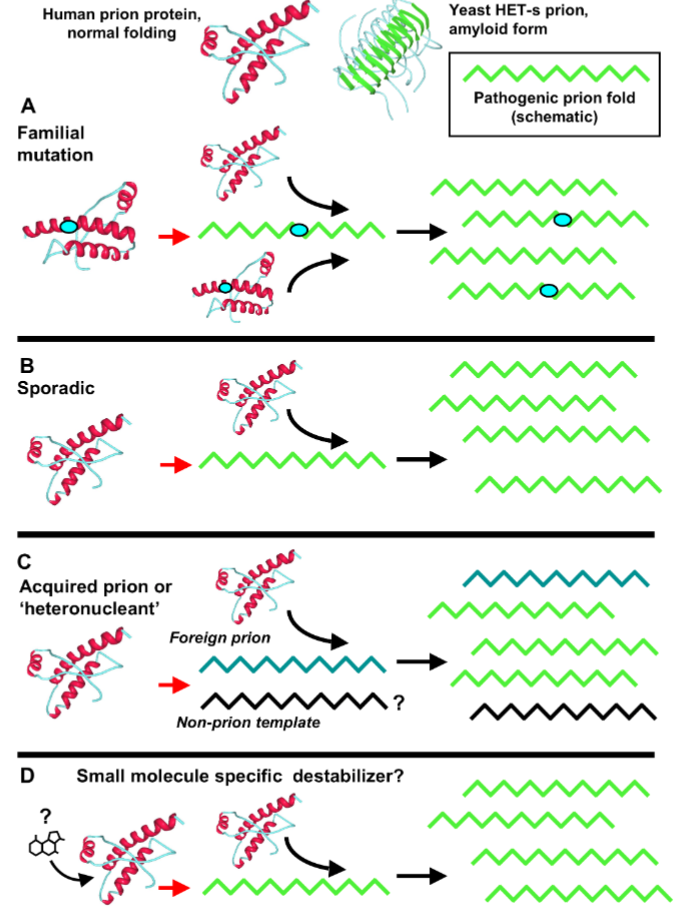
Properties of Infectious Prions (PrPSC )
Resistant to degradation by proteases → leads to accumulation
B-sheet structure of PrPSC have high affinity for other B-sheet structures (in other proteins or other PrPSC) → forms oligomeric, insoluble aggregates that in turn form toxin amyloid plaques → interferes with cell and tissue function and death of cells and tissues
PrPSC are extremely resistant to heat and chemicals
PrPSC are very difficult to decompose biologically
Survive in soil for many years
Prions are not nucleic acids (ex. DNA or RNA0
How do infectious prions propagate>
During oligomerization the prions corrupt the native form of the protein into a transmissible disease conformation
Formation of Infectious Prions PrPSC
Intro to Immunology and Innate Immune System 03.12.24
Basic Concepts of Immunology
Immunology is the study of the body's defense against infection. We are continually exposed to microorganisms, many of which cause disease, and yet become ill only rarely
How does the body defend itself?
When infection does occur, how does the body eliminate the invader and cure itself?
Why do we develop long-lasting immunity to many infectious diseases
Components of the Immune System
Basic defense system of the body
Composed by specialized cell types and organized structures that coordinate defense mechanisms
Protects from harmful pathogens and disease
Prevent and limit infection
When unsuccessful in curving the infection disease arise
History of Immunology
Edward Jenner, developed the first vaccine against smallpox (18th century). Develop the first vaccine by infecting a patient with cowpox and demonstrating that the patient became immune to smallpox.
Louis Pasteur, France, 19th century: Demonstrated that infectious diseases are caused by microorganisms (Germ theory of disease). Pasteur developed the first vaccine against rabies by attenuating the rabies virus, making it less harmful.
Robert Koch, Germany, 19th and early 20th centuries: Koch's work on tuberculosis and anthrax led to significant breakthroughs in our understanding of these diseases and their treatment. Laid the foundation for modern microbiology and the development of antibiotics.
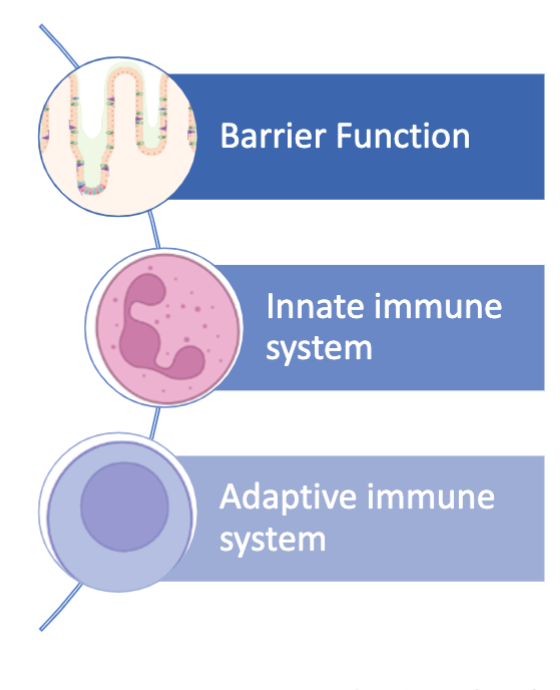
Components of the Mucosal Immune System
Several immune factors function in concert to:
Stratify luminal microbes
Minimize bacterial-epithelial cell contact
Tolerance towards food antigens and commensal microorganism (“oral tolerance”)
Prevent the induction of unnecessary systemic immune responses (“compartmentalization”)
Work in concert to:
Survival and respond accordingly to microbial threats
Maintaining a balance between tolerance and defense mechanisms
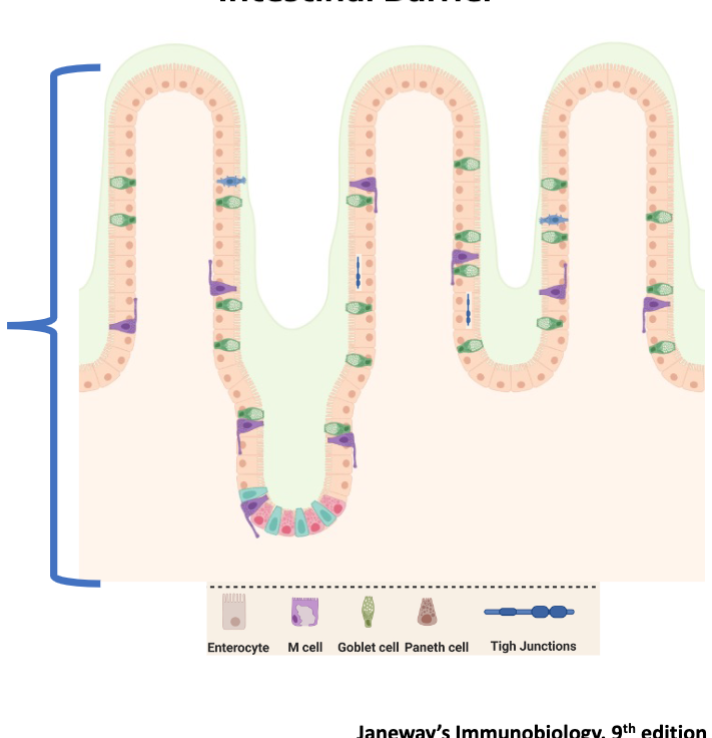
Components of Barrier Function
Intestinal barrier are made up of numerous different cell types
Enterocytes (absorptive)
Goblet cells (mucus-producing)
Paneth cells (found in crypts, produce antimicrobial compounds)
All these cell types develop from a common stem cell at the base of the intestinal crypts
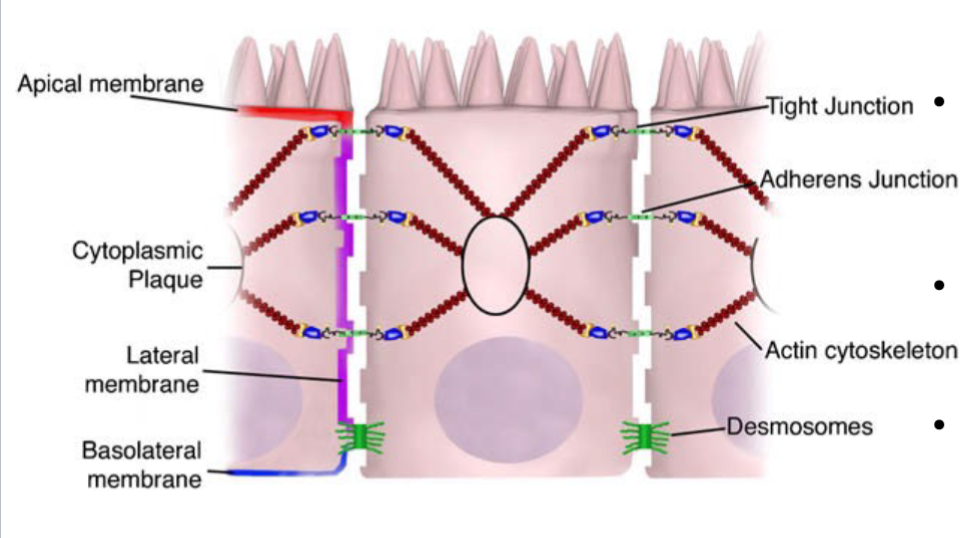
Structure:
One layer of epithelial cells tightly adhered to each other
Epithelial cell shedding
Unidirectional flushing
Inteintal barrier: surface area 25000 sq ft
The junctional complex
Transcellular proteins connected through adaptor proteins to the actin cytoskeleton
Important in maintaining cell polarity
Desmosomes are localized dense plaques that are connected to keratin filaments
Components of Barrier Function: Mucus Layer
Glycoproteins called mucin by goblet cells
Protein core with several polysaccharide molecules attached
2 layer of Mucin
Outlet layer: colonized by microorganisms
Inner layer: high concentrations of antimicrobial peptides prevents microbial colonization “Killing Zone”
Microbial Sensing of Intestinal Environment
Presence of microorganisms close to epithelial surfaces are recognized by APCs via PRRs
Activated Dcs stimulate IL-22 secretion by innate lymphoid cells
Stimulates epithelial cell proliferation and the secretion of antimicrobial peptides:
Defensins, REGIII, Lactoferricin
AMPs act as lytic enzymes disturbing microbial cell membrane
AMPs disrupt microbial cell membrane by forming pore
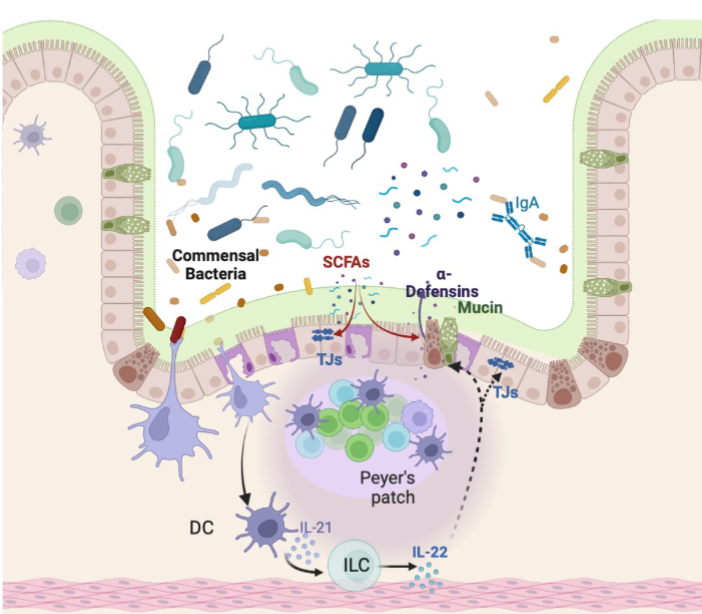
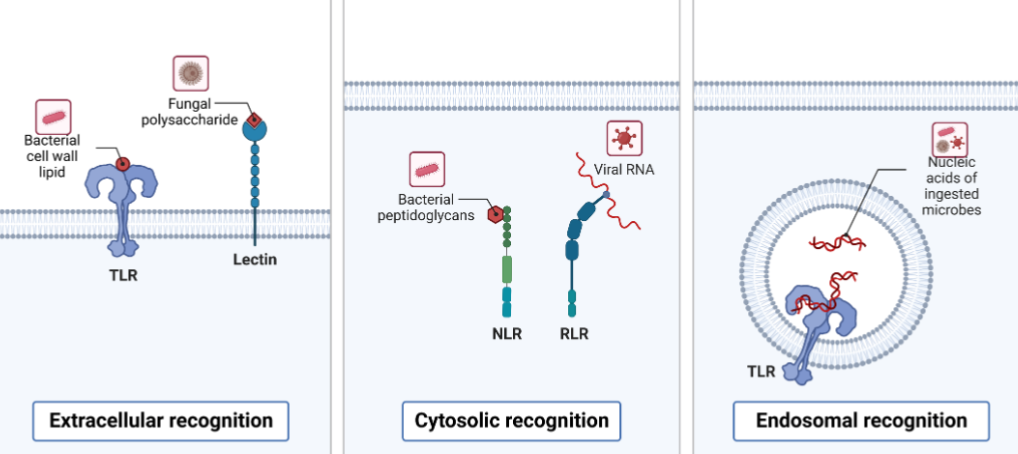
Pattern Recognition Receptors (PRRs)
Several germline-encoded receptors used to recognize different MAMPS
Sensing of MAMPS through the PRRs induces tissue repair and epithelial cell proliferation
Immunological Components of Barrier Function:
Lamina Propria
Loose connective tissue
Contains several immune cells
Antigen-presenting cells (APCs)
T cells and B cells
Innate lymphoid cells
Other immune mediators (complement, chemokines, cytokines)
Microbiology Research: Pre-NGS Periods
After Louis Pasteur discovered bacteria, medical research focused mainly on their role in causing disease.
The bacteria that reside in and on our bodies were generally regarded as either harmless commensals, or pathogens.
Tools available only allowed us to study these microorganisms one at a time, rather than as communities.
Research focused on the few bacteria that could be grown in vitro. But the majority of microbes that live in our bodies are extremely hard to grow in vitro
Microbiology Research: POST-NGS
Culture-independent techniques (NGS) allow the study of the ecological complexity of the intestinal microbiota and its impact on host physiology.
Have revolutionized the field of microbiology and broadened the lens to explore the functional roles of microbiota on host health and disease.
Intestinal microbiota serves an important role in protecting health, by shaping metabolic and immune function both locally and systemically
Microbial cells in intestine surpass human cells by a factor of 10
Microbial genome is 100 x more extensive than human genome
Intestinal Microbiota role in Health and Disease
The intestinal microbiota have likely evolved under selective pressure to effectively degrade nondigestible plant carbohydrates enhancing the host digestive efficient
Additionally
Metabolic: Vitamin B, K and SCFA
Structural: promote epithelial cell proliferation mucin production
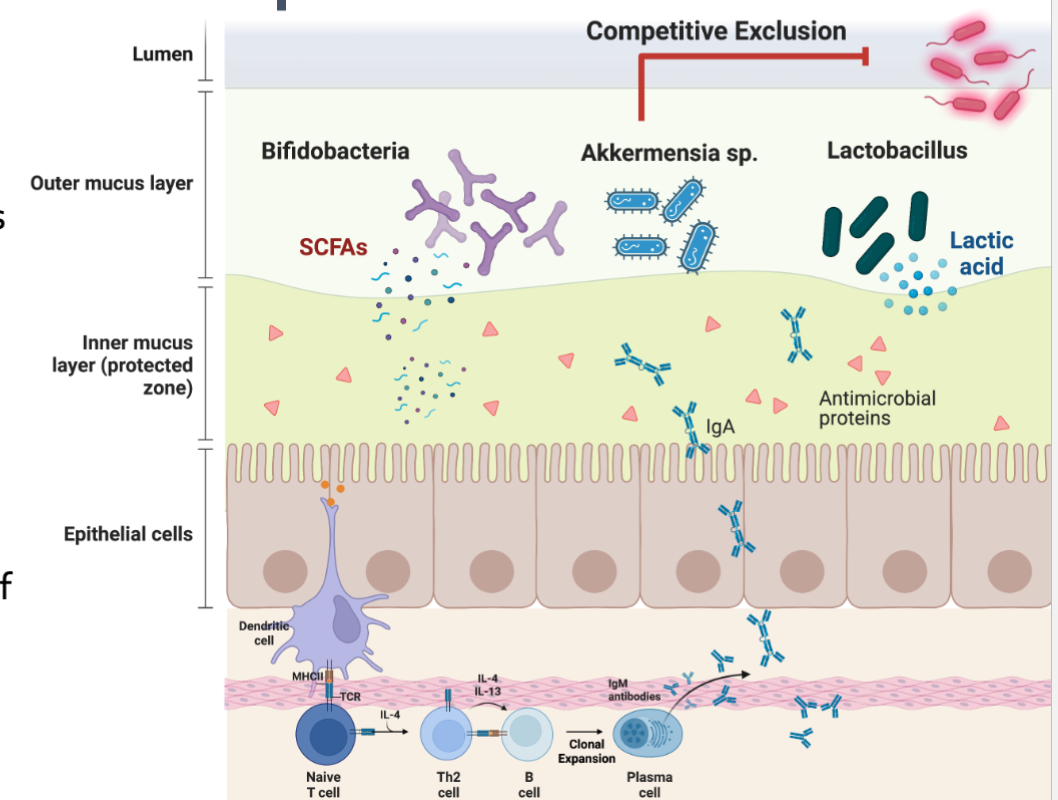
Protective: Competitive exclusion of non-resident bacteria and pathogens
Bidirectional communication between the host and intestinal microbiota via a wide range of metabolites (SCFA, Indoles, BA)
Niche Occupation and Competitive Exclusion
Mucin glycans are nutrients for some bacteria
Establishing a physical barrier (niche Occupation) excluding potential pathogens
Reduce pH by production of SCFAs acetate and lactate
Components of the Immune System
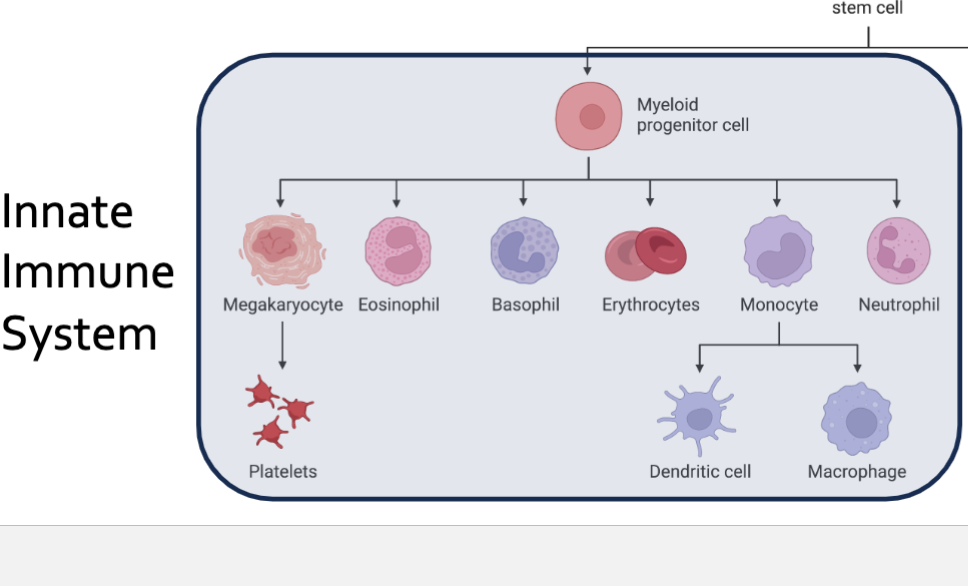
Where do the cells of the immune system come from?
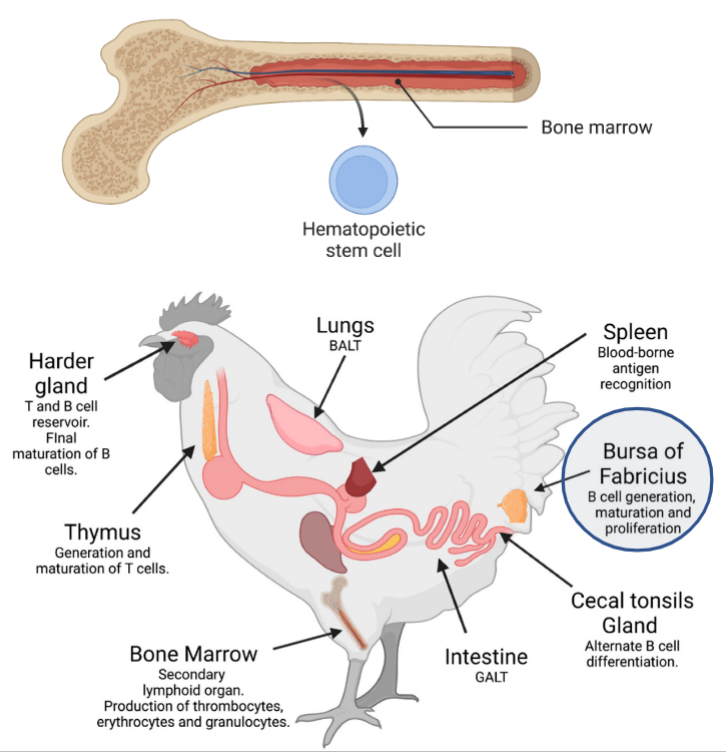
Hematopoiesis in the Bone Marrow
Production of blood cells
Myeloid
Lymphoid
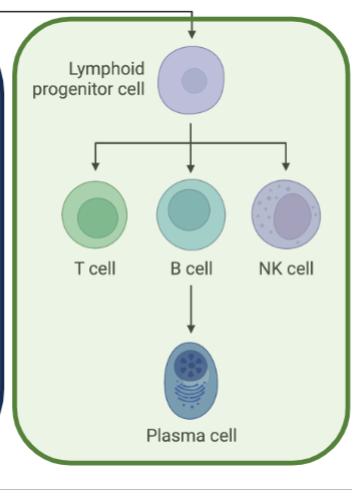
Maturation of Lymphocytes occurs in Central Lymphoid Organs
Bone marrow of animals (Bursa of Fabricius in birds) for B cells
Thymus, for T cells
Immune Cells
Stem cell differentiation from bone marrow
All lymphoid and myeloid cells are derived from hematopoietic stem cells in the bone marrow
