2. Surfactants and self-assembly
Surface tension and surface active molecules- Interactions between the molecules on the surface give rise to surface tension.
• If the molecular composition is altered → the surface tension is changed
• Adsorption at the surface of surface active molecules
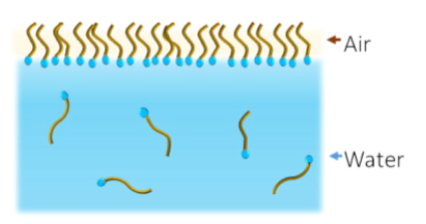
Surfactants - Molecules that interact with surfaces or interfaces . Surfactants are usually amphiphilic organic compounds, that have hydrophobic part (tail) and hydrophilic part (head). Surfactant= surface active agents
Example: sodium dodecyl sulfate (SDS) or fatty acid salts in common hand soap
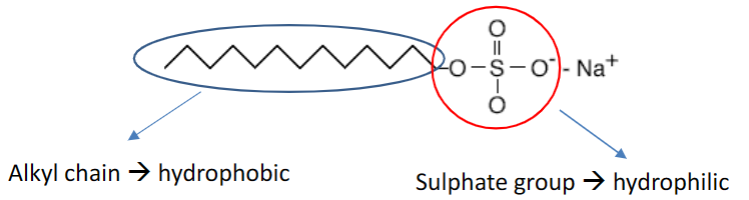
Some common types of polar head groups
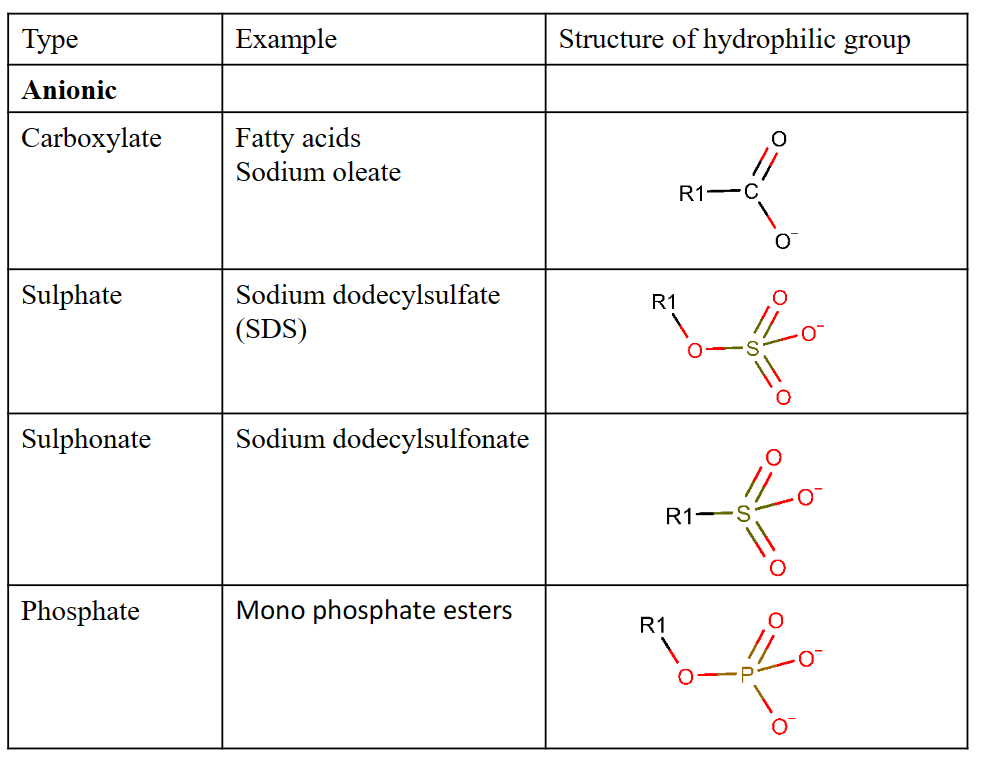
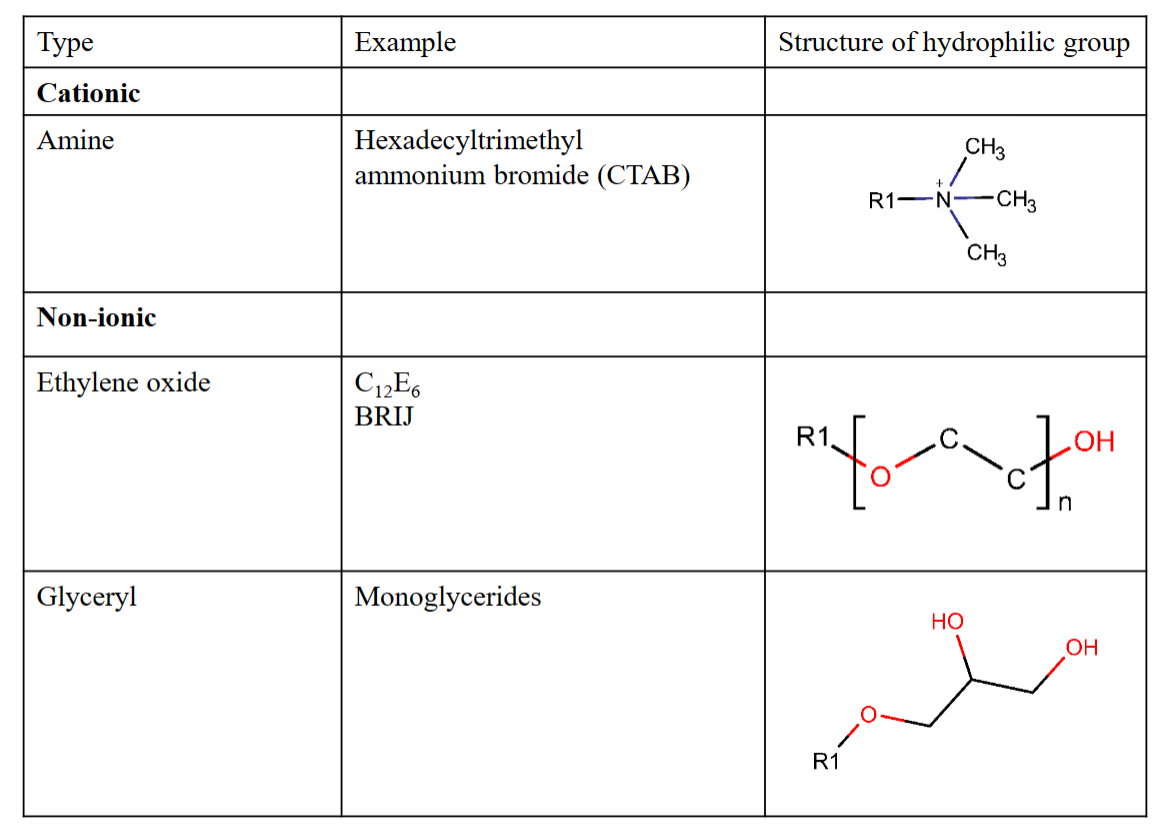