Lymphoid and myeloid cells in circulation are collectively referred to as leukocytes or WBCs
Cells are differentiated by appearance and surface markers, clusters of differentiation: CD4, CD8, T cell receptor and C cell receptor
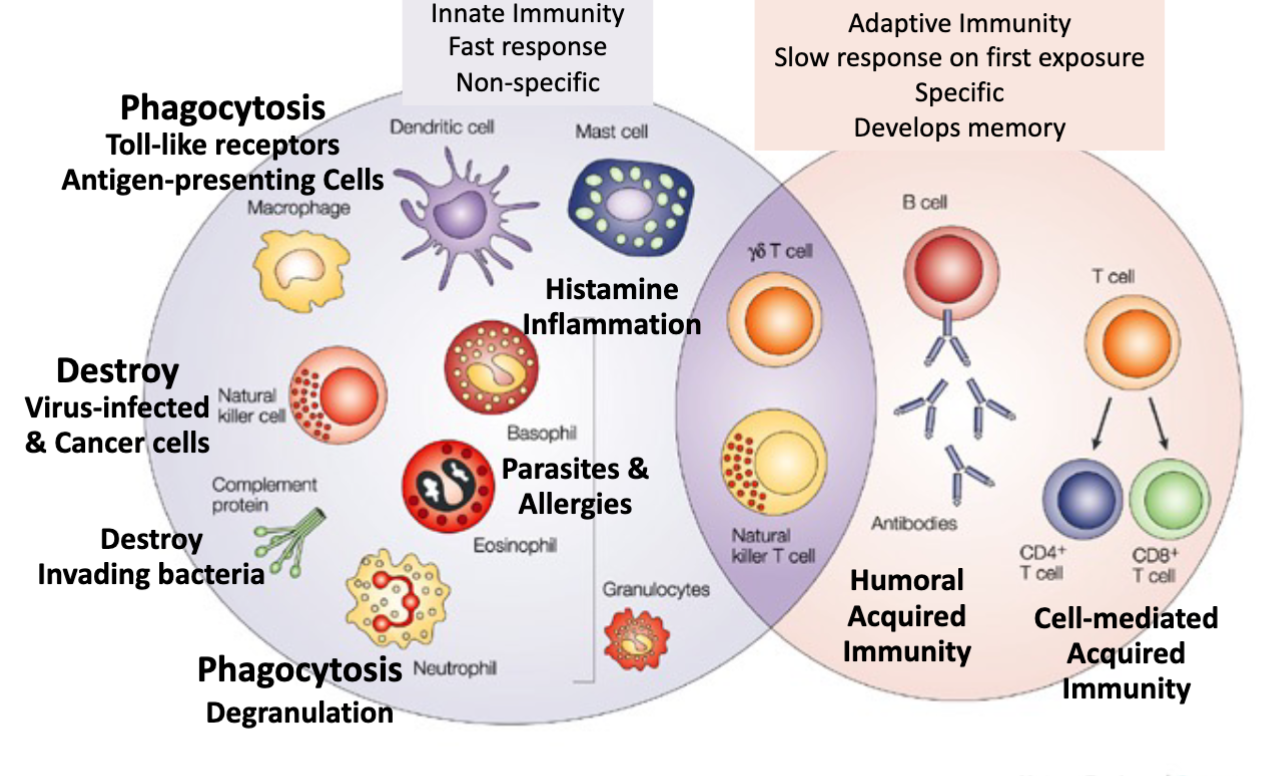
Innate Immunity Faster Response NonSpecific vs Adaptive Immunity Slow response on first exposure specific, develops memory
Innate Immune system Part 2 03.14.24
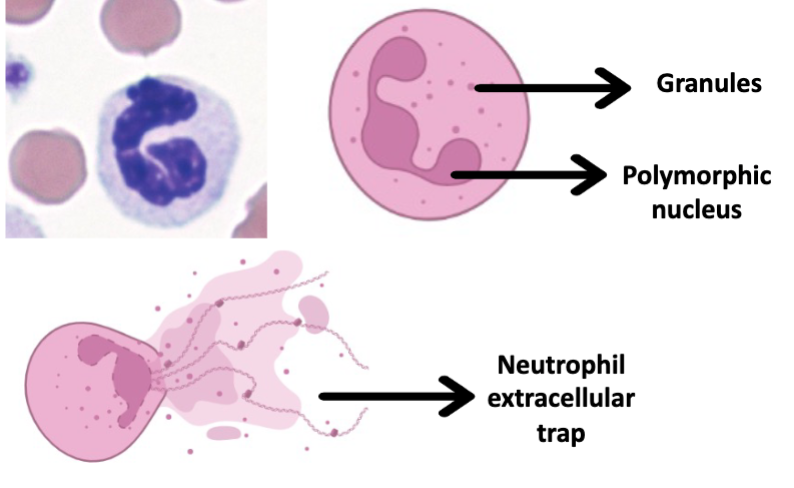
Neutrophils
Components of the immune system innate immunity
50-60% of circulating immune cells
Produce potent antimicrobial toxins, stored in vesicles
First responders to infection
Release mesh like structure composed of cytoskeleton and DNA that traps microorganism
Miltibulate nucleus
Lysosomal granules
Activation of bactericidal mechanism
Phagocytosis and kill
1st white blood cell to infection site
Die and release their contents
Irritate surrounding tissue/recruit other cells
Phagocytosis is improved by opsonization with immunoglobulins
Neutrophils enter site of injury and infiltrate
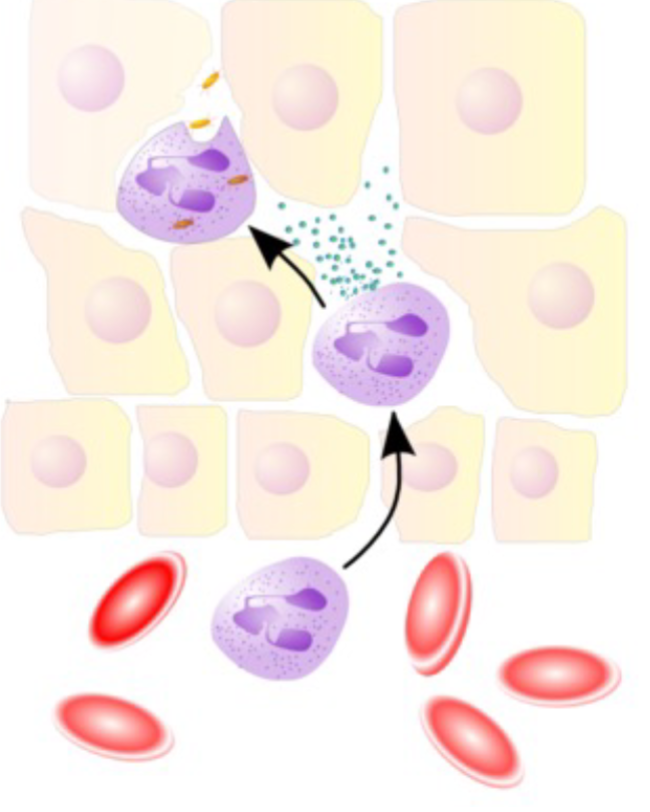
Release factors important in immune response
Eosinophils and Basophils
Eosinophils
Granules
0.2-3% circulating immune cells
Effective in killing parasites
Basophils
Granules
<0.5% circulating immune cells
Coordinate immune response to parasitic infection
Macrophages and Monocytes
Tissue resident immune cells (sentinel)
Constantly patrolling extracell spaces
Engulf and phagocyte microbes
Coordinate immune activation and promote recruitment of other immune cells
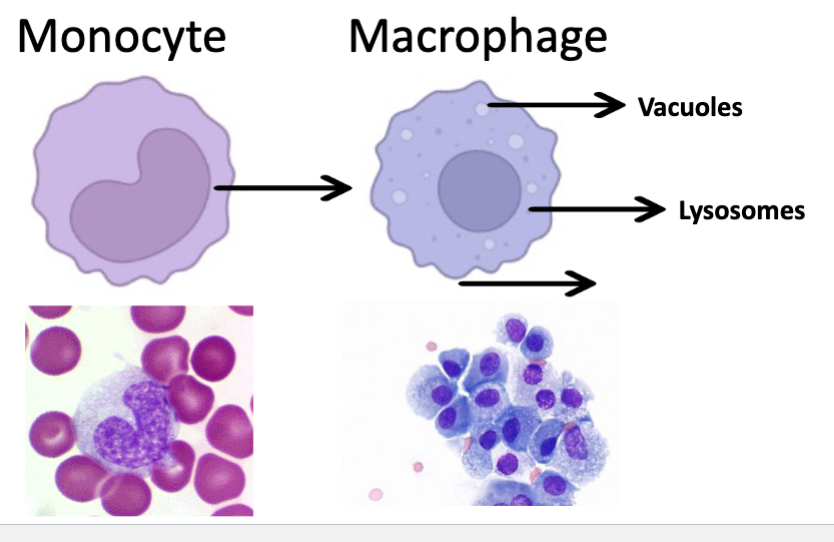
Macrophages
Large cell (10-25 uM diameter)
Main purpose: phagocytosis/kill
Act non-specifically
Chemotactic capability
Release cytokines
Potent phagocytosis when activated by T lymphocytes
Express antigen on surface to T/B cells
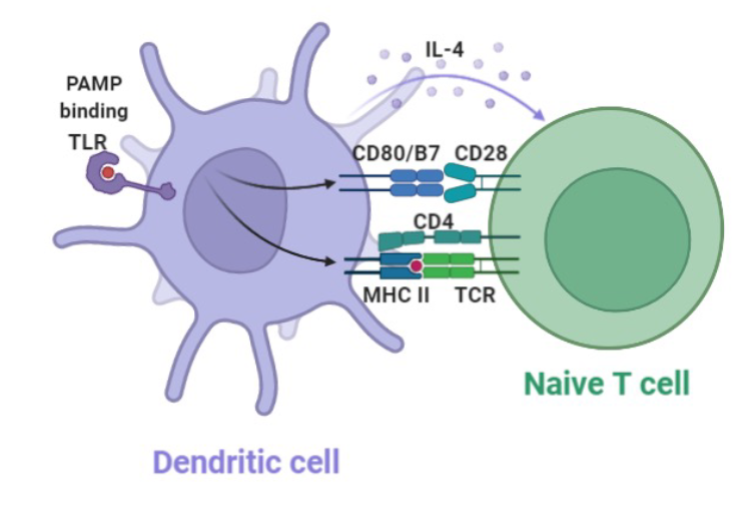
Dendritic Cell
“Professional” antigen presenting cells
Derived from monocytes
Located at barrier sites and lymphoid tissues (Lymph nodes, peyer’s patches)
Main function is to present antigens to T and B cells
Initiate immune response
Promote tolerance towards harmless microorganism or to self antigens
Sensing Mechanisms of Innate Immunity
Coordination of the innate immune response relies on the information provided by many types of receptors
Pattern recognition Receptors (PRRs)
Evolutionarily ancient pathogen system of recognition and signaling
Toll Like Receptors
Limited specificity compared with the antigen receptors of the adaptive immune system
Recognize elements of most pathogenic microbes
Are expressed by many types of cells:
Macrophages
Dendritic cells
B cells
Stromal cells
Certain epithelial cells
Enabling the initiation of antimicrobial responses in many tissues
Toll-like receptor family (10 in humans and in cattle, 12 in mice)
Extracellular and intracellular
Bacterial, viral, year associated molecular patterns
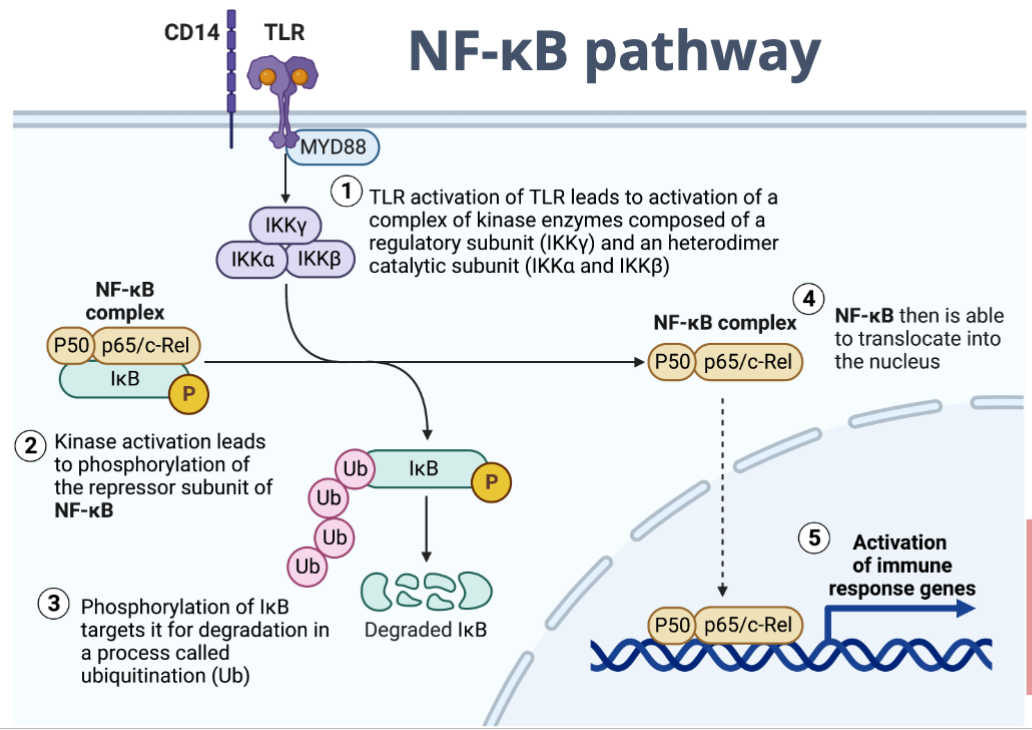
NF-kB pathway
Cytokines and chemokines responsible for initiation of innate immune response
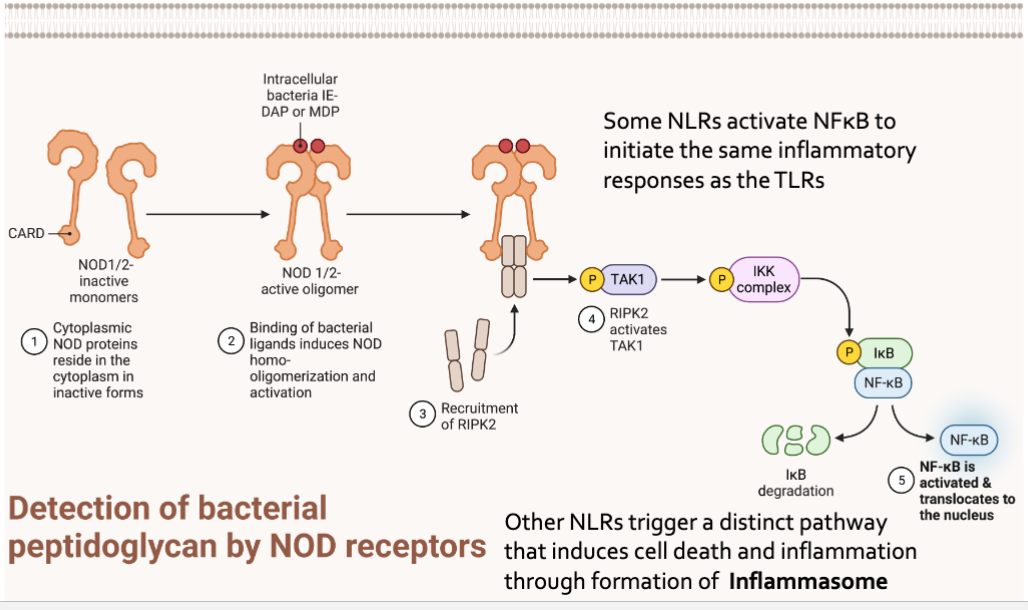
NOD Like Receptors
Nucleotide binding oligomerization dimer (NOD)- like receptors
Intracellular
NOD recognizes muramyl dipeptide from peptidoglycan
Detection of bacterial peptidoglycan by NOD receptors
Some NLR activate NFkB to initiate the same inflammatory response as the TLRs
Other NLRs trigger a distinct pathway that induces cell death and inflammation through formation of Inflamasome
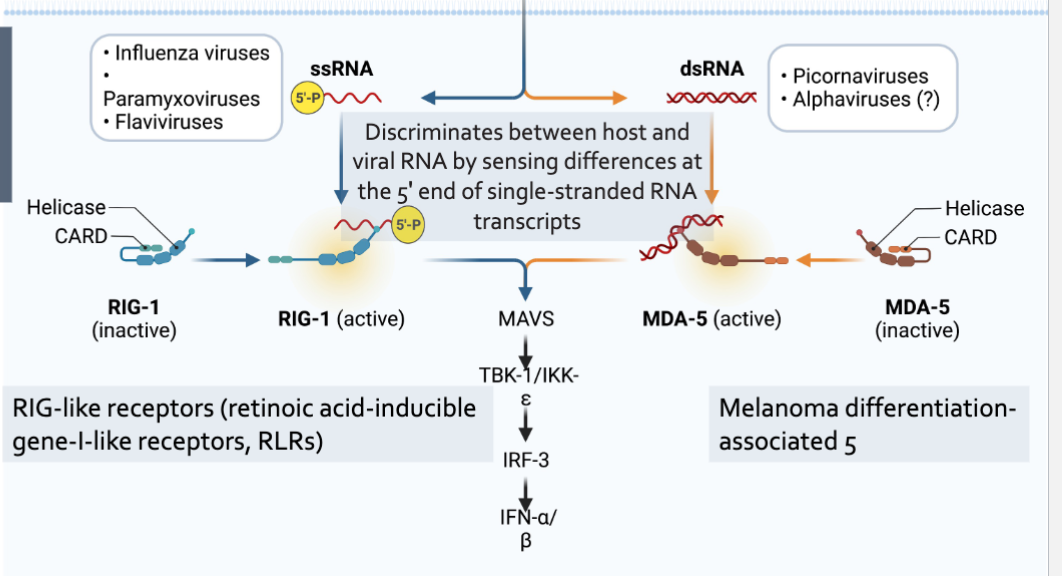
RIG-I and MDA-5
Intracellular pattern recognition receptors
Involved in viral recognition
Sentinels for intracellular viral RNA produced within the cell in contract with TLRs
Provide frontline defense against viral infections in most tissues
Cytokine Signaling
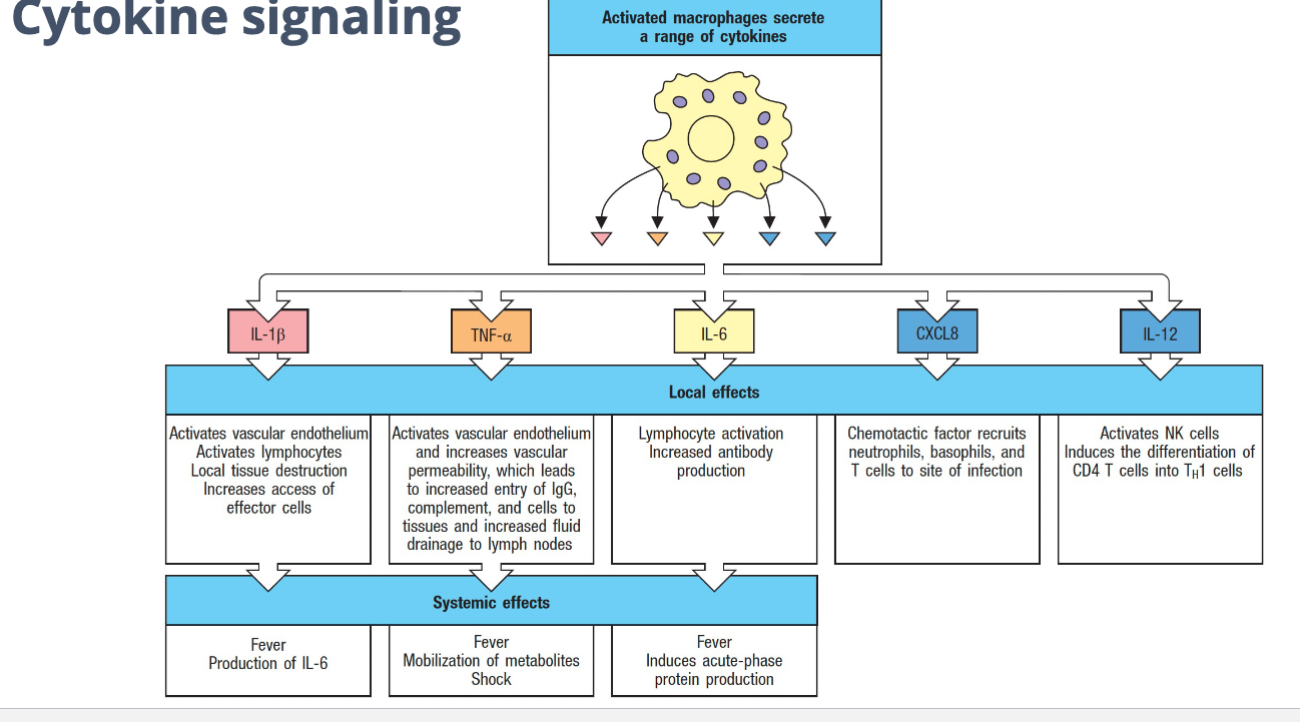
Activated macrophages secrete a range of cytokines
Local effects
Systemic effect
Cytokines signaling molecules of the immune system
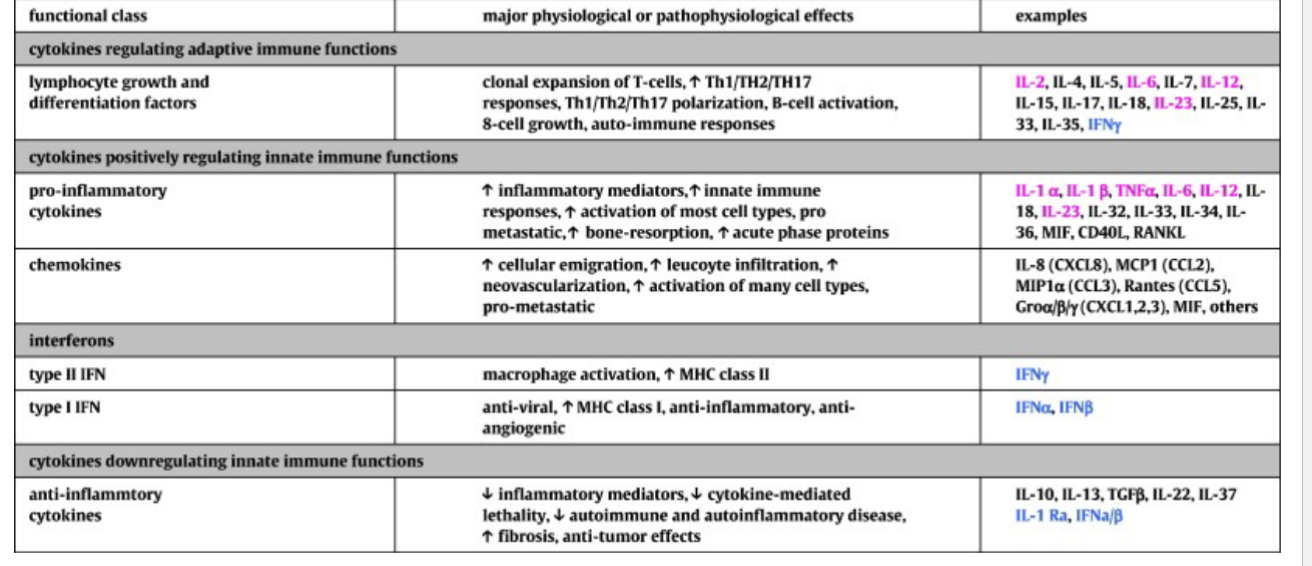
Mammalian Inflammatory response in response to bacterial infiltration through the physical barrier
Inflammatory response
Sensed by the integrated mast cells; neutrophils. Macrophages and effectors cells
Production of cytokines and chemokines
Interleukin-1beta
Interleukin-6
Interkelin-12
TNF-alpha
Vasodilation
Immune cell recruitment
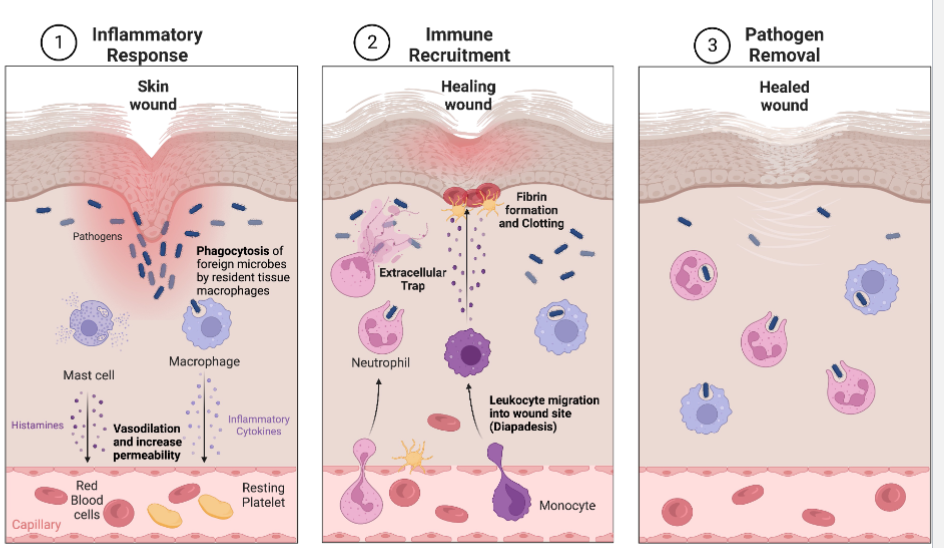
Wound Healing
Pathogen infiltration through wound
Mast cells secrete factors that mediate vasodilation and vascular constriction. Delivery of blood, plasma, platelets and cells to injured area increases
Platelets from blood release blood-clotting proteins at wound site
Neutrophils secrete factors that kills and degrade pathogens
Neutrophil and macrophages remove pathogens by phagocytosis
Macrophages secrete hormones called cytokines that attract immune system cells ot the site and activate cells involved in tissue repair
Inflammatory response continues until the foreign material eliminated and the wound is repaired
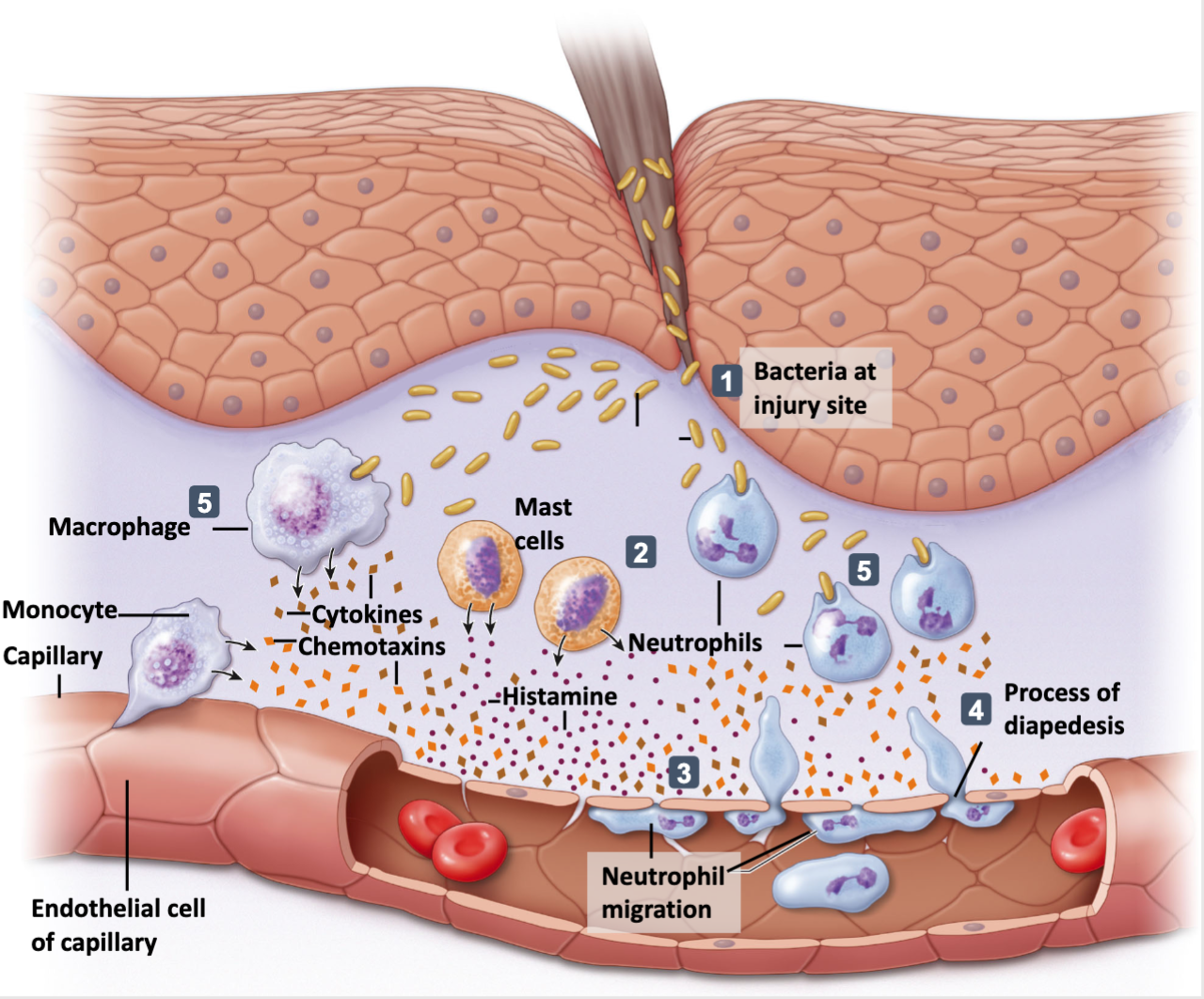
Map
Inflammatory trigger → inflammation → physiological purpose → pathological consequences
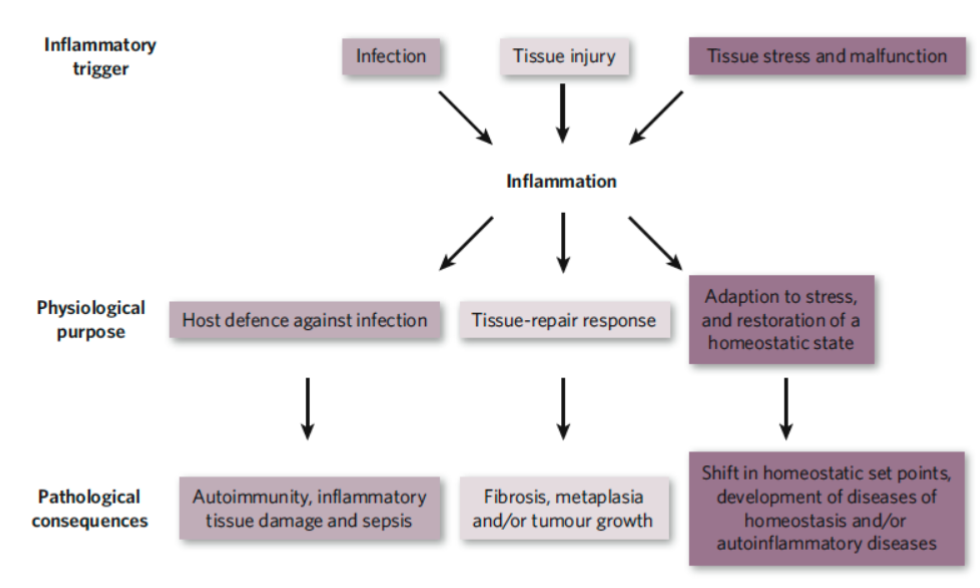
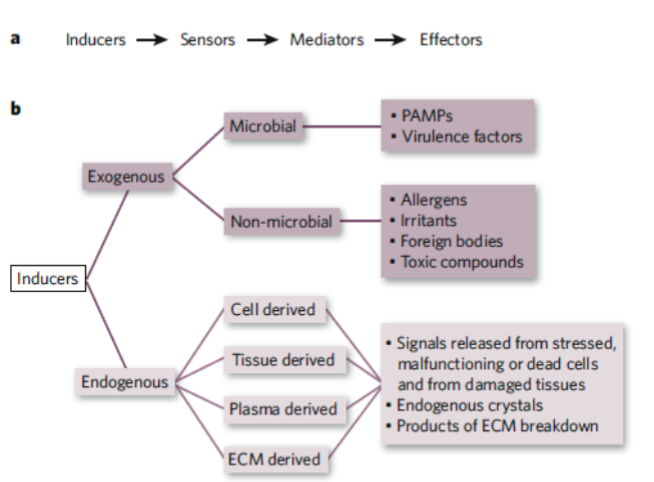
Inducers of Inflammations
Exogenous Inducers
Pathogen associated molecular patterns (PAMPS)
Virulence factors from microbial inducers
Commensal bacteria which can also induce inflammation (through TLRs)
Non-microbial origin: allergens, irritants, foreign bodies, toxic compounds
Foreign bodies
Endogenous Inducers
Sequestration of cells or molecules kept in intact cells and tissues
Necrotic cell death
Epithelial-mesenchymal transition
Damage to vascular endothelium
Chronic inflammation
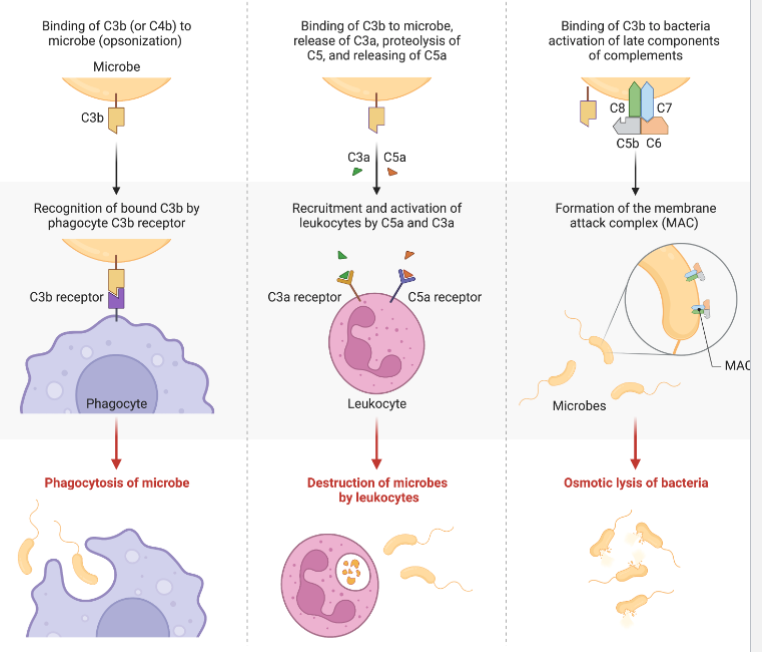
Complement System
Nine plasma proteins (C1 to C9) that are produced by the liver and circulate in the blood in inactive form
Sequential activation leads to assembly of large donut-shaped membrane attack complex (MAC) with the assembly of the C5-C9
MAC inserts in microorganism to form pores
3 outcomes of complement activation
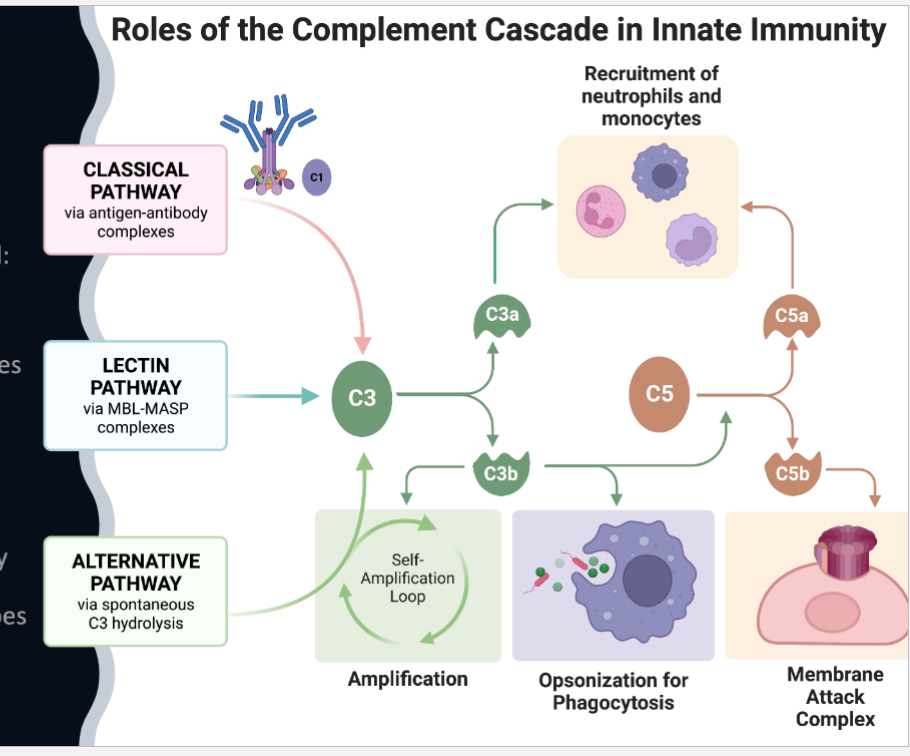
Complement System
Antimicrobial response continued: complement pathway
Cascade sequences that amplify
Classical:
binding to antibodies (adaptive)
Alternative:
directly binds to foreign invader (innate) - polysaccharides from yeast, gram negative bacteria
Lectin Pathway:
stimulated by mannose containing proteins and carbohydrates on microbes (viruses and yeast)
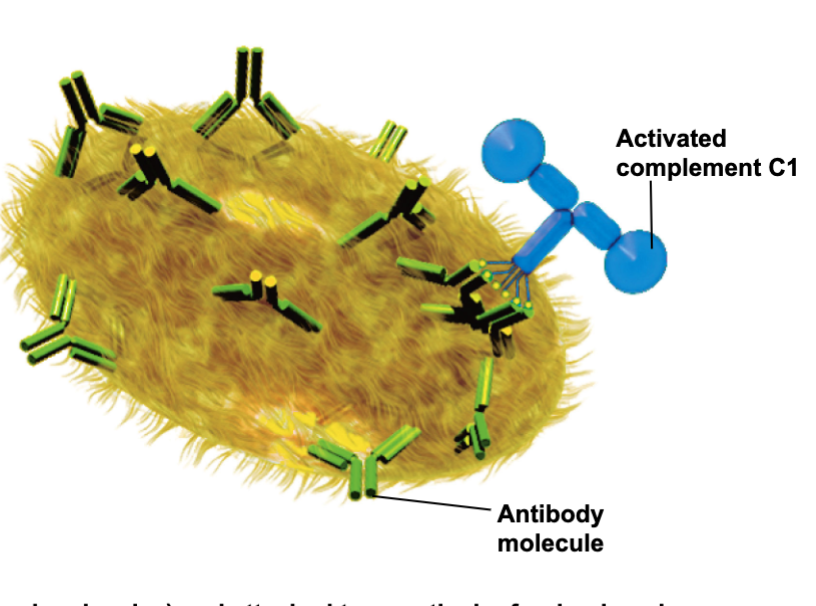
Complement System: Classical pathway
Binding to antibodies(Y-shaped molecules) and attached to a particular foreign invader
Initiates complement activation cascade
Activated complement C1
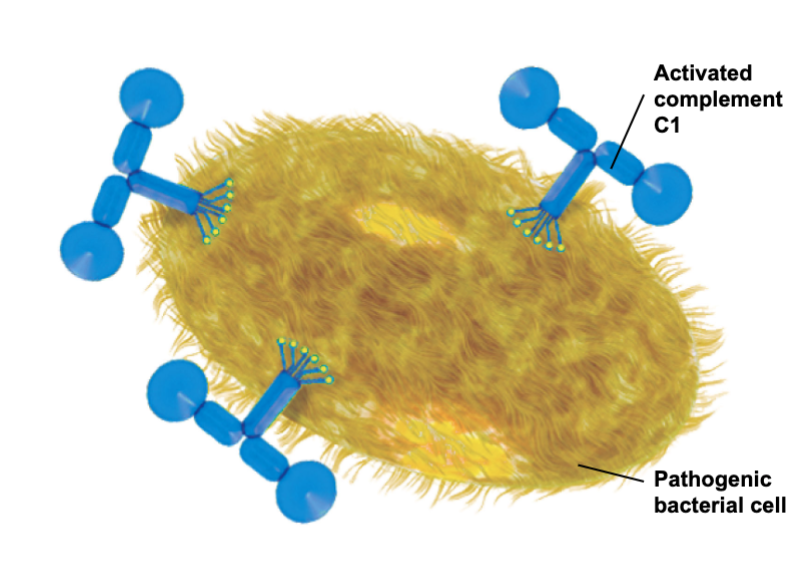
Complement System: Alternative Pathway
Binding directly to a foreign invader non specifically activates the complement cascade
Activates complement C1
Pathogenic bacterial cell
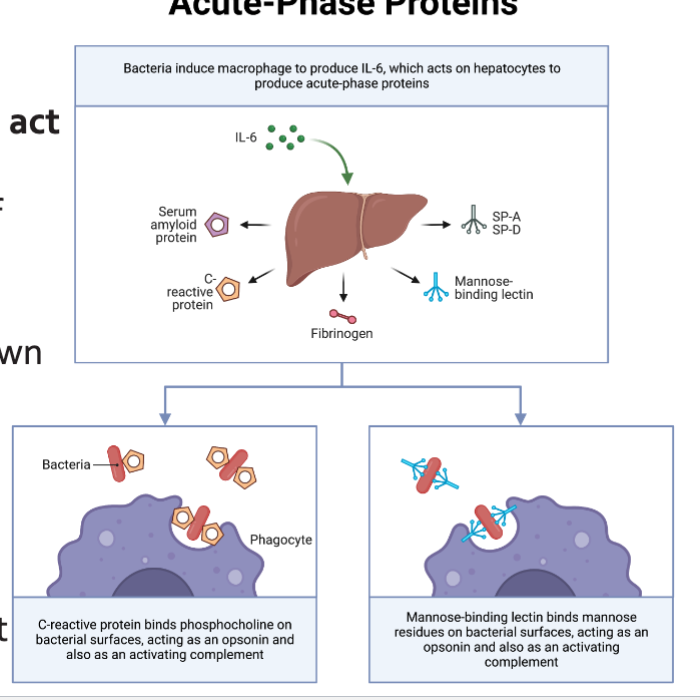
The Acute phase response
Cytokines (TND-a, IL1B, and IL-6) act on hepatocyte toL
Promotes a change in the profile of proteins that they synthesize
Blood levels of some proteins go down (Albumin), whereas levels of others increase markedly (SAA)
C-reactive protein: opsonization and complement activation
Serum Amyloid A: chemoattractant and inflammasome activation
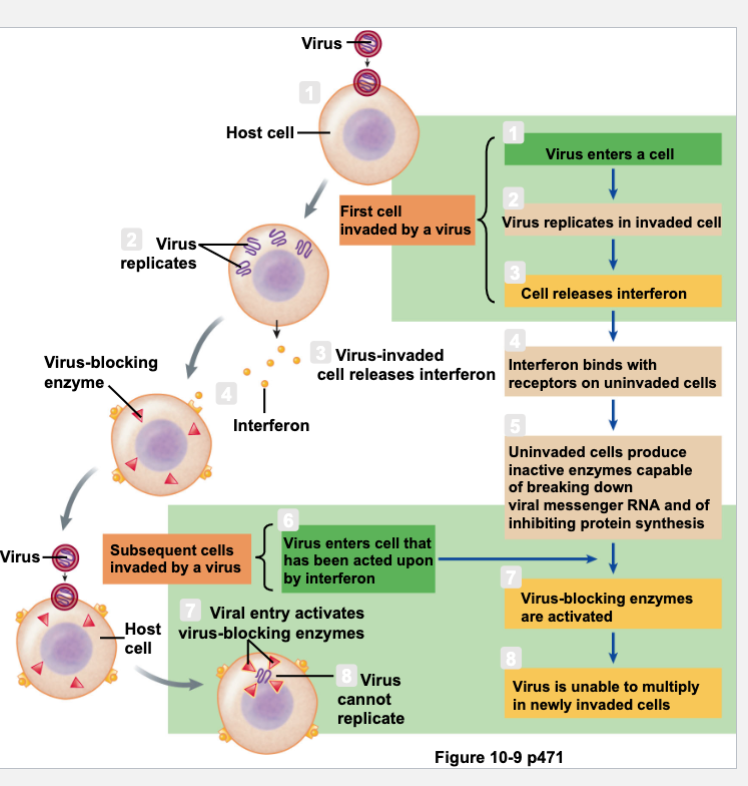
Role of Interferon in Viral Defense Mechanisms
Called interferon because it interferes with viral replication
Block spread of viral replication on neighboring cells
Signal neighboring cells to put up barriers
Signal infected cells to die
Recruitment of white blood cells to stimulate long lasting immunity
Released from virus-infected cells
Transiently interferes with viral replication
Enhance actions of NK cells
Slows cell division and suppresses tumor growth
SARS-COV-2 interferes with interferon signaling
SARS-CoV-2 inhibits multiple arms of the type I IFN response: Reduce IFN-b production during infection. Inhibits recognition of the foreign viral RNA by RIG-I Inhibits phosphorylation of repressor subunit of NF-kB Reducing import into the nucleus and reducing transcription of type I IFN
Effects of SARS-CoV-2 on respiration
Moderate damage: accumulating fluid, reduced gas exchange
Severe damage: build up of protein-rich fluid, vert limited on gas exchange
Cytokine Storm
Coronavirus infects lung cells
Immune cells, including macrophages, identify the virus and produce cytokines
Cytokines attract more immune cells, such as white blood cells, which in turn produce more cytokines, creating a cycle of inflammation that damages the lung cells
Damage can occur through formation of fibrin
Weakened blood vessels allow fluid to seep in and fill the lung cavities leading to respiratory failure
Adaptive Immune System-T Cell Development and Function: 03.19.24
Stages of Adaptive Immune Response 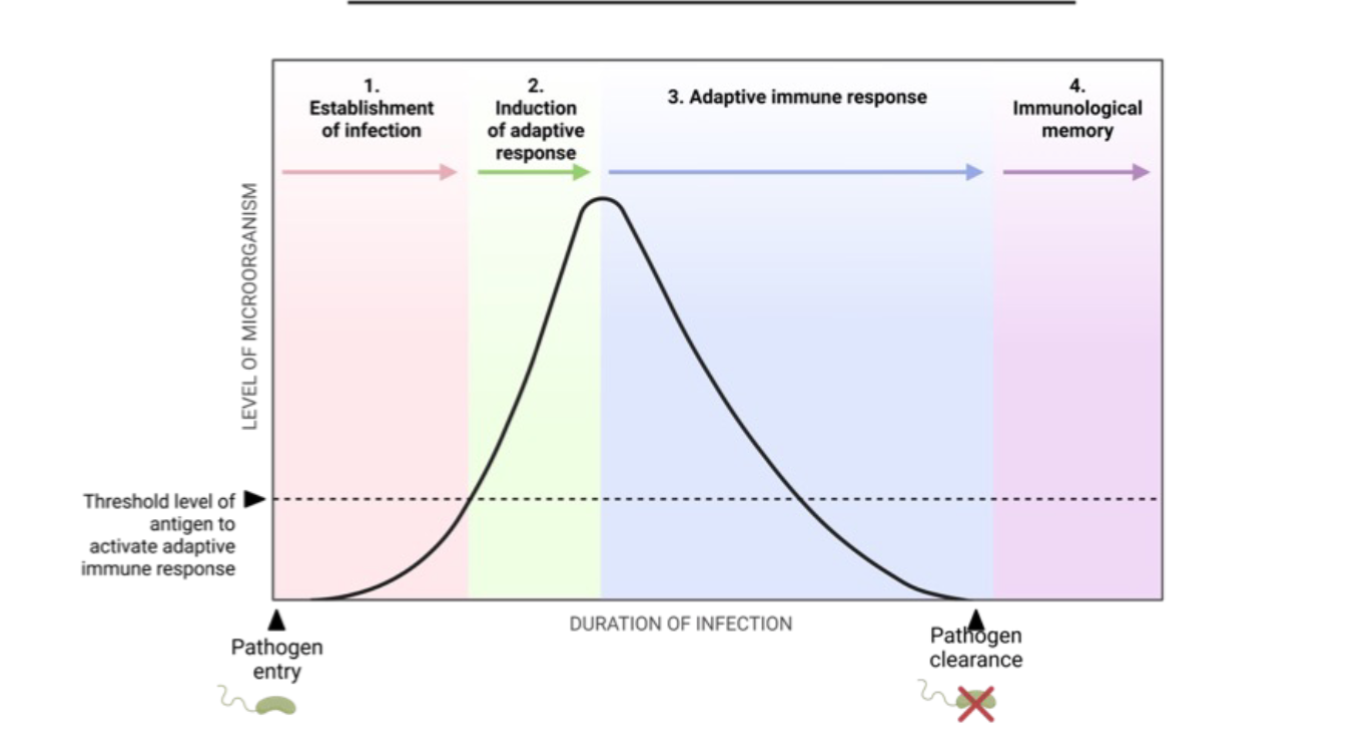
Components of the immune system: immune cells
Innate Immune System
Both have hematopoietic cell in bone marrow
Adaptive Immune System
Both have hematopoietic cell in bone marrow
Components of the immune system: Immune Cells
T cells recognize specific antigen fragments presented by other cells
Orchestrate immune responses to eliminate pathogens, infected cells, and abnormal cells
The vast diversity of function between different T cell subsets ensures that responses are tailored to the specific need
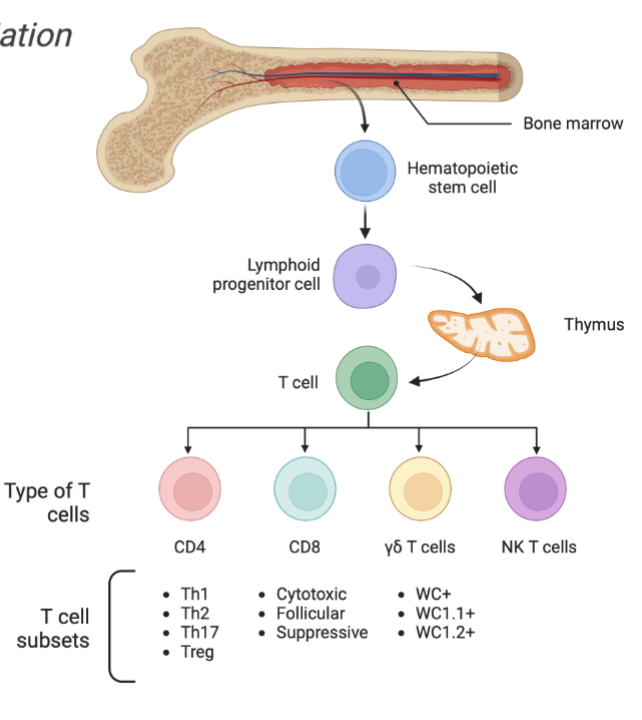
T Cell Development in Thymus
Selection process
Positive selection
Negative selection
Enter circulation as
CD4+: T Helper Cell
CD8+: Cytotoxic T cell
Treg: T regulatory cell
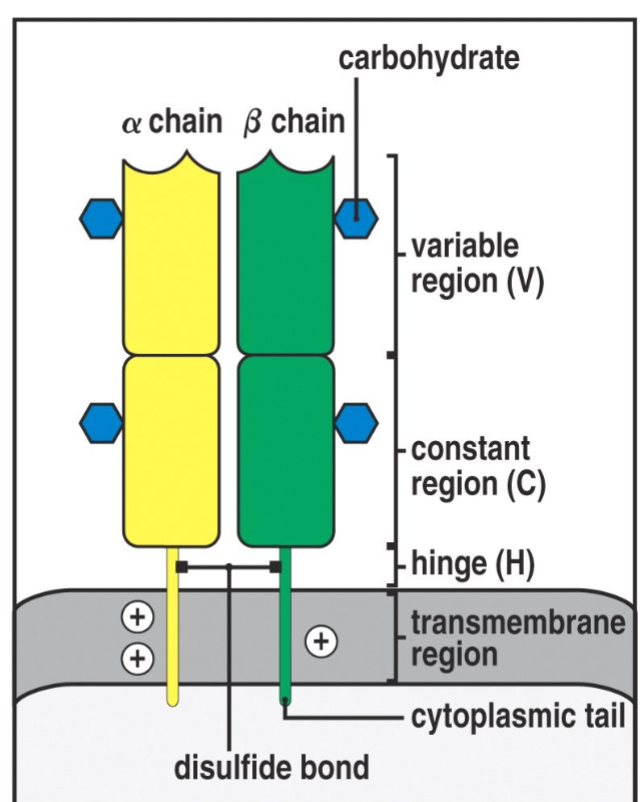
T Cell Receptor
Recognize specific antigen via the T CELL RECEPTOR (TCR)
TCR is a multi-subunit surface protein
Constant region
Variable region
How million of different antigen receptors can be generated from a limited amount of coding DNA in the genome
Gene Recombination
Diversity of TCR is accomplish by unique random genetic rearrangement of germline gene segments
Functional receptors are created during T cell development in the thymus by randomly choosing V, D and J gene segment
When progenitor T cell arrives in the thymus thymic factors (thymosin, thymopoietin) activate RAG genes
RAG genes encodes for recombinase enzyme that induces recombination of germline encoded DNA
Terminal deoxynucleotidyl transferase (TdT) binds V-D-J sections by randomly adding new bases, Junctional diversification
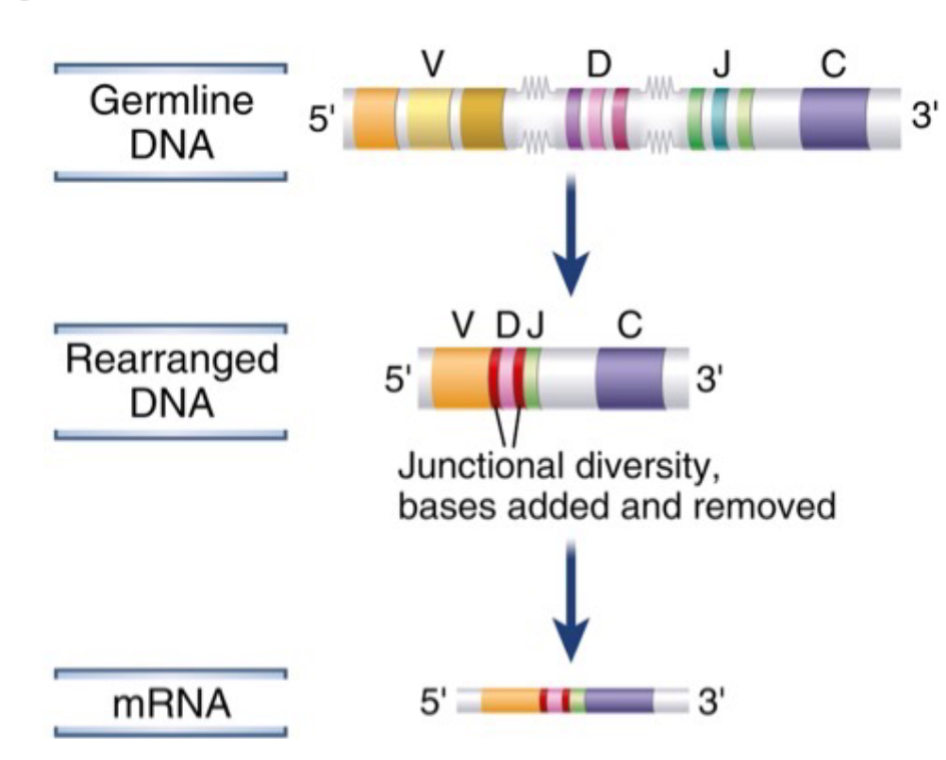
This gene rearrangement and junctional diversification occurs on both the alpha and beta chains of TCR
This creates a virtually unlimited TCR repertoire allowing to recognize millions of different antigens from aMHC- limited coding region of the DNA
However, almost ⅔ of the time junctional diversification will result in a non-functional TCR
T Cells recognize antigens in the context of MHC molecules
MHC MOLECULES (Major Histocompatibility complex) present antigens to T Cells
2 classes of MHC
MHC-I expressed in all nucleated cells present antigens to CD8+ T cells
MHC-II expressed by antigen presenting cells (APCs), present antigens to CD4+ T cells
Discovered to be responsible for tissue graft rejection in transplantation studies
T cells only recognize “own” MHC molecules
MHC complex is an extended genetic locus that contains several polygenic genes that are involved in antigen presentation to T cells
In humans MHC complex is called HLA (Human Leukocyte Antigen) and is located in chromosome 6
In Cattle MHC complex is called BoLA (Bovine Leukocyte Antigen), and is located in chromosome 23
MHC Molecules that most polymorphic gene in the mammalian genome
polygenicL the NHC region contains multiple genetic loci encoding proteins with the same function:
In humans (chromosome 6)
HLA-A (14,000 alleles), B, or C molecules can all present peptides to CD8+ cell
HLA-DPm DQ or DR molecules can present to CD4+ T cells
Polymorphic: each gene in the MHC locus has several alleles within the population
Co-dominant expression: Alleles from each haplotype are expressed in any one individual
“Polygeny”, “Polymorphism” and “Co-Dominant expression” ensure a diversity of MHC molecules providing protection of a populations from virtually unlimited diversity of microbes and therefore prevents loss of entire population from emerging infections
MHC Class I Molecules
Consist of an alpha chain associated noncovalently with a b2-microglobulin chain
Expressed in all nucleated cells (not expressed by red blood cells)
Main function is to present peptides to CD8+ T-lymphocytes, which kill virus-infection cells
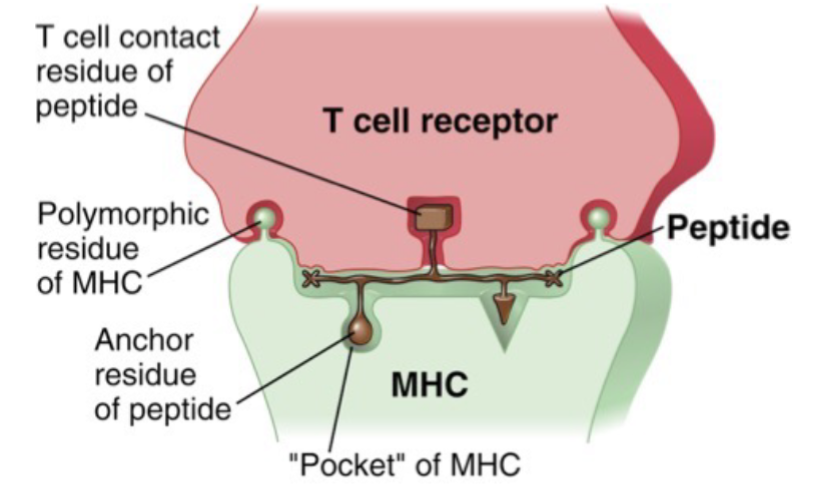
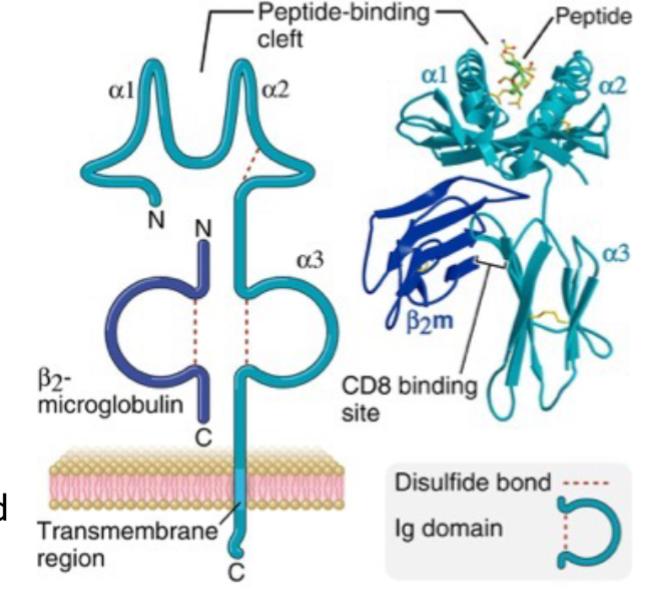
Viral peptides produced within the cell are digested by proteasome
Antigen peptide fragments are transported to endoplasmic reticulum with the help of TAP (transporter protein) and loaded into MHC-1
When MHC-I binds to an antigen it detaches from TAP and migrates from ER to cell surface
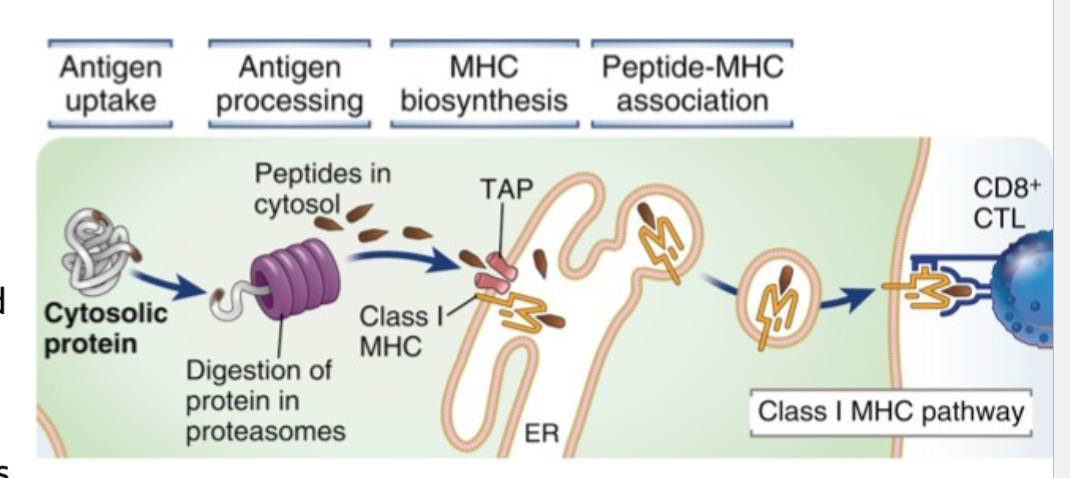
MHC Class II Molecule
Expressed only on “professional” antigen presenting cells such as DCs and Macrophages
Noncovalent association of polymorphic alpha and polymorphic beta chain
Main function is to present antigens derived from extracellular pathogens to CD4+ T cells, which once stimulated activate macrophages and B cells to generate inflammatory and antibody responses respectively
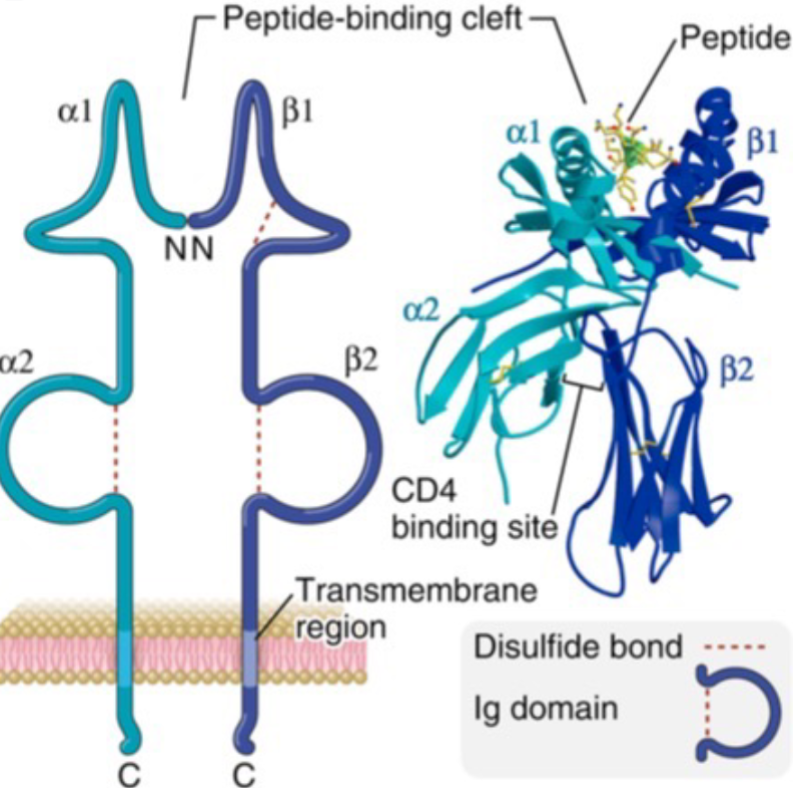
Antigen is taken up via endocytosis
Endosome becomes increasingly acidic which activates digestive protein called endosomes/lysosomes denaturing the protein fragment into small peptides
MHC0II molecules resides in specialized compartment hold in placed by an invariant chain
Once the endosome containing peptide fragments duse with the MHC-II endosome invariant chain is cleaved leaving a small fragment called CLIP
CLIP is replaced by antigen peptide and MHC-II is loaded to cell surface
T-Cell Development in Thymus
A gene present in the thymus allows thymic epithelial cells to express many self antigens to developing T Cells
If a double positive developing T cell engages first with MHC-II to commits to CD4+
If they bind with high affinity to MHC they are eliminated
Central Tolerance and Peripheral Tolerance
A subset of self-reactive T cells are allowed to survive and develop into regulatory T cells that promote and maintain tolerance towards self antigens (Central tolerance)
In the periphery certain signals from antigen presenting cells can induce mauve T cells to differentiate into regulatory cells (Peripheral tolerance)
T Cell Function: CD8 T cells
Once a T cell recognize an antigen, naive T cells differentiate into several functional classes of effort T cells that are specialized for different activities
CD* T cells recognize pathogen peptides presented by MHC class I molecules
Naive CD8 T cells differentiate into cytotoxic effector T cells that recognize and kill infected cells
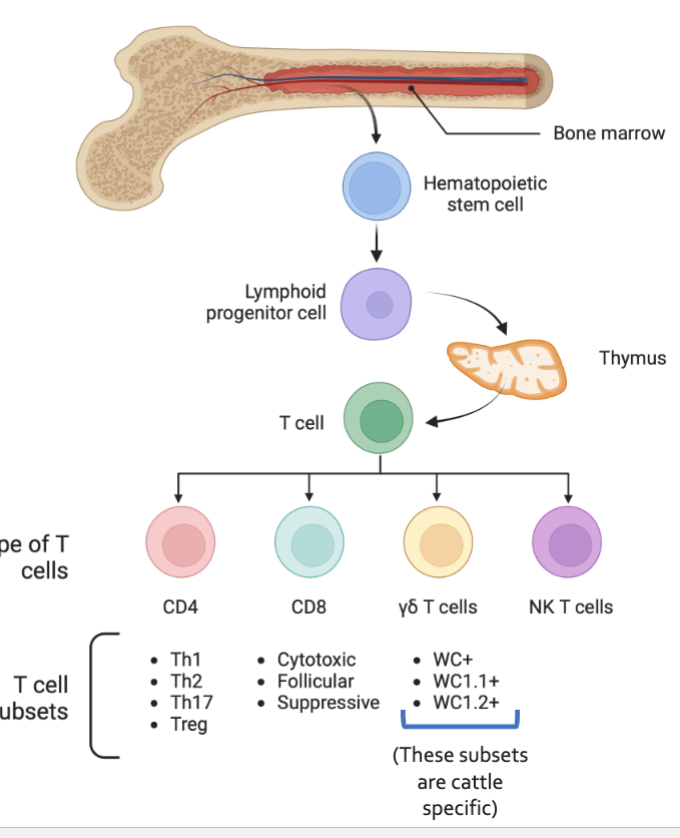
T Cell Function: CD4 T Cells
CD4 T cells have more flexible repertoire of effector activities
Once they recognize pathogen peptides presented by MHC class II molecules
Naive CD4 T cells can differentiate into many different subsets with different immunological functions
Main CD4 effector subsetsL TH1, TH2, TH17, and TFH, which activate their target cells
Regulatory T cells or Treg cells inhibit the extent of immune activation
yS T Cells, a major lymphocyte subset in calves
Unconventional T cells
Major subset in calves (40-60% lymphocytes)
Directly bind antigen
Not MHC restricted
Effector yS T cells, WC 1.1+
Early protection against intracellular infections while alpha and beta T cells effector function is blunted
Regulatory yS T cells, WC 1.2+
Immune tolerance (IL-10, TGF-Beta)
T Cells activation by APCs
T cell activation occurs in 3 steps
APCs present antigens to T Cells in context of MHC molecules
Level of expression of B7 on APC dictated by inflammatory signals influence T cell polarization
Secreted co-stimulatory signals from APCs determines CD4 T cell fate
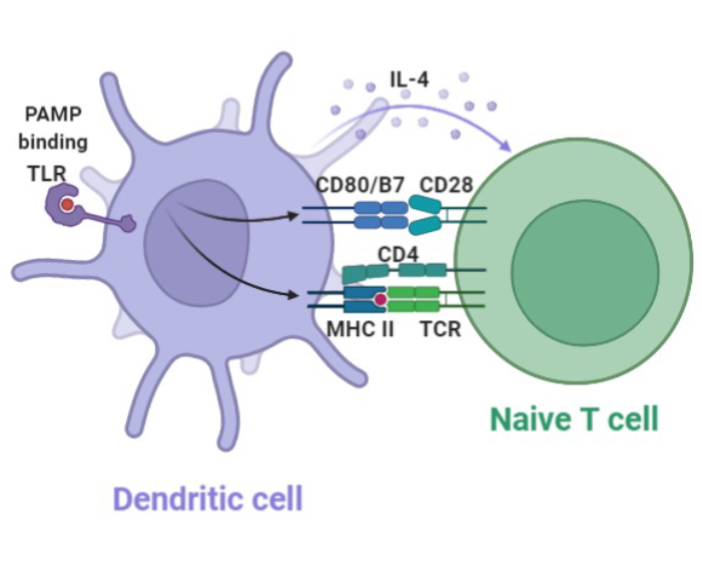
Development of Peripheral tolerance
With no inflammatory signals APCs don't express B7
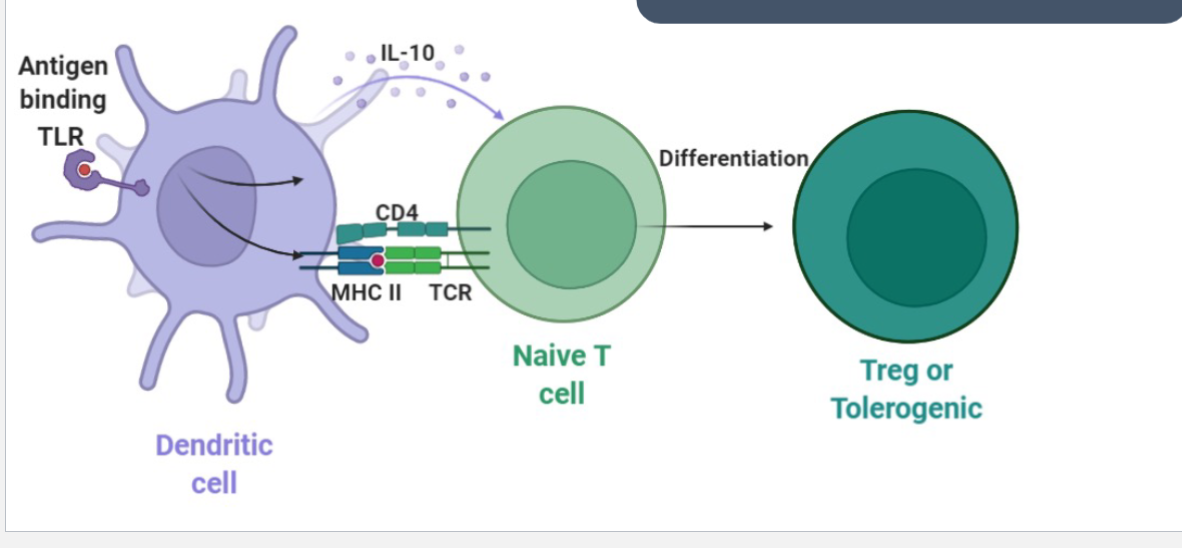
Development of effector function
When inflammatory signals prime APC activation B7 expression increases\
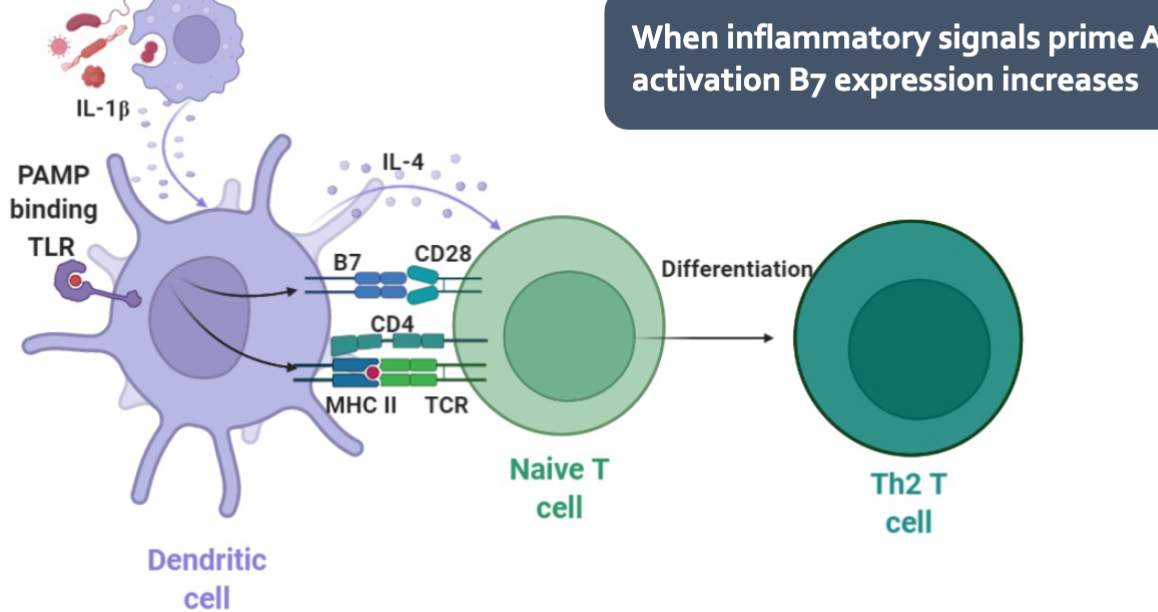
T Cell Activation
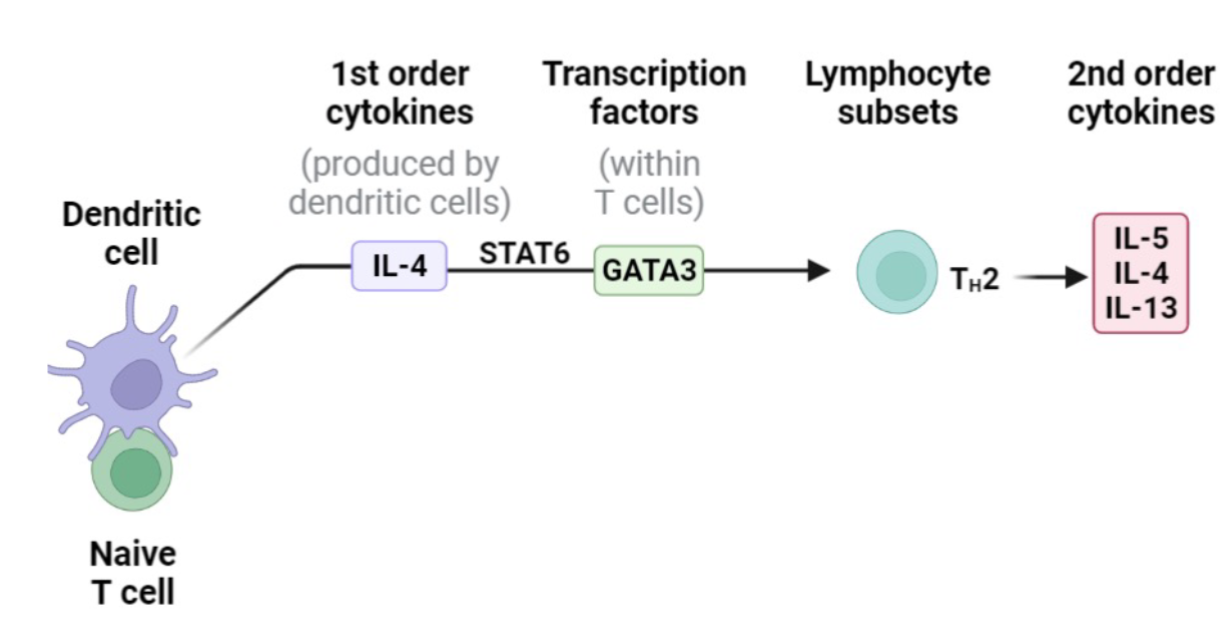
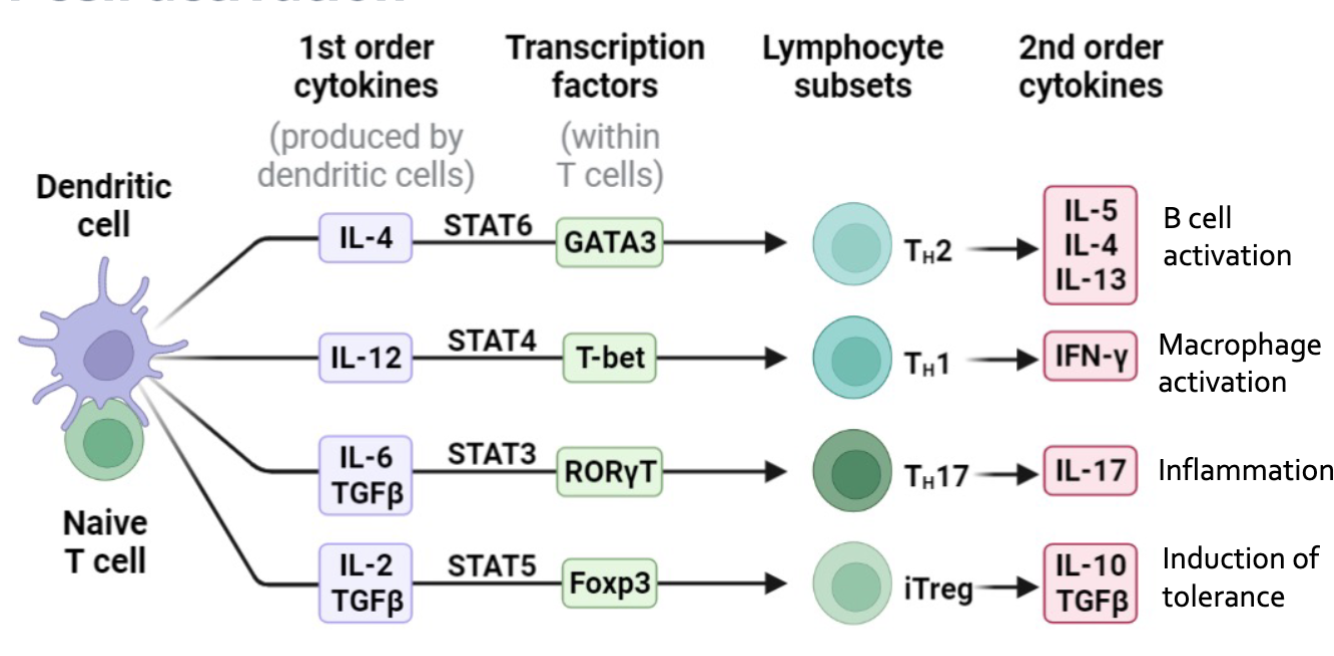
T Cell activation
Antigens are transported by dendritic cells to lymph nodes
Naive T lymphocytes recirculate through these lymph nodes
If a T cells has a receptor that recognizes the specific antigen, it becomes activated
Once activated it will rapidly expand (Clonal Expansion) and differentiate
Activated T cell can remain in lymph node (T Follicular Cell) to help with B activation
Or migrate to sites of infection
C4-B cell function and Development - 03.21.
Stages of Adaptive Immune Responses
Establishment of infection
Induction of adaptive response
Adaptive immune response
Immunological memory
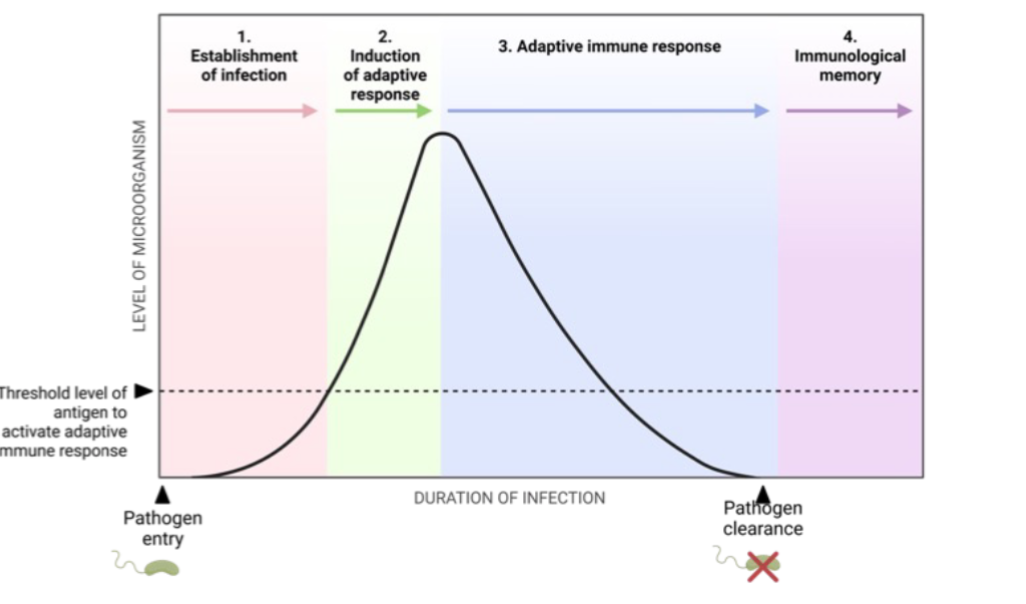
B cell function and development
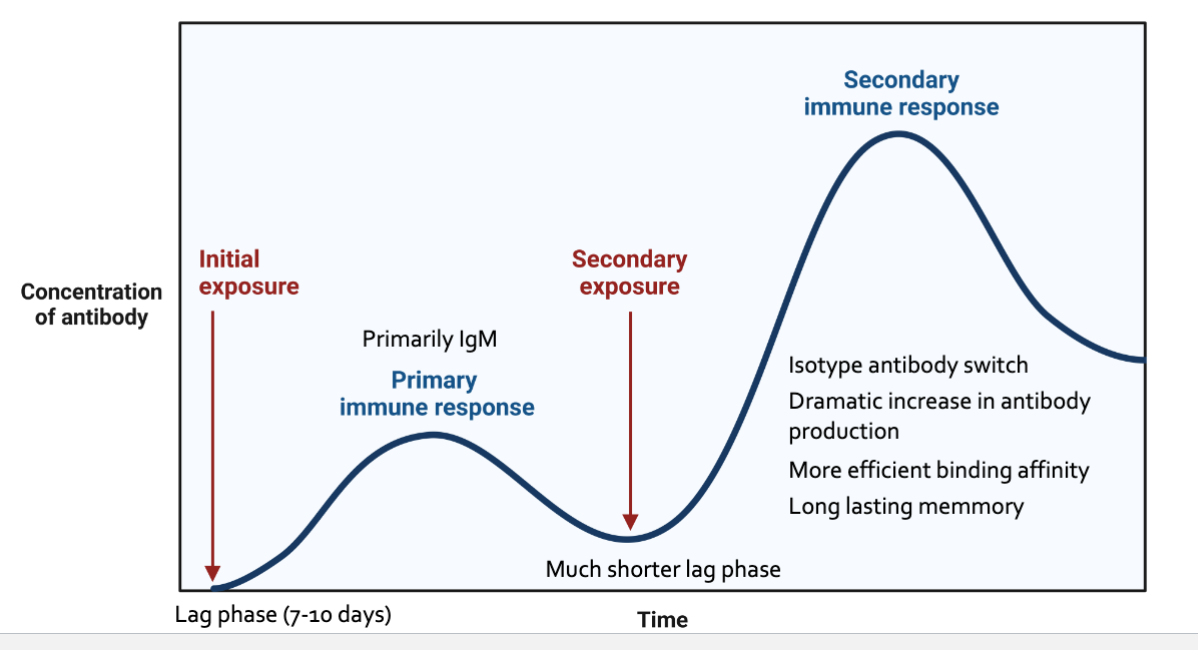
Initial exposure
Primary immune response
Primarily lgM
Secondary exposure
Much shorter lag phase
Secondary immune response
Isotype antibody switch
Dramatic increase in antibody production
More efficient binding affinity
Long lasting memory
B Cell Antibodies
B cells can directly recognize specific antigens via B cell receptors/antibodies and does not need antigen presentation by MHC
BCR: membrane bound
Antibody: secreted
BCR/Antibodies
Constant region
Variable region (antigen specificity)
Antigens
Carbohydrates
Lipids
Protein / peptide
B Cell Development
Fetal development of B cells
Fetal liver (FL)
FL HSC
Pro-B
Pre-B
Immature B
All into B-1B cell
Postnatal Development of B cells
Bone Marrow (BM)
BM HSC
Pro-b
Pre-b
Immature B
Into spleen
Transitional B-2B cell intoL
Follicular B-2B Cell
Marginal zone B cell
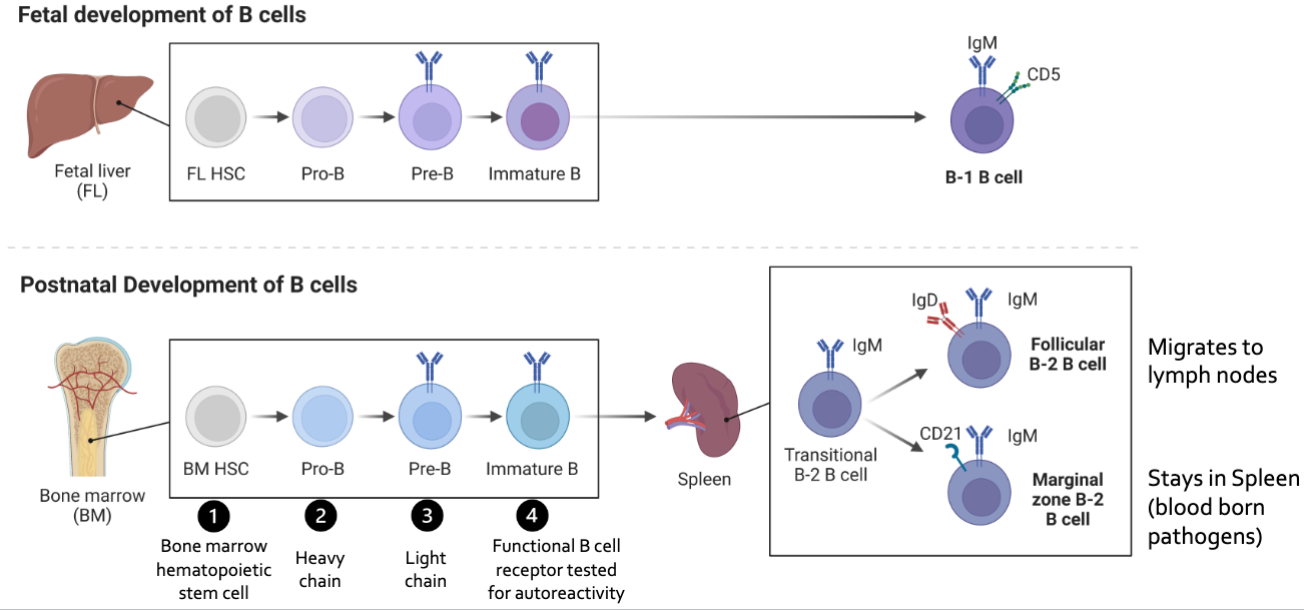
B Cell Development
B Cells receptor undergoes a similar genetic rearrangement as the T cell receptor
For a B cell to become activated more than 1 BCR must recognize the antigen and become cross link
This requires than antigens that have repetitive epitopes so that more than one BCR can bind at the same time
Most self protein lack repetitive epitopes providing a mechanisms of self tolerance
B Cell Activation
Once a fully functional B cell develops in spleen it migrates to lymph nodes (Follicular B cell) or stays in Spleen (marginal B Cell)
In germinal center of lymph node if a B cell recognizes an antigen through if a B cell recognizes an antigen through its BCR it quill rapidly start to divide (Clonal Expansion)
After this step it requires stimulatory signals from T follicular cells to survive
High rate of cell division induces mutations in BCR (Somatic hypermutation)
Some mutations will lead to better binding affinity to antigen (Affinity maturation)
Every time B cells divides in germinal centers i needs to present antigens to Tfh cells to keep dividing
The only sources of antigen comes from “reservoirs” from Follicular DCs
If the hypermutated BCR has low affinity it cannot take antigen from Dcs and it is programmed for cell death
If new BCR has high affinity it can then present antigen to Tfh cells
B Cell Activation Summary
APCs are continuously sampling luminal antigens
Leads to antigen-specific immune responses that promote the production of dimeric lgA
slgA aids in antigen sampling from the intestinal lumen
1. TLR ligand recognition promotes naive T cell differentiation
2. Tfh cells promote B cell differentiation into lgA+ B cells inside the Peyer's patch
3. lgA+ B cells become plasma cells and secrete lgA to regulate gut microbiota
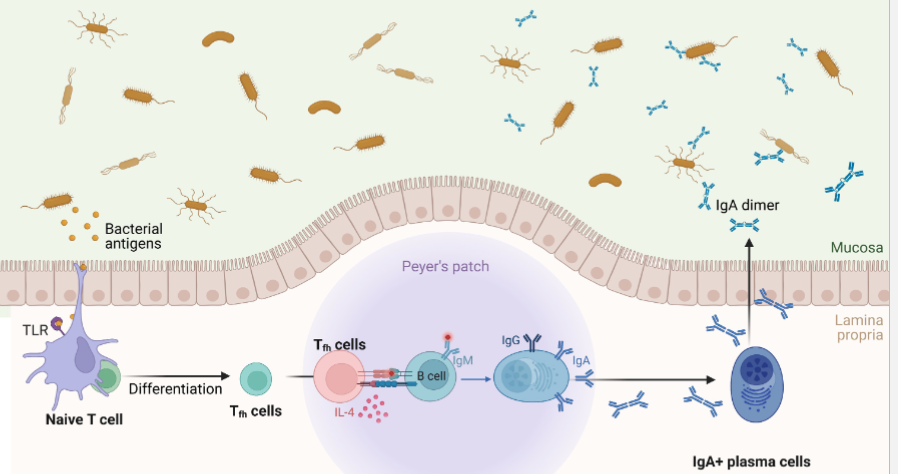
Antibody Classes; lgG
Principle Ab in serum
14-18 mg/mL
lgG1: 11 mg/mL
lgG2: 7 mg/mL
Fixes complement
Late response to antigen
lgG1
Selective transfer (colostrum)
fetal/neonatal defense
Toxin inactivation
Principal milk /colostrum lg
Fc portion involved in endotoxin neutralization and complement fixation
lgG2
Primary opsonin for phagocytosis
Fc portion: Opsonization and neutrophil phagocytosis
In cattle lead from blood to infected mammary gland and act as key opsonin to help neutrophils target and clear mastitis causing bacteria
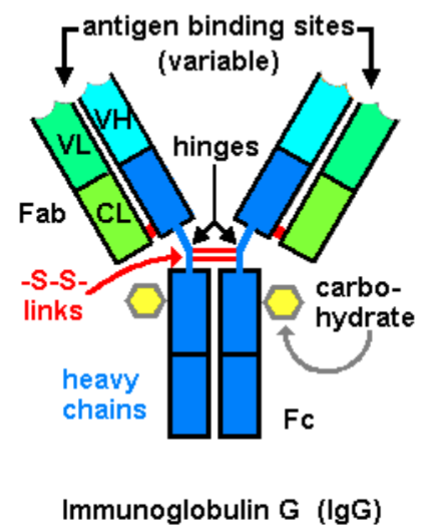
Antibody Classes: lgM
Largest lg
Serum (1-3 mg/mL)
Fixes complement
1st lg produced to antigen challenge
Fc portion is involved in blocking and complement fixation
Antibody classes: lgM
Bind to masts cells
Binds to basophils
Activation
Release of
Histamine
Involved in allergy progression
Antibody Classes: lgA
Main antibody at mucosal sites
When secreted into intestinal lumen (secretory component)
Activates complement system in serum, but not in milk
Local immunity an secretions
Prevents bacterial adherence
Maternal milk: very important
Primary Ig in colostrum of humans
Antibody Effort Functions
Class switching promoted by Tfh cells allows to tailor antibody responses to the specific need
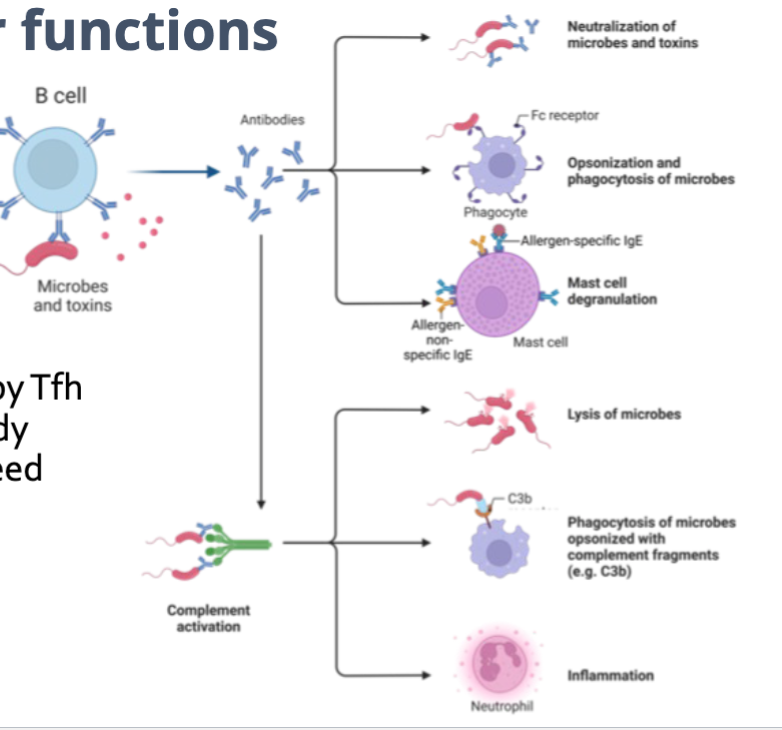
Allergy Induction
Early life dysbiosis can result in aberrant immune development
Local intestinal conditions drive immunoglobulin class switch to lgE rather than lgA
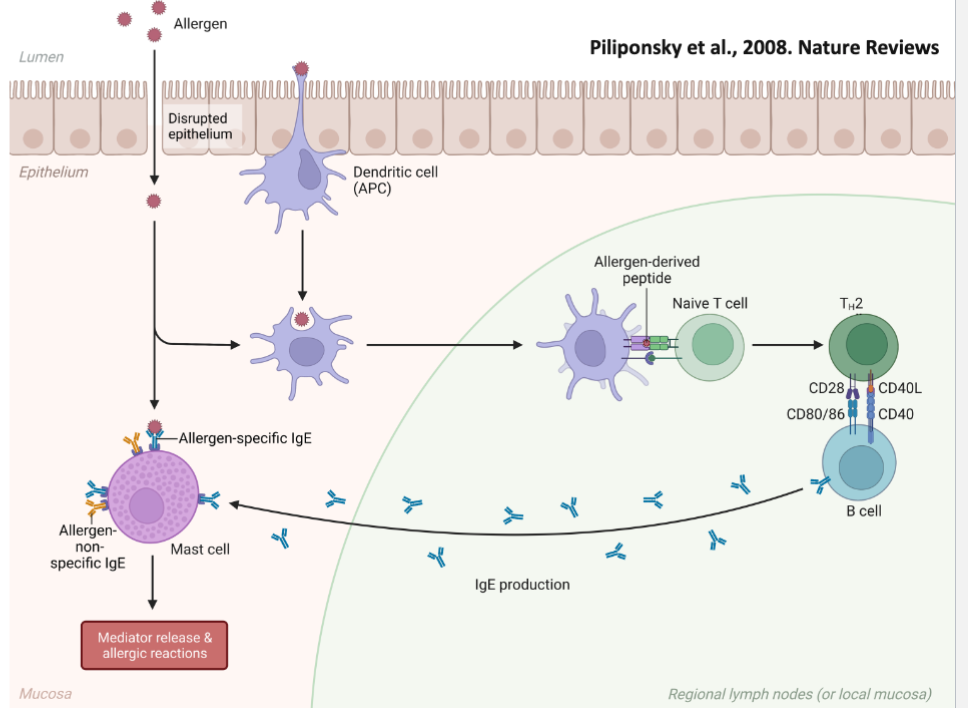
Allergens enter via inhalation and bind to lgE on masts cells
Mast cells are activated, leading to increased mucus production, nasal irritation, and contraction of bronchial smooth muscle.
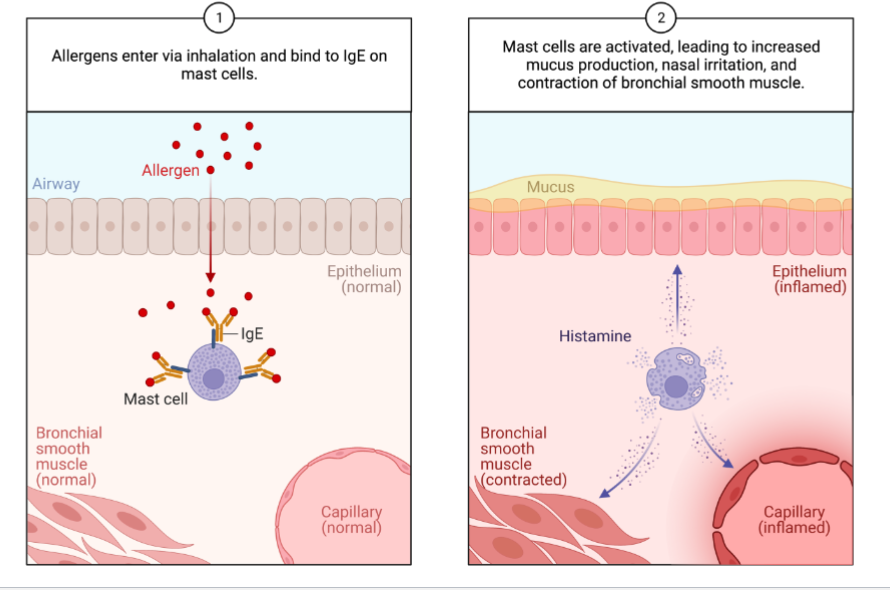
Immune System - Antibodies April 2nd
Antibodies
Cell Mediated and Humoral Immunity
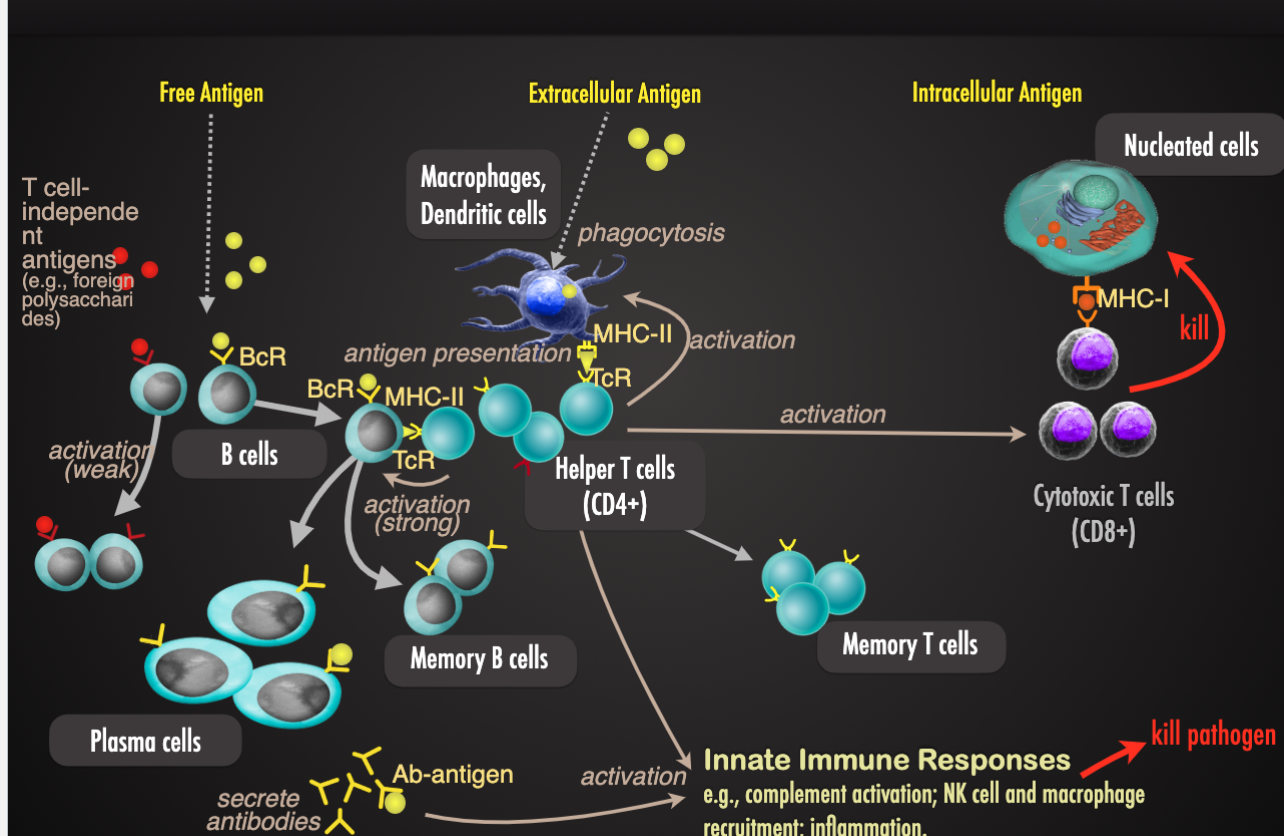
Function of Antibodies
Opsonization
Bacterium
phagocyte
Complement system activation
Complement system protein
Bacterium
Opsonization by C3b
Inflammatory response
Lysis of foreign cells
Immobilization and Prevention of Adherence
Bacterium
Flagellum
Cross-linking
Bacterium
ex. aggregation
Antibody-dependent cellular cytotoxicity (ADCC)
Natural killer cell
Kills cell
Infected “sell” cell
Neutralization
Virus
Toxin
Antibodies (Ab)
Antibodies are also called immunoglobulins (lg)
Y-shaped protein
Two copies of heavy chain and light chain
Two identical variable regions that bind antigen: specificity
Stem is constant region: immune system recognition
Antibody Classes
Five major classes: lgM, lgG, lgA, lgE, lgD
Have some basic monomeric (single unit) structure
Monomer: single Y shaped molecule
Each class has different constant region of heavy chain
Some classes from multimers (binding of multiple units).
Each class has distinct functions and properties
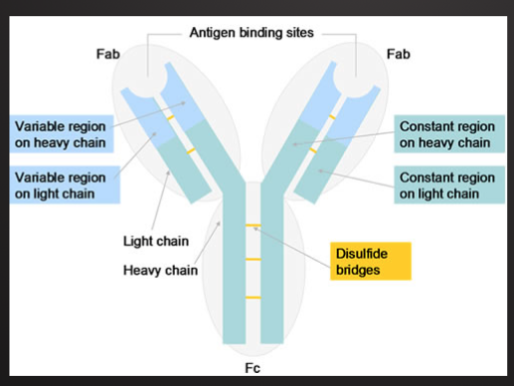
Immunoglobulin G (lg G)
Monomer (2 binding sites)
Most common Ab (80% of total Ab in serum): half-life: 23 days (longests protection)
Location: blood, lymph, intestine
Can cross placenta from mother to child (in humans, and some animals, NOT in cattle, sheep, pigs and horses)
Can activate complement (classical pathway)
Fc region binds phagocytes
Function:
Neutralization, agglutination, complement activation, opsonization and antibody-dependent cell-mediated cytotoxicity.
First and most abundant during secondary response
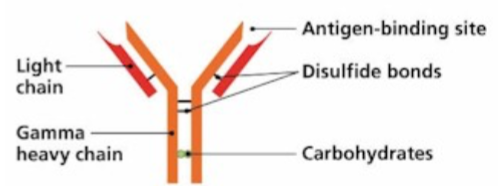
Immunoglobulin M (lg M)
Pentamer (10 binding sites)
5-10% of total antibody in serumL half-life: 5 days
Location: blood lymph, B-cell surface (monomer)
Cannot cross placenta
Can activate complement ( classical pathway)
Function
First antibodies produced in response to first exposure to antigen
Neutralization, agglutination, and complement activation
Large size percents crossing from bloodstream to tissues. Primary role therefore in bloodstream infections
Monomeric form serves as B-cell receptor
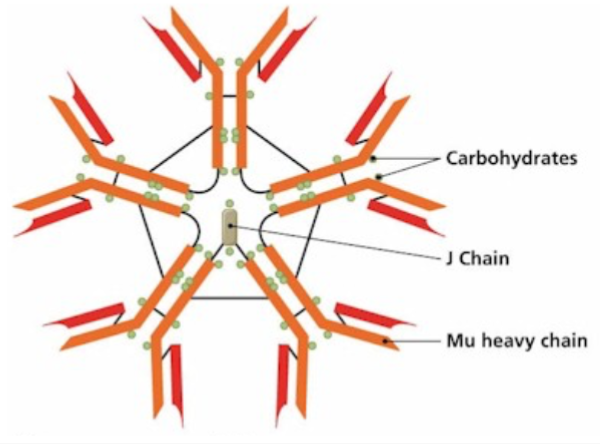
Immunoglobulin A (lg A)
Dimer with secretory component (4 binding sites)
10-15% of total antibody in serum; half-life: 6 days
Location: secretion (ex. Tears, saliva, mucus, intestine, respiratory tract, milk): monomer: blood, lymph
Cannot cross placenta
Cannot activate complement
Function
Protection of mucosal surfaces: neutralization and trapping of pathogens in mucus, prevents attachment of pathogens to epithelial cells.
Protects breast-fed infants against intestinal pathogens. Not absorbed by a gut after gut closes, protects the gut barrier from within the lumen
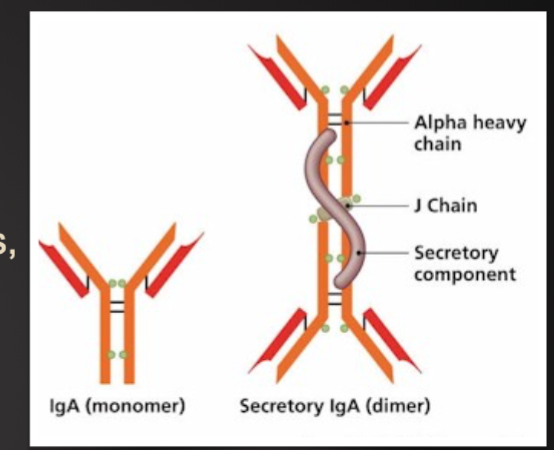
Immunoglobulin D (lg D)
Monomer (2 binding sites)
0.2% of total antibody in serum: half-life: 3 days
Location: blood, lymph and B-cell surface
Cannot cross placenta
Cannot activate complement
Function
Serves as B-cell receptor: initiation of acquired immune response
Function in serum is largely unknown
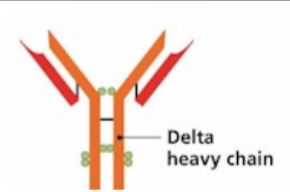
Immunoglobulin E (lg E)
Monomer (2 binding sites)
0.002% of total antibody in serum: half life: 2 days
Location: bound to mast and basophils through body and blood (Fc regions binds mast cells and basophils)
Cannot cross placenta
Cannot activate complement
Function:
Activation of basophils and mast cells against parasites and allergens
When bound to antigens, triggers release of histamine (and other compounds) from mast cells and basophils that contribute to inflammation and some allergic responses
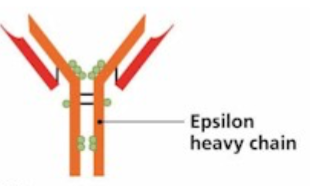
Class Switching
Plasma cells normally secret lgM when first activate
T helper cells can induce some activated B cells to become plasma cells that secrete other antibody classes
B cells in lymph nodes usually switch to lgG
B cells in mucosal tissues often switch to lgA
Primary vs. Secondary Response
First response to antigen is the primary response.
Adaptive immune system “remembers” specific antigen
If encountered again, a stronger secondary response results
Secondary response to antigen is strong and faster because the body can remember the first encounter and fight back
Combination of rapid proliferation of memory cells and on-going affinity maturation on subsequent exposures
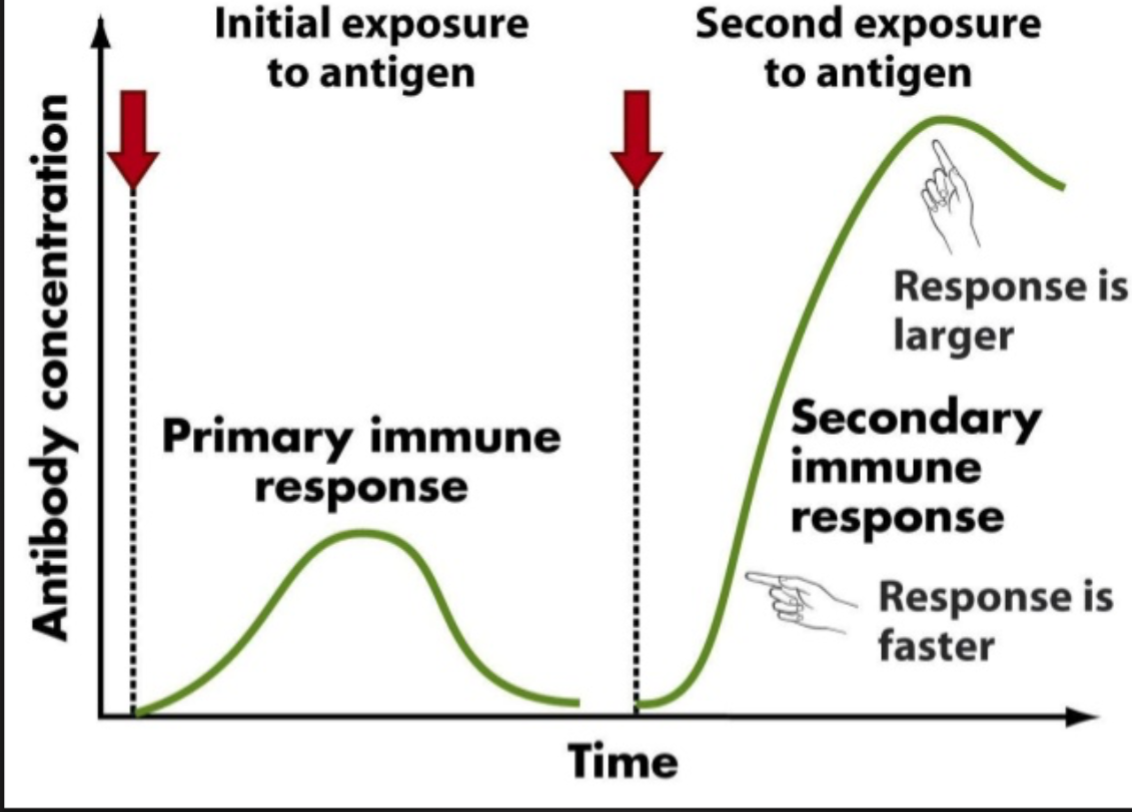
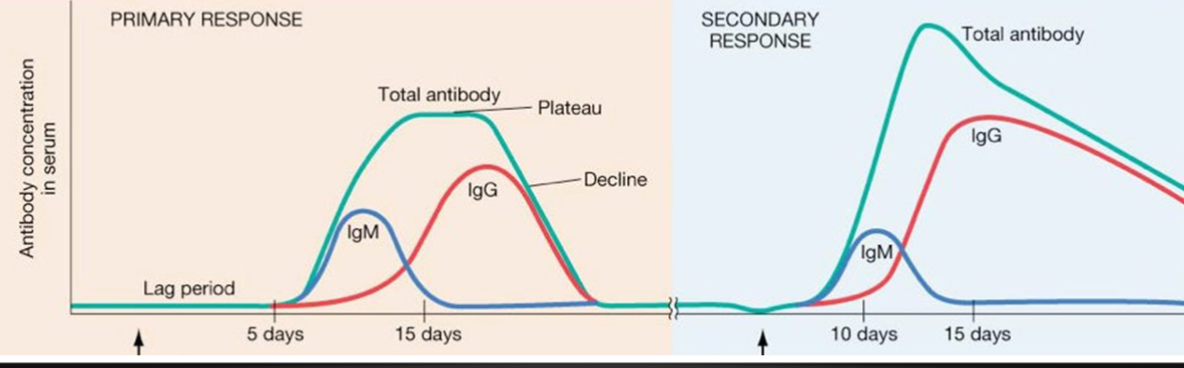
Maternal Transfer of Immunity and Vaccination 04/04
Modes of Adaptation Immunity
Maternal transfer of antibodies to infant via placenta or breast milk
Administration of pre-formed substances to provide immediate but short-term protection (immunoglobulin, antitoxin)
Passive Immunity
Give rapid protection within 48 hours
Protection is temporary and wanes with time (usually few months)
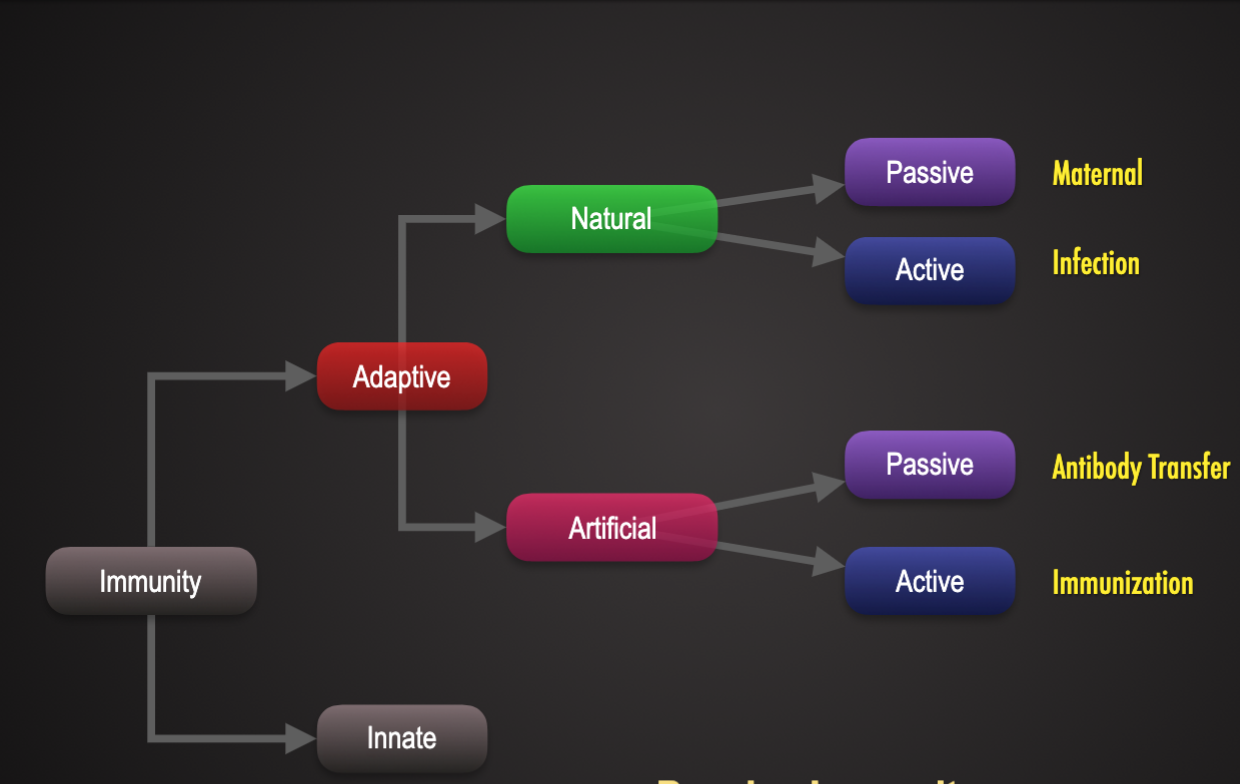
Natural Passive Immunity: Maternal Transfer
Maternal transfer of antibodies to offspring via:
Placenta (lgG)
Colostrum (primarily via lgG, short (24h) window of opportunity)
Breast milk (primarily secretory lgA, mostly protects against enteric pathogens)
No memory: protection is lost once antibodies degrade.
No evidence that some maternal lymphocytes may be transferred via breast milk: these lymphocytes may take residence in neonates' peyer's patches (role in immunity is not well-understood.)
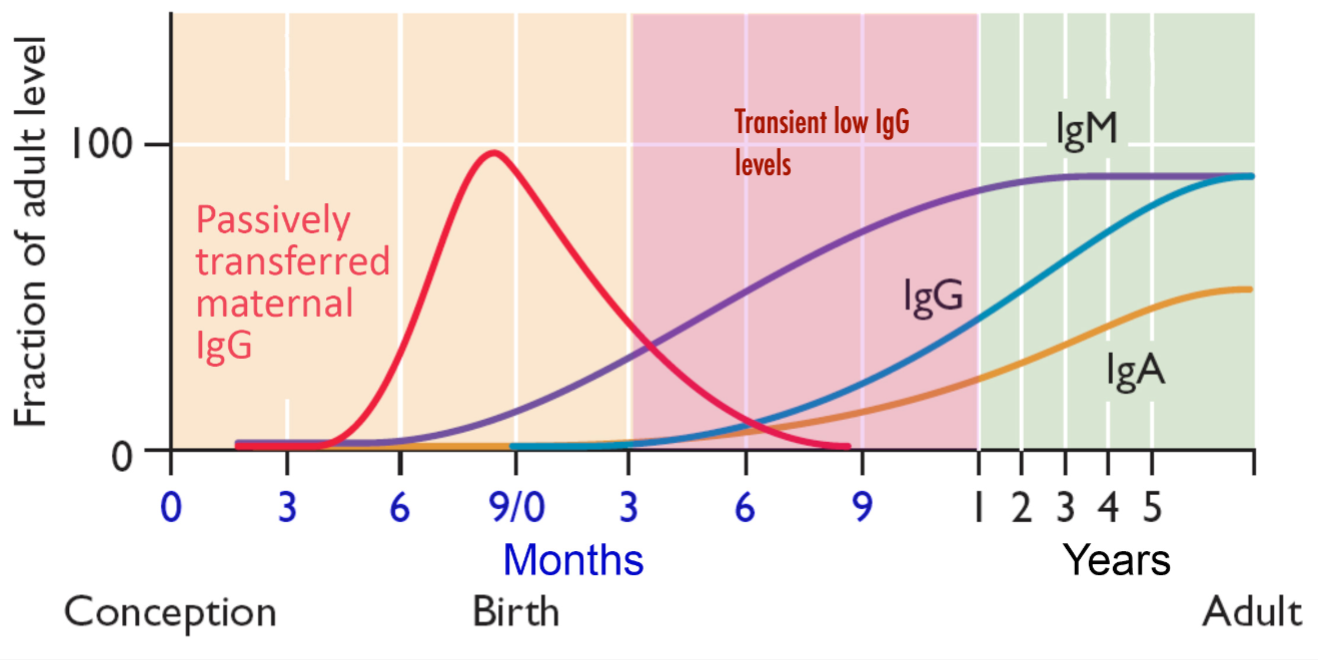
Not all animals are capable of trans-placental transfer of antibodies (lgG)
These animals are critically reliant on colostrum for passive immunity.
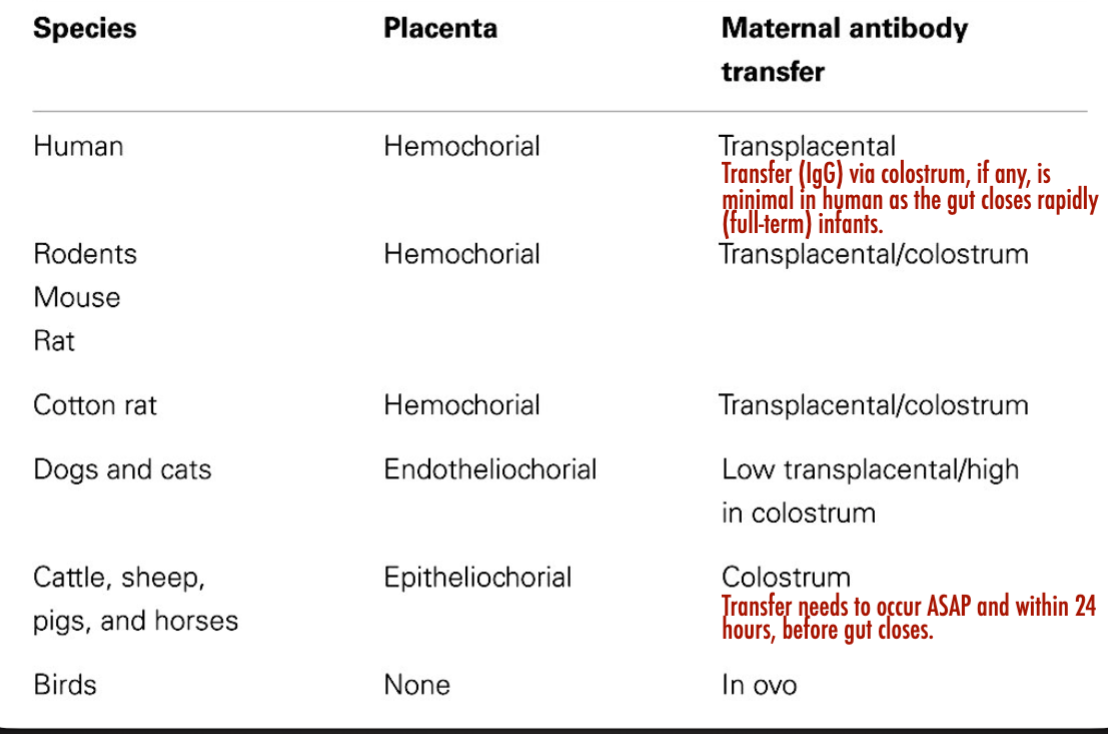
Artificial Passive Immunity: Immunoglobulin Therapy
Provided by administering immunoglobulins (lg) for post-exposure prophylaxis
Ex.
human normal immunoglobulin (HNlG). Collected from pooled human donations – contains antibodies to infectious agents common in the community
Hepatitis B immunoglobulin (HBIG)
Varicella Zoster immunoglobulin (VZIG)
Rabies immunoglobulin
Also used to treat some immunodeficiencies/ immune disorders
Lupus, multiple sclerosis, after bone marrow transplant)
Involves use of blood-derived products
Safety concerns, possible side effects such as serum sickness
Vaccination: Artificial Active Immunity
Variolation
Variolation or inoculation: first method used to immunize an individual against smallpox (Variola)
Edward jenner noticed milkmaids who recovered from cowpox rarely got smallpox
In 1796, Jenner exposed a boy (James Phipps) to material from cowpox lesion: then six weeks later exposed him to smallpox
Phipps did not catch smallpox. He was immune
Jenner and others worked to spread variolation: the use of less dangerous cowpox material to protect against smallpox.
Louis Pastuer honored Jenner by using the term vaccination (vacca is latin for cow) to describe artificial induction of a community against any infectious disease.
Vaccination
A vaccine is a preparation of pathogen or its products that elicits an immune response
Vaccines establish immune memory without the pathogenic events that are typical of first encounter with virulent pathogen
One of the most effective strategy for controlling infectious disease
Responsible for dramatic declines in childhood diseases
Diseases sometimes reappear and spread as a result of failure to vaccinate children
Primary and Secondary Antibody response
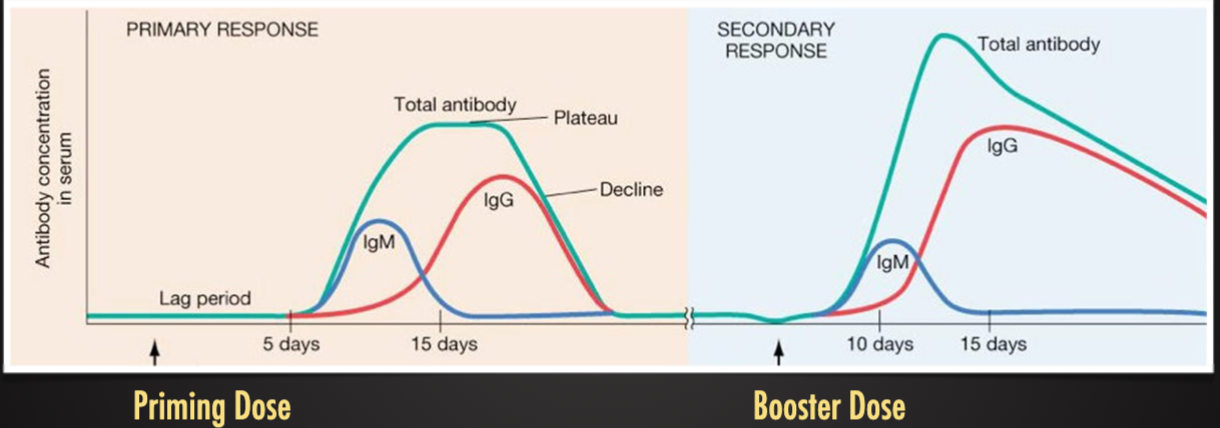
Vaccination: Boosting
A booster dose is an additional vaccine dose administered after the priming dose
For some vaccines, only receive one or a few more boosters
For others, receive boosters every X years or when the antibodies titer in blood is below acceptable level
Given to increase circulating antibodies and capacity for strong immune response
Often necessary because priming dose insufficient to elicit protective immunity. Immune response to vaccine may be weak, particularly to certain types of vaccines
Goals of Vaccination: Individual
Decrease severity of clinical signs after exposure
Most vaccines do not entirely prevent infection
Elicit humoral.cellular immunity that limits pathogen replication/spread and lessens clinical signs
Prevent mortality by reducing severity of pathogenesis
Increase total number of pathogens (infectious dose) needed to cause illness in a vaccinated individual
Decrease shedding of pathogen
Vaccinated individuals may still shed virus when vaccinated, but do not become clinically ill
Reduce spread by reducing shedding (concentration or duration of shedding)
Goals of Vaccination: Population Level
Not all individuals can be vaccinated
Population sometimes to large: sometimes diffuse to reach all
Allergies adverse response to vaccination
Immunocompromised (young, old, or ill)
Herd immunity develops when critical portion of population is immune to disease – protects unvaccinated individuals
Infectiou agent unable to spread due to insufficient susceptible hosts
In a freely, randomly mixing population, this threshold is ~70% but can be lower or higher depending on population and pathogen traits
Herd Immunity:
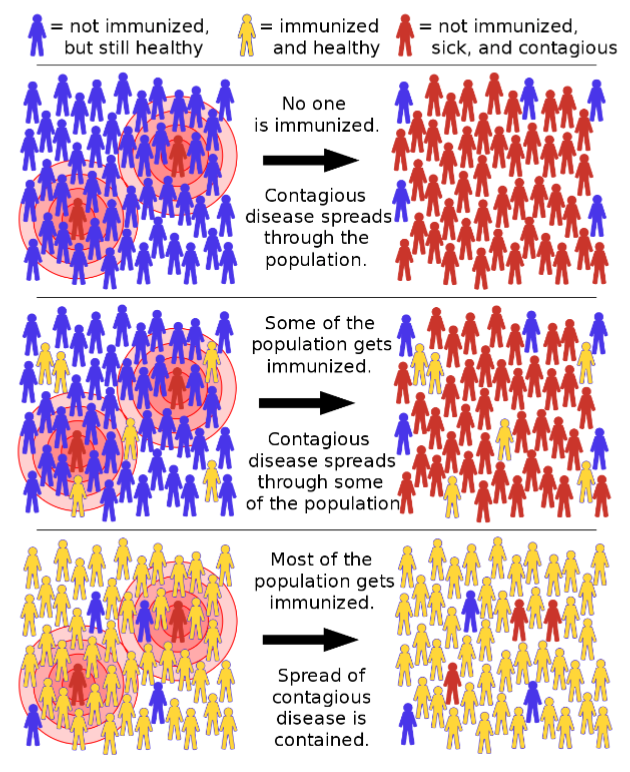
Requirements of a “perfect” vaccine
Must be safe: must not cause disease, minimal side effects
Give long lasting protection, preferably with a single dose
Protect against both illness and death
Prevent shedding (reduces spread)
Provide rapid protection after administration
Ideally low cost, stable, easy to administer (oral vs. needle)
Can differentiate infected from vaccinated (i.e DIVA: no impact on surveillance efforts)
Types of Vaccines
Attenuated whole-agent vaccines (Modified live (MLV) )
Inactivated whole-agent vaccines (“Killed”)
Subunit (proteins or carbohydrates)
Recombinant: antigen inserted into another virus vector
Conjugated: carbohydrate antigen attached to a protein to help immune system “see” the poorly antigenic component
Toxoid: inactivated toxin used as a vaccine
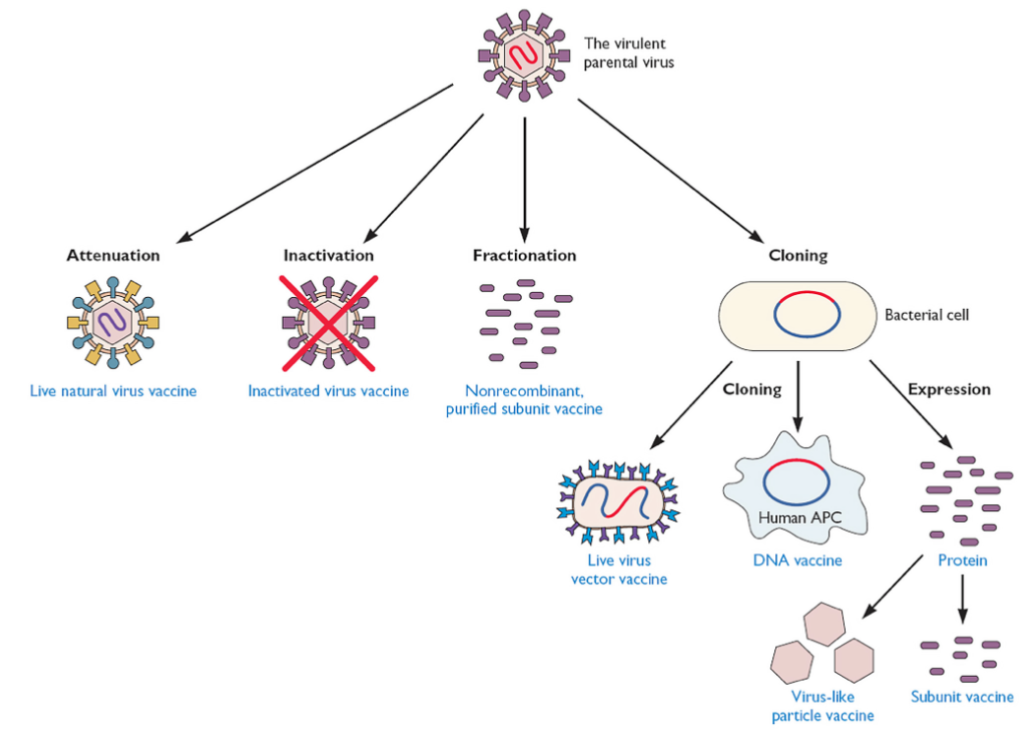
Attenuated Whole-Agent Vaccines
Attenuated: weakened form of pathogen
Grow under conditions resulting in mutations, or genetically manipulated to replace or delete pathogenicity genes
Replicates in recipientL disease undetectable or mild
Often stimulates both humoral and cellular immunity
Advantages: single dose usually induces long-lasting immunity
Can also inadvertently immunize others by shedding of live vaccine
Disadvantages: can sometimes cause illness in vaccine recipient
Can occasionally revert or mutate, become pathogenic
Cause cause overt symptoms in immunocompromised individuals
Generally not recommended for pregnant women
Usually require refrigeration to remain potent – live organism
Inactivated Whole-Agent Vaccines
Inactivated: unable to replicate “killed”
Treated with formalin or other chemical that does not significantly change surface epitopes
Primarily stimulate humoral immunity (antibodies)
Advantages: cannot cause infection or revert to pathogenic forms
Generally safer and fewer adverse events or side effects
Are sometimes more heat stable than inactivated
Disadvantages: no replication, so no amplification in vivo: less robust immune responses
Cannot spread vaccine by shedding (only vaccinate recipient)
Several booster doses usually needed for adequate immunity
Often contain adjuvant to enhance immune response
Subunit Vaccines
Break virus into components, immunize with purified components
Clone appropriate viral gene, express in bacteria, yeast, insect cells, cell culture, purify protein
Antigen usually a capsid or membrane protein
Advantages:
Proteins produced by recombinant DNA technology
Contain no viral genomes that can replicate or escape
No contamination with infectious virus or foreign proteins
Disadvantages:
Expensive
Poor antigenicity (low, level, short duration response).
Usually stimulate production of antibody, not cytotoxic T cells
Lack of good delivery system (injections are best, not well liked)
Adjuvants
Adjuvants are non-specific stimulators of the immune system that are added to improve vaccine response
Mechanism of action
Depot effects: localization of antigen to the site of inoculation. Slow release and clearance of antigen: prolong immune response (ex. Mineral oil)
Surface Effects: through presentation of antigen as particles: activate and hence activity of APCs (ex. Particulate adjuvant: aluminum hydroxide)
Inflammation effects: direct stimulation of the immune response (ex. Monophosphoryl lipid A – non-toxic component of LPS)
Essential for efficacy of inactivated vaccines
Less commonly required for attenuated vaccines
Active replication extends period of immune system exposure allowing for adequate response
Comparison of Attenuated and Inactivated Vaccines 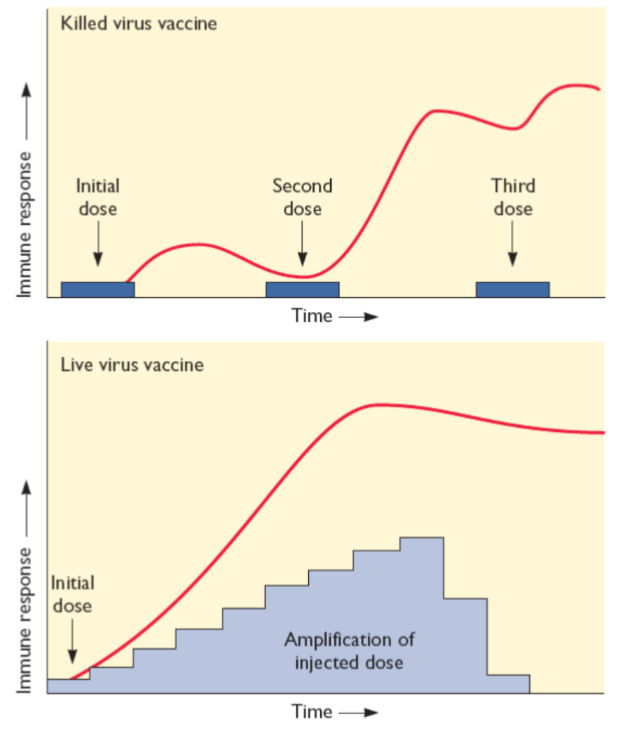
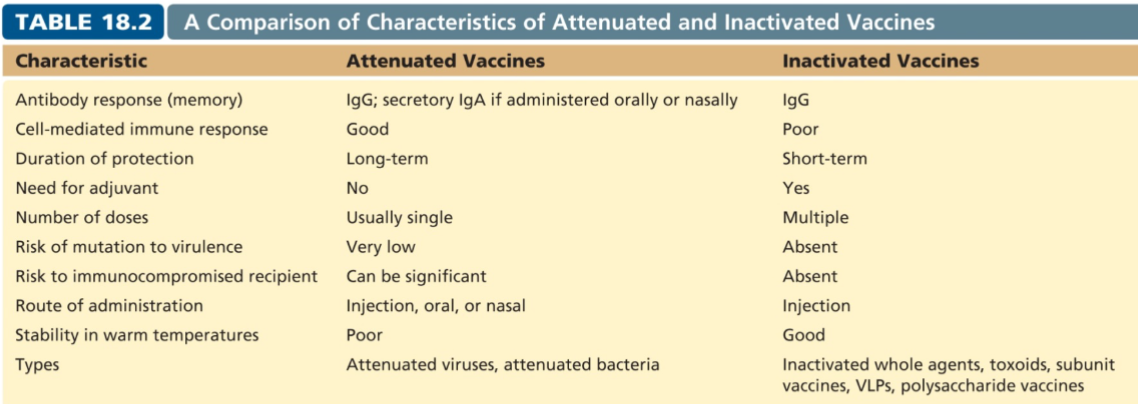
Routes of Administration
Vaccines are most commonly injected into the skin of muscle tissues
Needle and syringe (parenteral)
Needle-less administration easier and often cheaper
Oral
Intranasal
Intradermal
Mass delivery – aerosol or water
In ovo delivery to poultry – chicken hatches with immunity
Safety of Vaccines
Therapeutic index: risk-versus-benefit of receiving vaccine
All vaccines have side effects
Some are mild: fatigue, swelling and pain at site
Relatively common – counsel clients on these side effects
Some are severe: severe allergic responses, high fevers, seizures, vaccine reversion to wild-type, death
Exceedingly rare for most vaccines
Risks can outweigh benefits for some vaccines
Vaccine Failure
Bad vaccine - product problems
Incorrect delivery
Wrong vaccine, wrong route, wrong dose
Inactivated vaccine (inappropriate storage conditions)
Wrong animal/timing
Immunocompromised or sick animal
Maternal antibody interference (too young)
Given too proximate to exposure to achieve protection
At least 5-10 days to have sufficient response to protect
Poor responder: some animals just don't respond well to vaccines
Protect these animals by herd immunity
Too few boosters: one response may be insufficient for complete protection
Immune Disorders and Chronic DIsease - 04/09
Immune Hypersensitivity Reactions (tolerance)
An overreaction of the immune system: hypersensitivity reactions require a pre-sensitized (immune) state of the host
Includes allergies and autoimmunity
Reactions may be uncomfortable to damaging to occasionally fatal
Four classifications
Type 1: Allergy, Anaphylaxis and atopy (immediate)
Type II ; cytotoxic, antiBody-dependent
Type III: immune complex disease
Type IV: Delayed-type hypersensitivity
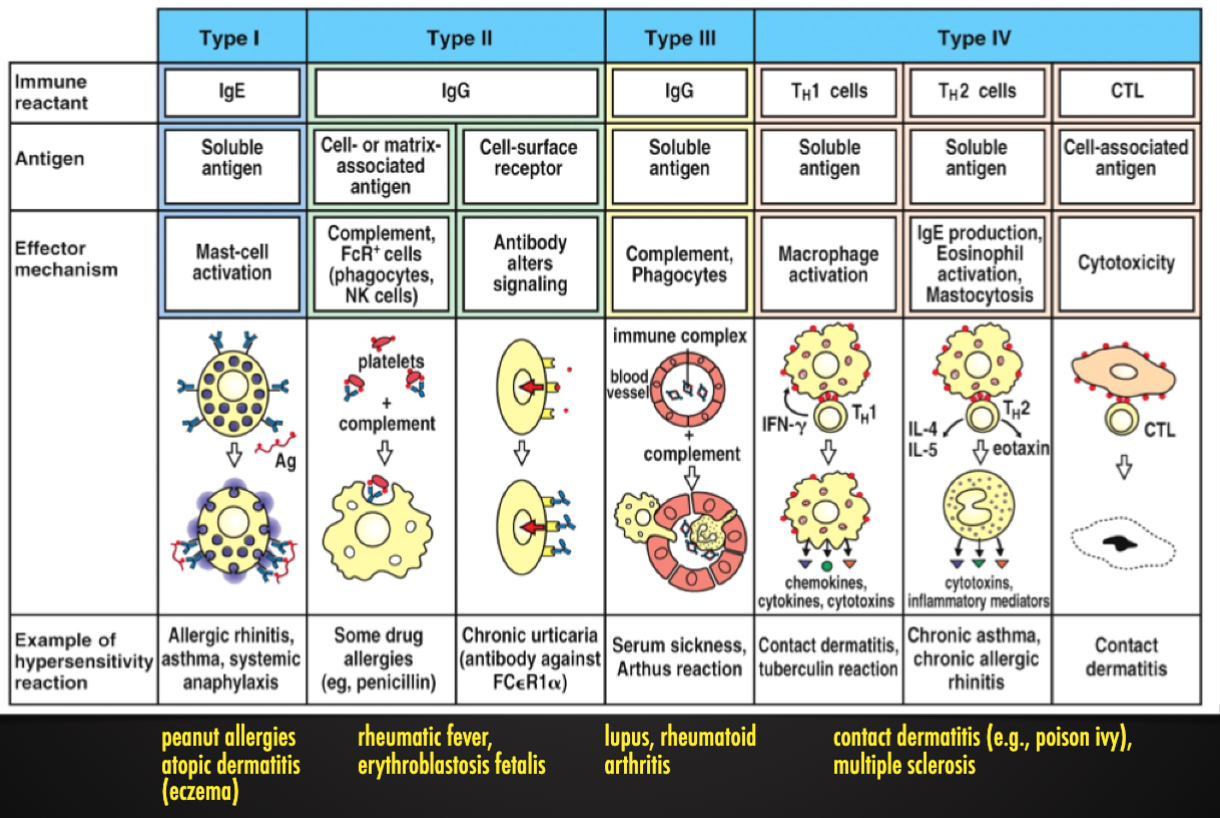
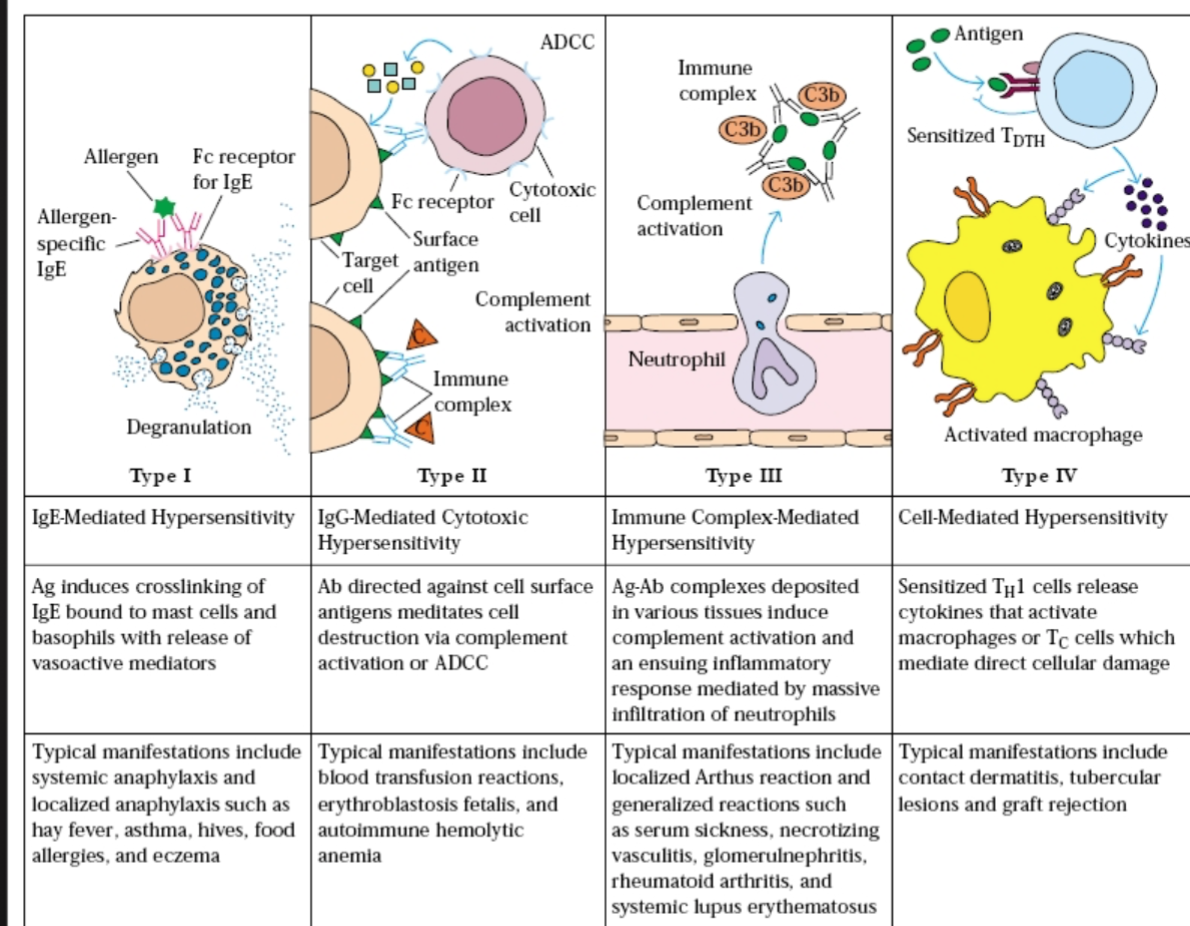
Immune System and Sterile Inflammation
Tissue injury but no infectious agent present
Mechanical trauma
Ischemia-reperfusion injury (IRI)
Injury to the tissues results from the initial hypoxia but also from the restoration of blood flow and re-oxygenation, which exacerbates inflammation
Acute myocardial infarctions, cerebral infarctions, acute kidney injury and solid organ transplantation
Gastric dilatation and volvulus (GDV) - commonly seen in larg, deep-chested dogs
Crystal-induced inflammation: crystal deposition within joints leads to gouty arthritis and elicit the classical signs of inflammation including redness, pain, heat, swelling and loss of function
Particle-induced lung disease
Asbestosis and silicosis
Toxin exposure
Innate recognition of tissue damage.
Involves damage-associated molecular patterns (DAMPs)
DAMP-Sensing and Sterile Inflammation
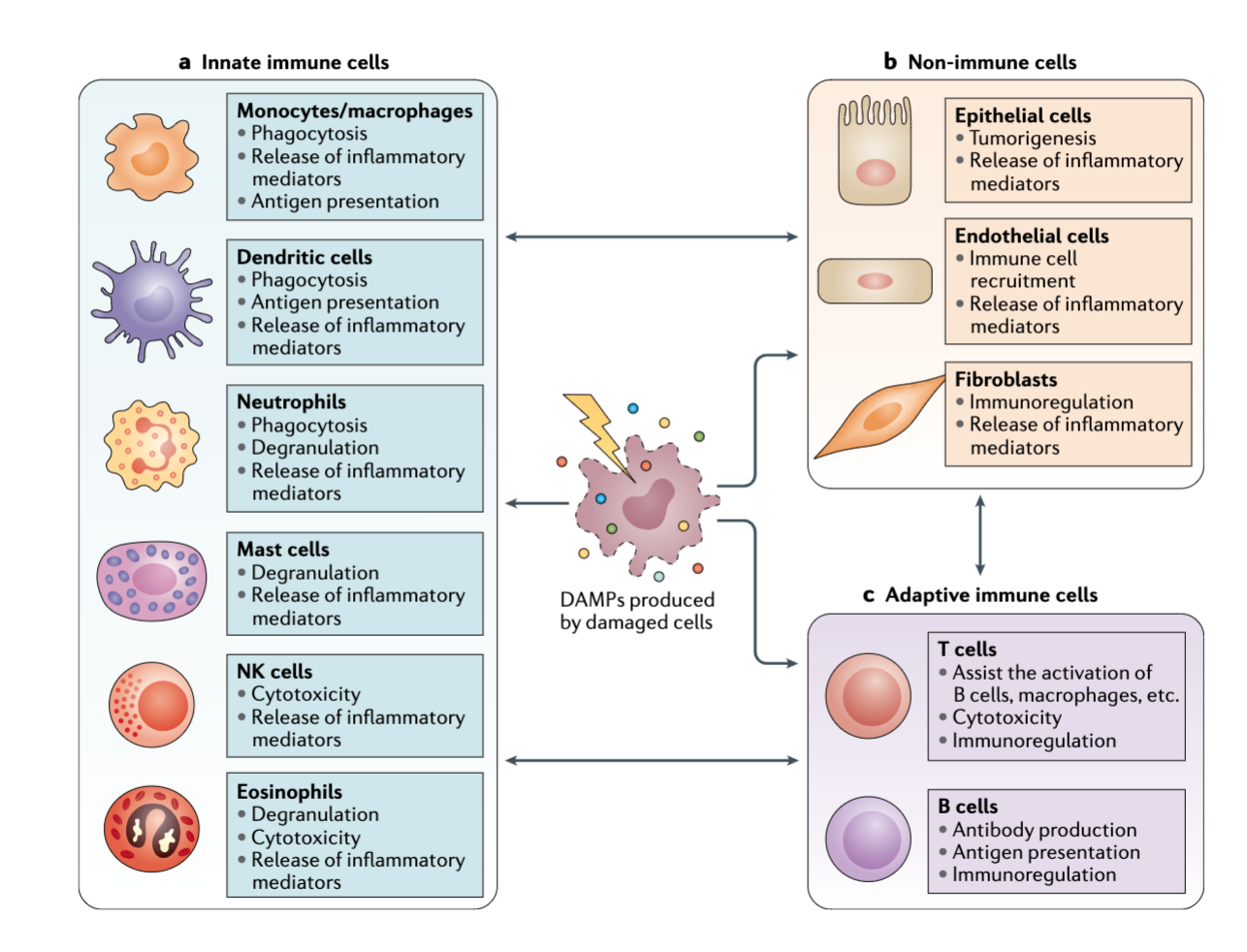
Immune System and Chronic Inflammation
Chronic inflammation
If antigen persist, antigen-reactive T cells can drive continued
Recurrent activation of immune processes (ex. inflammation)
Causes tissue damage (autoimmune diseases and inflammatory diseases), which then causes immune activation. Cyclical process
Adiposity is a major contributor to sub-clinical chronic inflammation
Important role of inflammation in pathogenesis of chronic disease: atherosclerosis, type 2 diabetes, Alzheimer's disease, cancer, etc.
Adipose and Chronic Inflammation
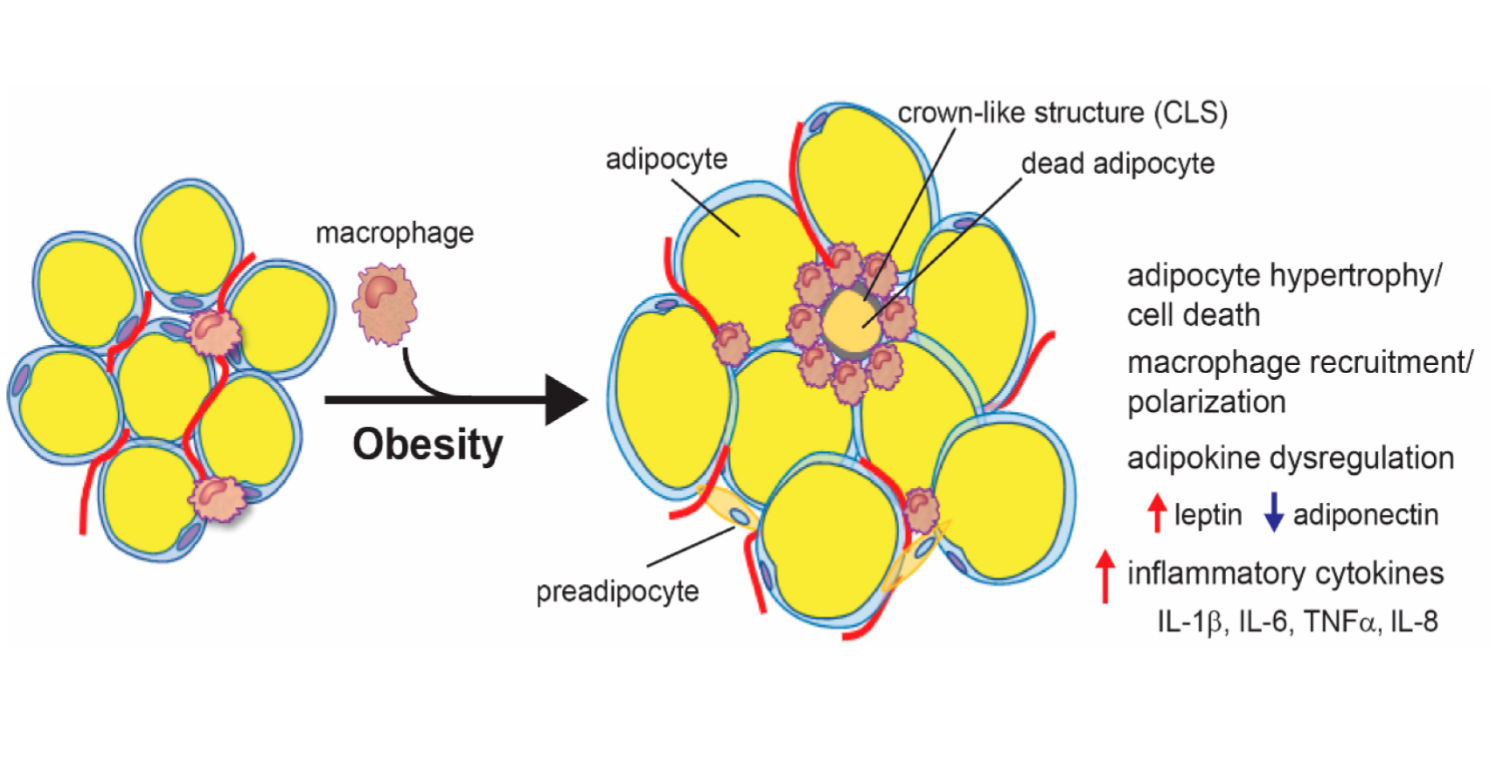
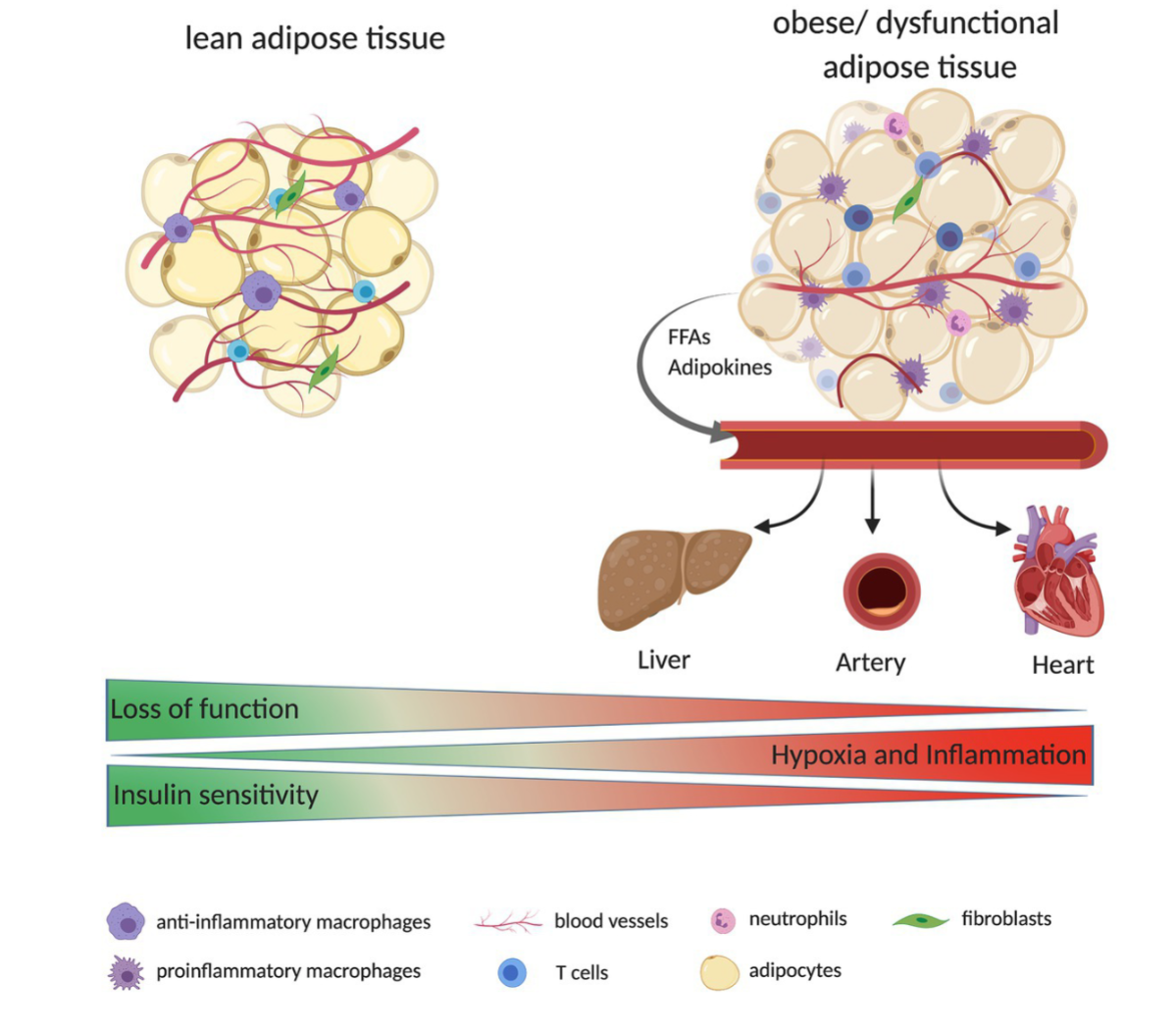
Chronic Disease in Animals
Some common examples
Arthritis and other orthopedic conditions
Chronic kidney disease
Hepatitis and other liver diseases
Skin allergies (atopy)
Diabetes mellitus
Cushing’s and addison's disease
Inflammatory bowel disease
Hyperthyroidism (cats) and hypothyroidism (dogs)
Alzheimer's disease
Antimicrobials and Antibiotics 04.16.24
Definitions
Sterilization
Complete elimination or destruction of all organisms and viruses by chemical or physical means
Sanitization
The process of reducing microbial contamination to an acceptable “safe” level
Decontamination
A treatment of an inanimate object/surface to kill microorganisms or remove contamination is safe to handle. No quantitative implication
Disinfectant
A chemical agent that is applied to inanimate objects to kill most pathogenic microbes, but not all microbes. They may not be effective against bacterial spores. Usually not safe on host tissues
Antiseptic
A chemical agent that is applied to living tissue to kill most pathogenic microbes, but not all microbes. Usually safe on host tissue
Antimicrobials
Inhibits growth of microorganisms
Antibacterial
Inhibits growth of bacteria
Antibiotic
Inhibits growth of microorganism
Made by other microorganism
Usually extended to include synthetic drugs
Bacteriostatic vs. Bactericidal
Bacteriostatic
Reversible inhibition of bacterial growth
When the antibiotic is removed, the bacteria can replicate
Some bacteriostatic drugs may be bactericidal at high concentrations
Examples: sulfonamides, tetracyclines, chloramphenicol
Bactericidal
Kills bacteria
When the antibiotic is removed, almost none of the bacteria can replicate
Examples: penicillin, vancomycin, rifampin
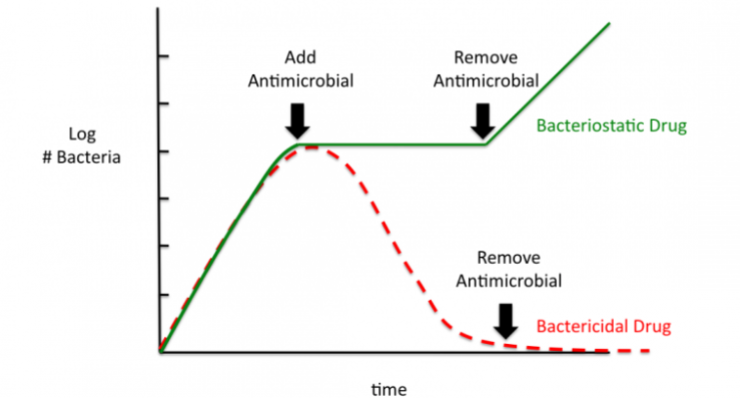
Time before “Modern” Antibiotics
First Treatments:
1500 BC
Egyptians used honey, lard and lint for dressing wounds
Enzymatic production of hydrogen peroxide in most honeys
High sugar content (high osmolarity)
Presence of other bioactive factors
Over 2,000 years ago
Moly bread was used in china, greece, serbia, egypt and other ancient civilizations as treatment for infected wounds
Metal Solutions
Arsenic
Used since antiquity
One of the first antimicrobial compounds and was effective against syphilis
Arsenic is very toxic to the patient
Mercury
Very effective antimicrobial agent
Used to sterilize surfaces and kill microbes
Still used as preservatives in vaccines
Bacteria can develop really high levels of resistance to metals (5-10mM)
Discovery of Penicillin
September 3, 1928: Alexander Fleming (Professor of Bacteriology) discovers that growth of Staphylococcus (cause boils, sore throats and abscesses) was inhibited by growth of mold (Penicillium notatum).
June 1929: findings published in the British Journal of Experimental Pathology, with only a passing reference to penicillin's potential therapeutic benefits.
Howard Florey, Ernst Chain and colleagues at Oxford University turned penicillin from a laboratory curiosity into a life-saving drug.
1940:
Florey showed that penicillin could protect mice against infection from deadly Streptococci.
1941:
a 43-year old policeman, Albert Alexander, became the first recipient of the Oxford penicillin.
Developed a life-threatening infection with huge abscesses affecting his eyes, face, and lungs.
Penicillin was injected and within days he made a remarkable recovery.
But supplies of the drug ran out and he died a few days later.
Plans to make penicillin available for British troops on the battlefield led to large scale production of the antibiotic.
Penicillin: Mechanisms of Action
A bacterial cell wall is composed of macromolecule of peptidoglycan composed of NAG-NAM chains that are cross-linked by peptide bridges between the NAM subunits
New NAG and NAM subunits are inserted into the wall by enzymes allowing the cell to grow. Normally, oher enzymes link new NAM subunits to old NAM subunits with peptide crosslinks
Penicillin interferes with the linking enzymes, and NAM subunits remain unattached to their neighbors. However the cell continues to grow as it adds more NAG and NAM subunits
The cell bursts from osmotic pressure because the integrity of peptidoglycan is not maintained
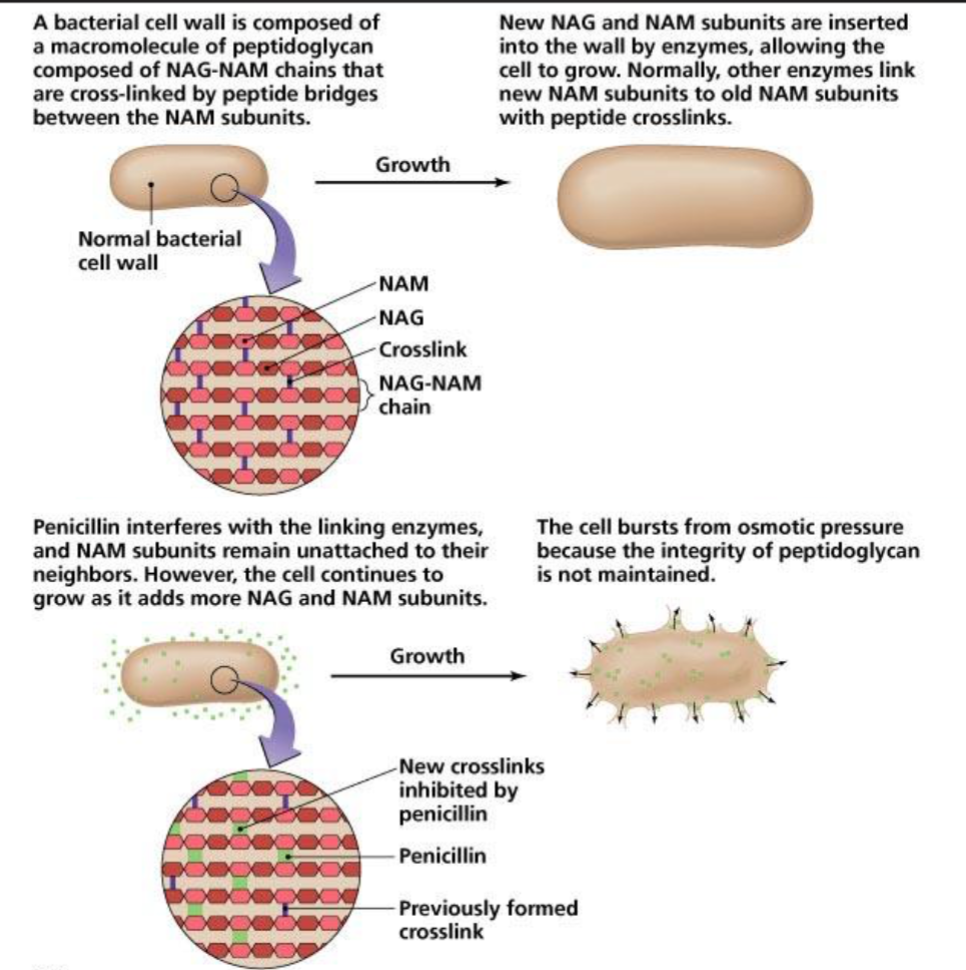
Antibiotics: Timeline of Discovery
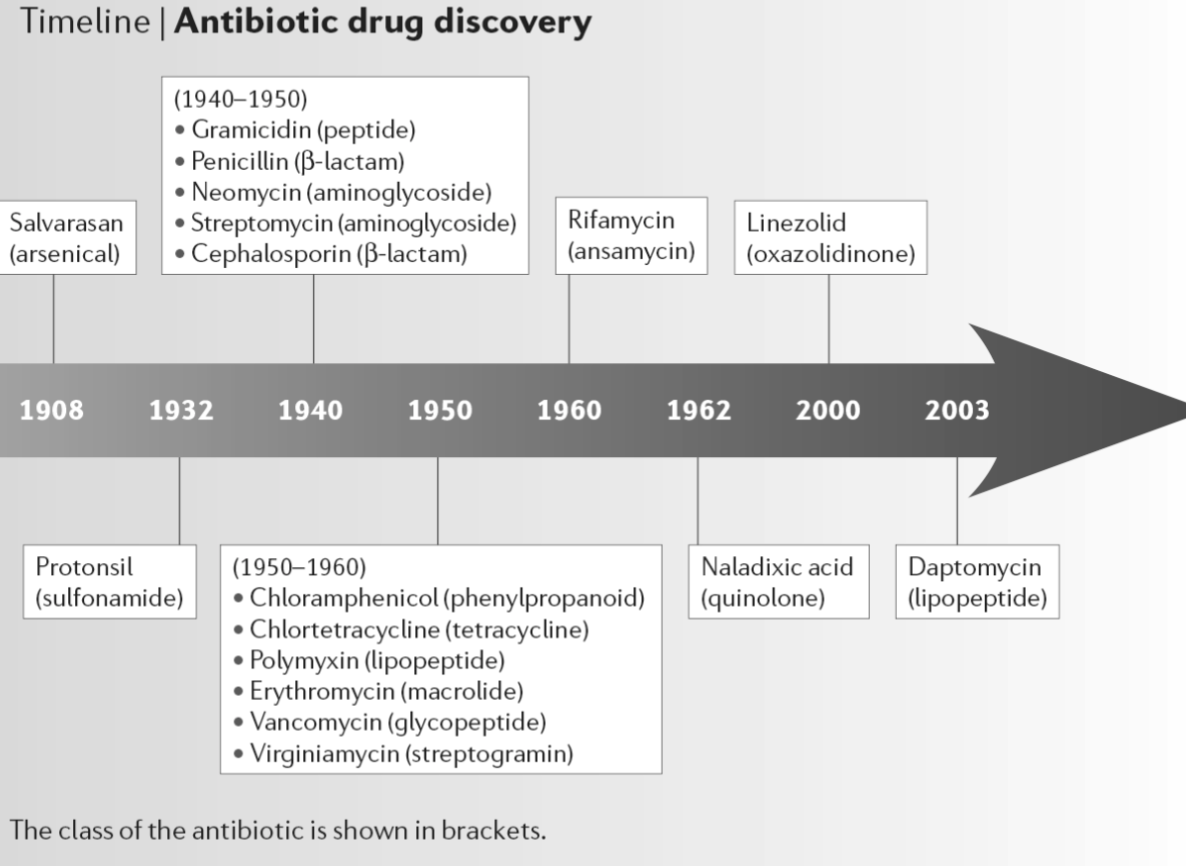
Antibiotics: Mechanisms of Action
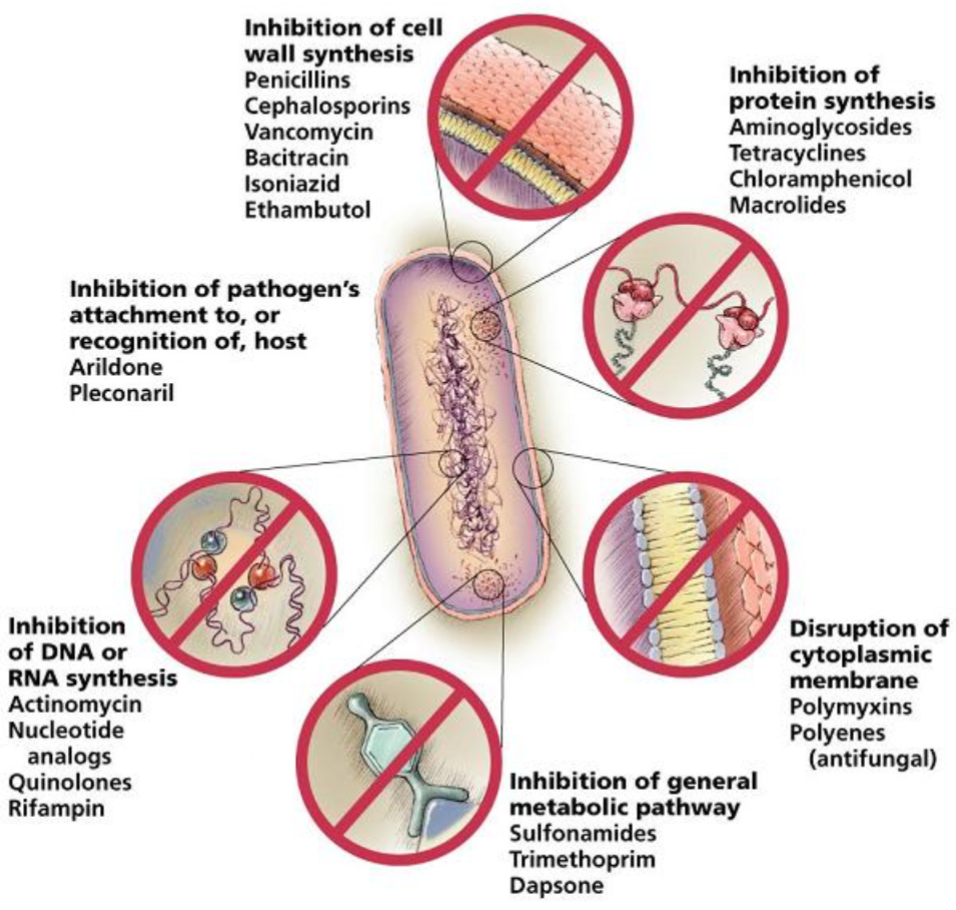
Spectrum of Activity
Broad Spectrum
Active against both gram-positive and gram-negative organisms
Examples: tetracyclines, phenicols, fluoroquinolones, “third-generation” and “fourth-generation” cephalosporins
Narrow Spectrum
Have limited activity and are primarily only useful against particular species of microorganisms
Example:
Glycopeptides and bacitracin are only effective against Gram-positive bacteria, whereas polymyxins are usually only effective against gram negative bacteria
Aminoglycosides and sulfonamides are only effective against aerobic organisms, while nitroimidazoles are generally only effective for anaerobes
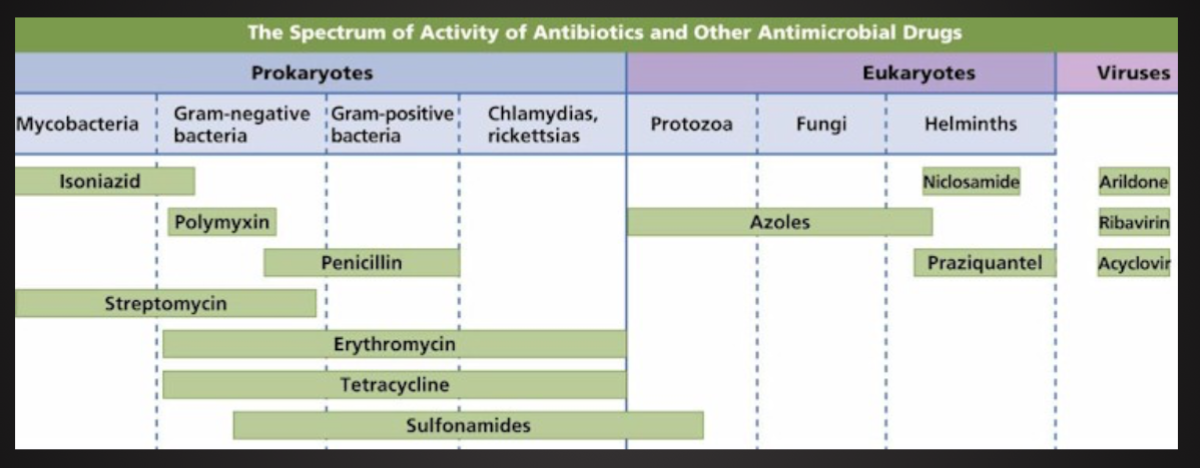
General Characteristics of Antimicrobial Drugs
Selective Toxicity
Ability of drug to kill or inhibit pathogen while damaging host as little as possible
Therapeutic Dose
Drug level required for clinical treatment
Toxic Dose
Drug level at which drug becomes too toxic for patient (i.e produces side effects)
Therapeutic Index
Ratio of toxic dose to therapeutic dose
Level of Antimicrobial Activity
Effectiveness of antimicrobial are expressed in two waysL
Minimal inhibitory concentration (MIC)
Lowest concentration of drug that inhibits growth of pathogen
Minimal lethal concentration (MLC)
Lowest concentration of drug that kills pathogen
Two techniques are routinely used to determine MIC and MLC
Dilution Susceptibility Tests
Inoculate media containing different concentrations of drug
Monitor growth by plate counts or optical density (OD) at 600 nm
Plot the OD 60 nm vs concentration
MIC:
The lowest concentration showing no growth
MLC:
Tubes showing no growth are subcultured into drug-free medium
Lowest corresponding original concentration from which microbe cannot be recovered is MLC
Kirby-Bauer Disk Diffusion Tests
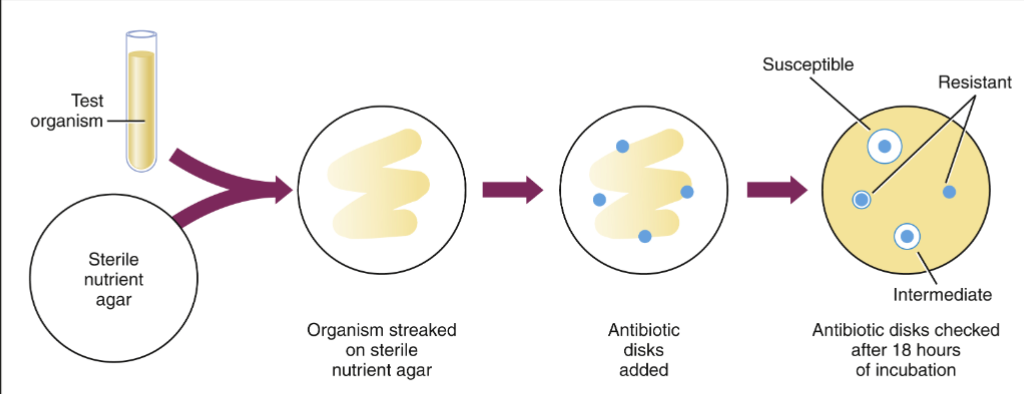
Disks impregnated with specific drugs are placed on agar plates inoculated with test microbe
Drug diffuses from disk into agar, establishing concentration gradients
Observe clear zones (no growth) around disks
Diameters of zone used to quantitate susceptibility or resistance
Synergism of Combination Antibiotic Therapy
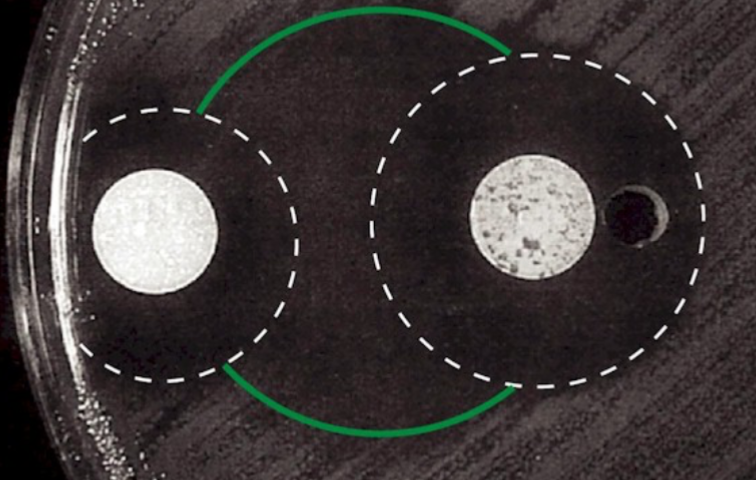
Antibiotic Combination Therapy
Indication for combination therapy may include:
Infections caused by multiple microorganism (abdominal and pelvic infections)
Nosocomial infections, which may be caused by many different organisms
Serious infections in which a combination is synergistic (ex. An aminoglycoside and an antipseudomonal penicillin for pseudomonas infections)
Likely emergence of drug-resistant organism if a single drug is used (ex. tuberculosis )
Fever or other signs of infection in immunosuppressed patients. Combinations of antibacterial plus antiviral and/or antifungal drugs may be needed
Effectiveness of Antimicrobial Drugs Therapy
Factors that influence effectiveness:
Susceptibility of pathogen to drug
Single vs combination therapies
Ability of drug to reach site of infection
Ability of drug to reach concentrations in body that exceeds MIC of pathogen
Factors Influencing the MIC in the body during treatment
Amount Administered
Route of administration
Duration of Therapy
Varies from single dose to year.
For most acute infections, ~7-10 days until the patient has been asymptomatic for 48 to 72 hours
Other Pharmacokinetics (fate of a substance in the body)
Rate of uptake
Route (kidney, liver) of clearance (elimination) from body
Half-life, which is affected by diseases (liver or kidney disease) and other drugs
Interactions with other drugs
Dosing schedule, particularly compliance
Side effects and idiosyncratic (“Type B reactions”) responses
Immune Status of Patient
Antibiotic Drug Resistance
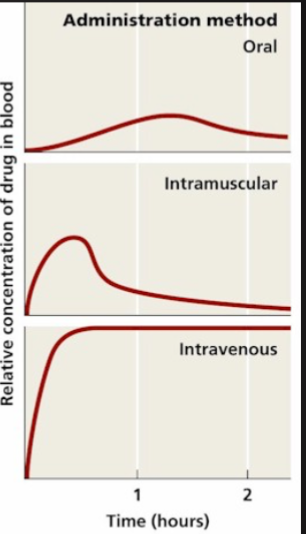
Antimicrobials and Antibiotics (continued) 04.18
Antibiotic Use in Agricultural Animals
Therapeutic vs non-therapeutic uses
Antibiotics are given in sub-therapeutic concentrations (below MIC) in feed to promote better feed conversion and growth
Estimated use in agriculture in United States
~16 million pound/year for non-therapeutic use in animals (institute of medicine, 1989)
24.6 million pounds/year for non-therapeutic purposes in chickens, cattle, and swine. Compared to just 3..0 million pounds for human medicine. (union of Concerned Scientists, 1989)
17.8 million pounds of antimicrobials used for animals, only 3.1 million pounds are used non-therapeutically. (Animal Health Institute: pharmaceutical industry-sponsored)
Many antibiotics used in animals are ones used in human medicine
Examples: tetracyclines, penicillins, and sulfonamides
Examples of exceptions: ionophores (used routinely in beef calves in feedlots and some dairy heifers)
Antibiotic Use in Agricultural Animals 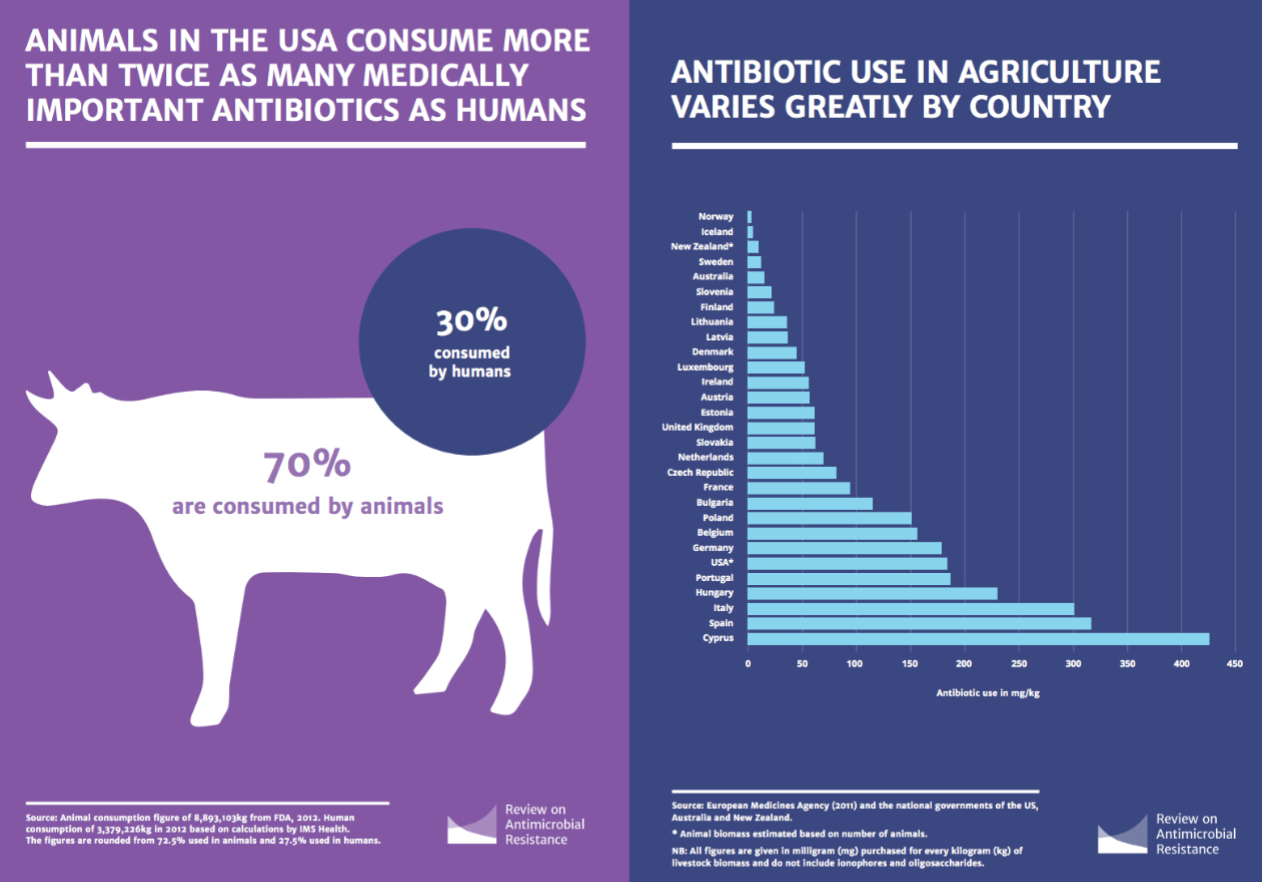
Misuse of Antimicrobial Drug Therapy
Alternation in microflora
Susceptibility to secondary infections
(children given antibiotics for routine upper respiratory infections often get diarrhea (ex. Susceptible to aggressive antibiotic-resistant C. difficile).
Long-term health effects
Obesity, allergy, atopic and autoimmune disease, infectious disease
Drug-resistant microflora
Chronic Impacts of Early Antibiotic Use
Antibiotics
Early in life
Multiple courses of broad spectrum antibiotics
Dysbiosis
Imbalance in gut microbiota
Loss of keystone species
Loss of microbial diversity
Alterations in metabolic capacity
Blooms of pathogens
Disease
Later in life
Obesity
Allergy and atopic disorders
Autoimmune disease
Infectious disease
Chronic Impact of Early Antibiotic Use
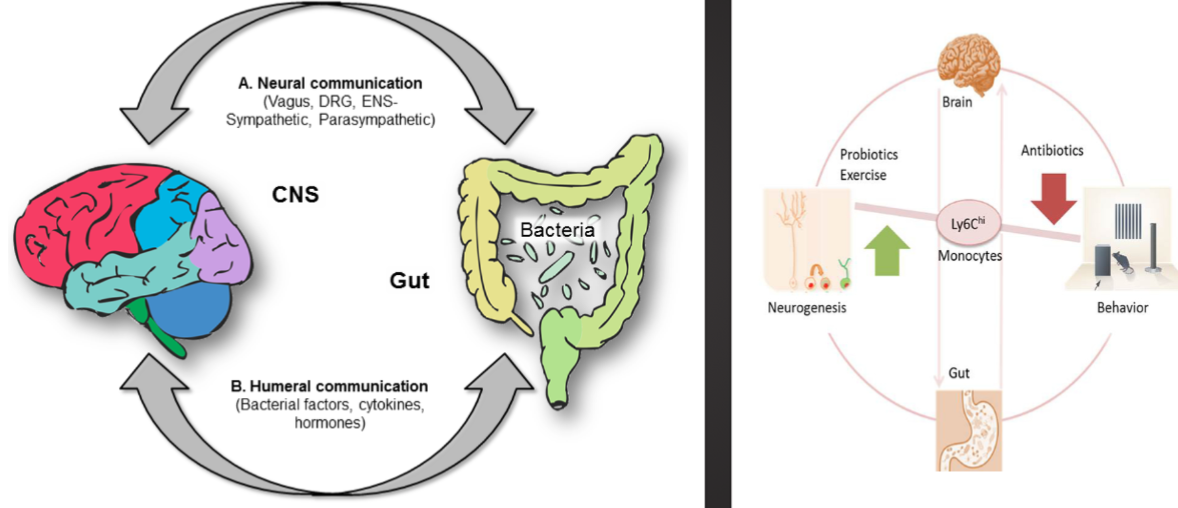
Differential Impacts of Antibiotics on Microflora
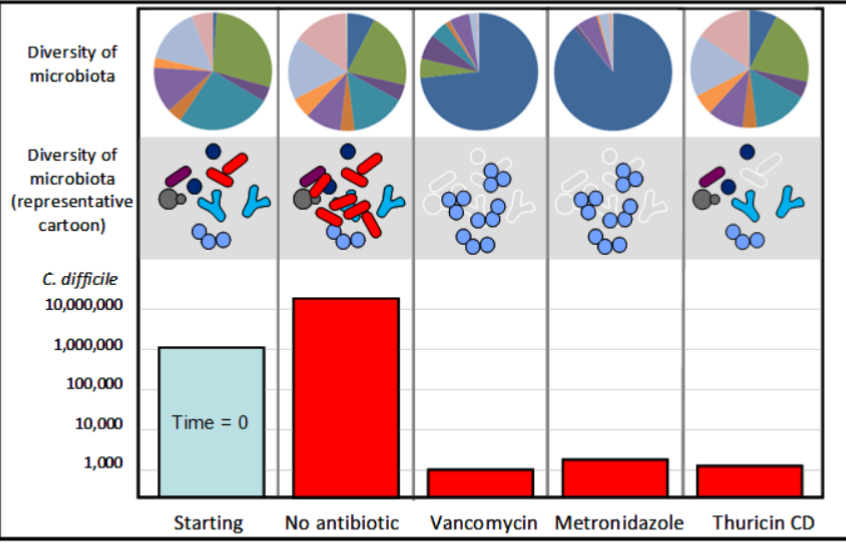
Antibiotics Drug Resistance
Terminology
Multiple drug resistance (MDR)
Organism is resistant to more than one drug in three or more antimicrobial categories
Extensively drug resistant (XDR)
Organism is resistant to at least one agent in all but two or fewer antimicrobial categories
Pan Drug-resistant (PDR)
Greek prefix “pan”, meaning “all”
Organism is resistant to all agents in all antimicrobial categories
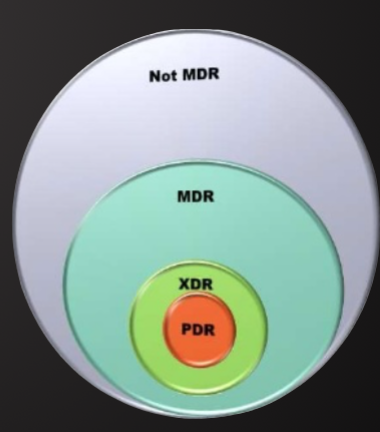
Emergence and Transmission of Antimicrobial-Resistance
Introduction of a resistant organism
Presence of a patient or animal with a resistant microorganism
Emergence of a new resistant organism
Selective pressure from antimicrobial use
Clonal Dissemination
Inadequate hand hygiene
Insufficient use of barrier isolation
Inattention to environmental reservoirs or vectors
How antibiotic resistance occurs
Lots of germs, a few are drug resistant
Antibiotics kill bacteria causing the illness, as well as good bacteria protecting the body from infection
The drug-resistant bacteria are now allowed to grow and take over
Some bacteria give their drug-resistance to other bacteria, causing more problems
Role of Poor Compliance in Antibiotic Resistance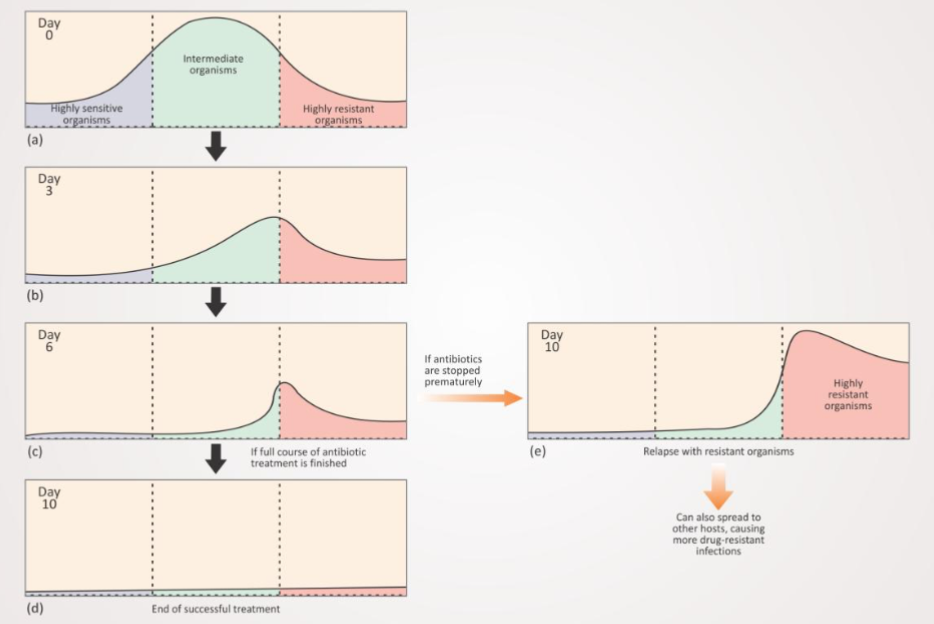
Strength of Biofilms
Predator evasion
Antibiotic tolerance/ resistance
Horizontal gene transfer
Biofilms Promote Antibiotic Tolerance and Resistance
Reversible phase
Motility
Adhesion
Irreversible phases
Mutration
Dispersion
Propagation
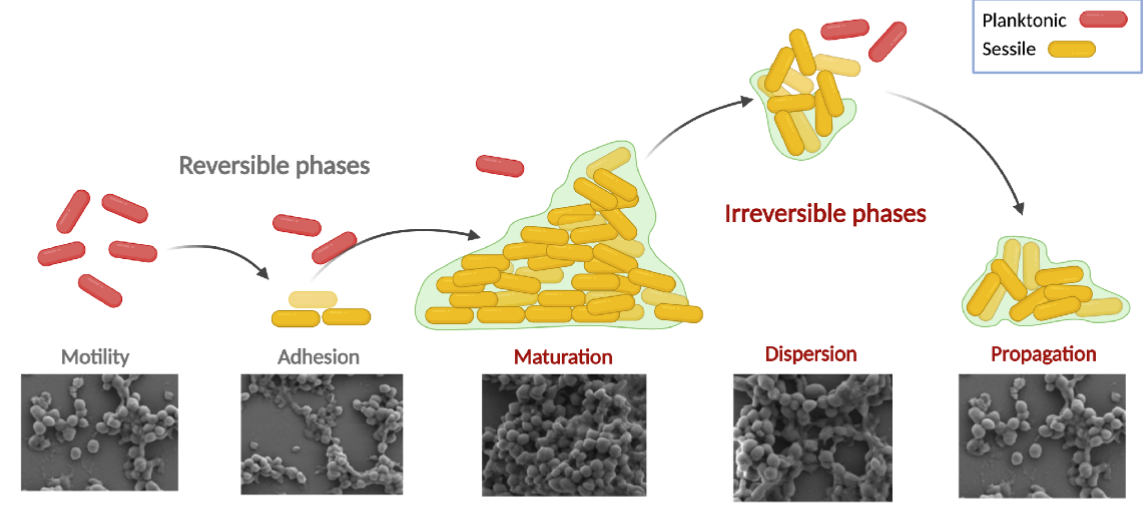
Mechanism of Antibiotic Resistance
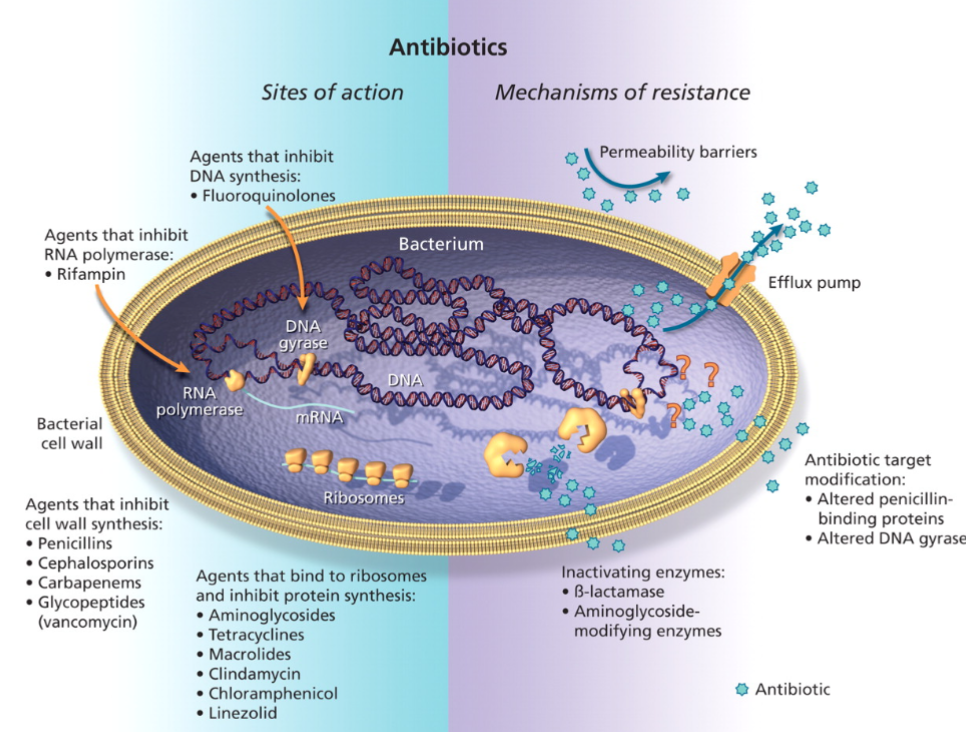
Dynamics of Drug Resistance
People or animals who receive an antibiotic are more likely to harbor bacteria resistant to that antibiotic and biochemically unrelated antibiotics
People or animals who frequent environments in which antibiotics are used are more likely to harbor drug-resistant bacteria, even if they have not received antibiotics, this applies to patients as well as to staff
The probability of harboring drug-resistant bacteria returns to normal within a few weeks after antibiotic therapy is discontinued or after absence from the antibiotic-rich environments. But not entirely eliminated
The prevalence of drug-resistant bacteria in the community is increasing due to increasing use of antibiotics in the environment ‘
Antibiotics, use them and lose them
Cost of Antibiotic Resistance
Every year in the United States, more than two million people get antibiotic resistant (ABR) infections. At least 23,00 people die as a result
In the united states, antibiotic resistance add $20 billion in direct healthcare costs, with additional societal cost of ~$35 billion a year in lost productivity
Reducing ABR infections by just 20% would save $3.2- $5.2 billion in health care costs each year and cut up to $11.3 million additional in-hospital days for patients with ABR infections
What is the impact of ABR to animals and veterinary care?
Not well-understood
State of New Antibiotic Drug Development

New Antibiotics: They're not profitable to make. Who pays?
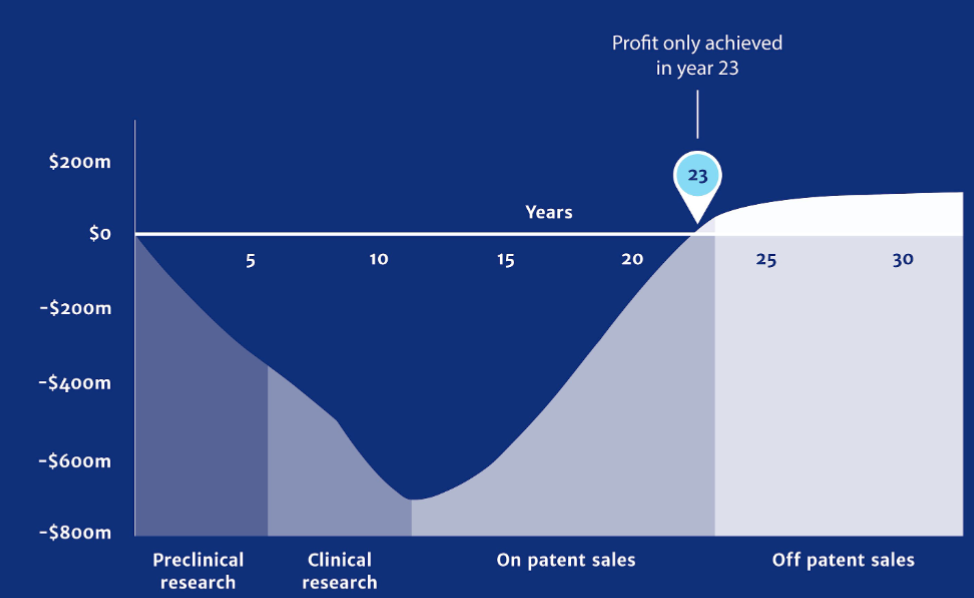
Retarding Emergence of Resistance
Maintenance of therapeutic levels
Ensure patient compliance
Avoid the use of drugs when the MIC is at or only slightly below the attainable level
Prevent biofilms and treat them aggressively
Use combinations of antibiotics when indicated (but not otherwise)
Avoid over and ill-advised use of antibiotics
Prescriptions for infections that won't respond
Tendency to use hot new drugs
Self medication
Spread of Antibiotic Resistance
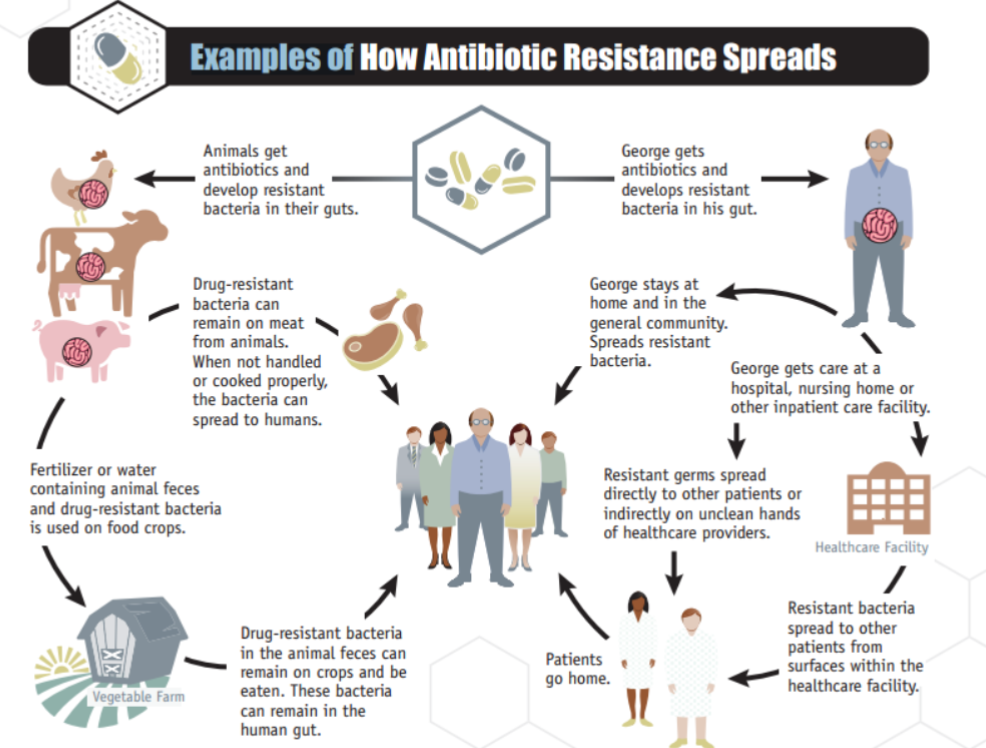
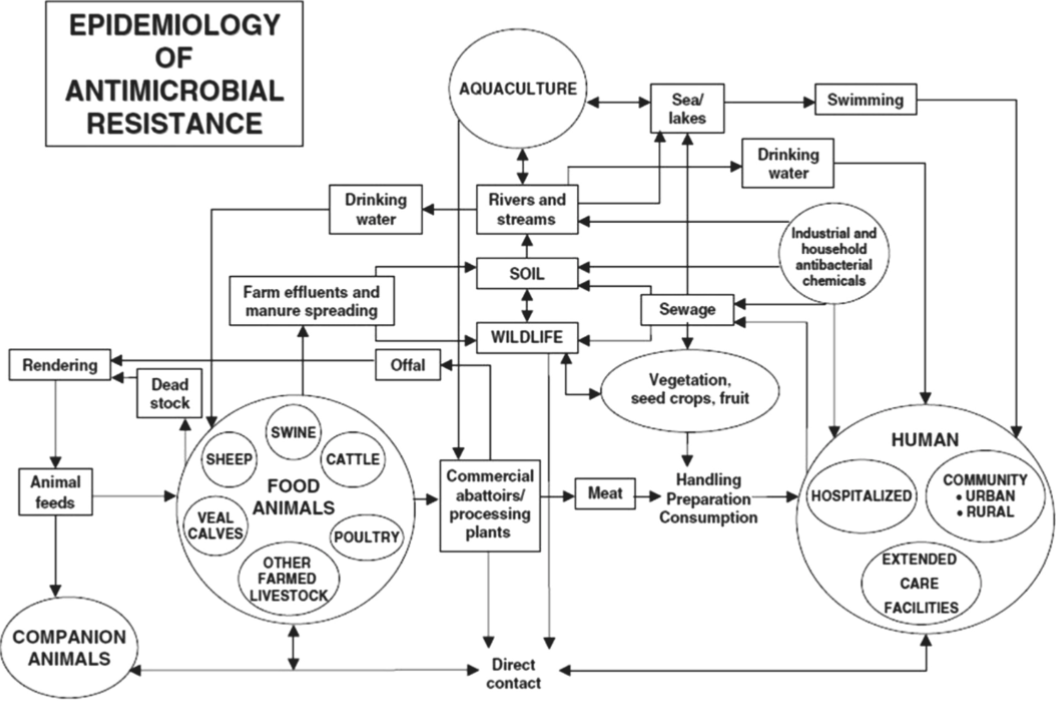
Biosecurity 04.23.24
Defining Biosecurity
Preventive measures designed to reduce risk of
Infectious diseases
Exposure to toxin and other contaminants
Bioterrorism
Zoonotic transfer
Importance of Biosecurity
Health animals for healthy people
To ensure safe food for the population
Protect the livelihood of individuals and families. Mant family owned farms and production
Protect a vital industry in the country
Meet policy requirements for competitive global trade
Mitigate economic consequences of a disease outbreak
Beef Cattle Production
Largest single segment of US agriculture
Great than 1 million businesses, farms, and ranches, 98% of US farms
$88.25 billion to US economy
26.4 billion pounds of beef
92.0 million head (all cattle and calves)
Pork Production
60,000 pork producers : 550,000 jobs
More than 110 million hogs/year
$23.4 billion to US economy
Exports over 2.2 million metric tons annually of pork and pork related products (26% of US production)
Poultry Industry
World's largest producer and second largest exporter of poultry meat and a major egg producer
Broiler meat: $45 billion and 36.9 billion pounds annually
Eggs: $13.5 billion and 96.4 billion eggs annually
Some Factors that influence Biosecurity
Globalization
Increasing travel and movement of people across borders
Increased trade in food and agricultural products
New agricultural production and food processing technologies
High dependence of some countries on food imports
Shift from country independence to county interdependence for effective biosecurity
Advances in communications and global access to biosecurity information
Great public attention to biodiversity, the environment and the impact of agriculture on both
Scarcity of technical and operational resources
Legal obligations: local, national and international laws and restrictions
Increased Risk of Exposure
Farm density
Other production facilities within a few miles
Animal movement
Especially if animals leave, then return to the premises
Traffic on and off the premises
Vehicles (feed, milk, garbage, rendering) and drivers
Human activity
Employees, service personnel, visitors
Equipment sharing
Between facilities, oro between animal groups within the facility
Access by wildlife
Such as insects, birds, rodents, feral animals
Housing difficult to clean
Well-thought out construction and facility lay out is important
Mortality disposal near animal housing
Some element of biosecurity planning
Biosecurity coordinators on premises identified and recorded
Organized training program with records of training
Lines of separation on buildings with required sanitation
Perimeter buffer areas defined: defining which areas of the facility are “hot” and “cold”
PPE on premise for employees
Vector control for multiple species of pests
Equipment control for multiple species of pests
A mortality management plan
Manure and other waste management
Replacement / new animals
Water management
Feed and new material management
A Biosecurity Plan
Embodies multiple components
Risk perception
Risk assessment
Risk management
Risk communication
Designed to improve disease control
Provide foreign and domestic diseases
Provide tools to minimize risk
Disease risk cannot be totally eliminated
Decrease exposure to existing disease agents
No one-size fits-all answer
Risk Perception
Different perceptions of risk
First identify what is viewed as a threat
Factors influencing perception
Previous experience
Media
Environment
Common beliefs to overcome
We have always done it this way
I've had most everything on this farm
It's too expensive
New beliefs to embrace
Disease outbreak can and do happen
Prevention is less costly than treatment
Too much is financially invested to lose
Prevention through awareness and management
Risk Assessment
Objective evaluation is critical
Possible vs. probable
Perform site specific hazard analysis (geographical, indoors versus outdoor raises animals)
Identify sources of potential infection
Consider the health status and species of animals
Identify strengths and weaknesses of existing infrastructure / practice
Identify areas needing protection
Ascertain site-specific pathways for potential disease movement
Be aware of the change over time. Regular re-assessment is important
Disease prediction is complicated
Depends on interaction of many different factors
Fundamental knowledge of disease is important
Route of transmission
Understand the epidemiologic triad of each disease
Risk Management
Evaluate facility / operation
Preemptively identify challenges
Develop systematic plan
Establish well-defined line of separation
Dirty (contaminated) / clean (protected)
Prioritization of biosecurity measures
Focus on highest risk first
Consider probability of occurrence
Consider ease/ cost of implementation vs. consequences
Easy to implement often means greater compliance
Inexpensive yet yield rewards
Tailored management plan: no common formula
Immediate challenges
Short term goals
Long term goals
Seek several expert advice, remain open to suggestions
Risk Communication
Communication is key
Plan must be understood and supported to be effective
Reporting and reporting requirements
Legislative requirements for animal health
The legal requirement to notify the local animal health divisional office at the first sign or suspicion of notifiable disease
Awareness of the legal requirements during a disease outbreak, such as foot-and-mouth disease (restriction of movement measures)
Familiarity with the legal requirements relevant to farm businesses
Knowledge of current legislation concerning high profile issues ex. Fallen stock, animal identification and traceability
An understanding of the food production regulations
Levels of a Biosecurity Plan
Conceptual
Location, geospatial sitting, orientation of the facility
Structural
Capital investment, construction, to prevent disease spread
Operational
Processes, management practices, standard operating procedure to exclude or contain diseases
Conceptual Biosecurity
Evaluate existing facility
Facility location, geospatial sitting, orientation, scope , size
Risk level
High risk: greater farm density, close to wildlife area, large groups managed as one population
Best practices
Separation, isolation with enhanced distance to neighboring livestock/ livestock facilities
Manage smaller groups of animals as biosecure units
Distance to wildlife habitats and roads
Mitigate / compensate for vulnerabilities
Eliminate (make less attractive) wildlife and pest habitat
Reroute traffic away from animal areas
Create smaller biosecure groups
Structural Biosecurity
Construction and capital investment
Physical design and maintenance
Paved parking away from barns
Fences, barriers leading to entrances to conduct biosecurity protocols
Locations for cleaning / disinfecting
On-site laundry for outerwear
Specialized anteroom at entry
Structural Biosecurity: Danish Entry system
Visible line of separation
Site-specific biosecurity attire
Appropriate biosecurity protocols for entering and exiting
Operational Biosecurity
Processes, management practices, standard operating procedures to exclude or contain disease
On-farm movements and managements
People, animals, supplies, equipment, vehicles, and other items
Understand effectiveness of your mitigation looks (disinfectants)
Should be based on general specific risk assessments
Mitigation of conceptual and structural vulnerabilities, and know disease
Apply strategic actions at critical control points
Focus on inputs and outputs
Entrance and exits
Work paths
Processes
Clearly identify separation of clean and dirty.
Development of a Biosecurity Plan
Step 1: prioritize the disease agent
Consider species/ susceptibility, housing, management, wildlife exposure
Step 2: conduct a facility assessment
Identify pathways/movements
Step 3: Implement processes to minimize impact of disease
Prevent movements (and practices) that carry disease
Assessment of Animal Health
Physical conditions
Condition and smell of hair coat and skin, body scoring
Sleeping habits
Time of day, time spent standing or lying
Attitude
Posture
Movement or weight shifting from one foot or side
Eating and drinking habits
Time of day, amounts, time spent eating and drinking water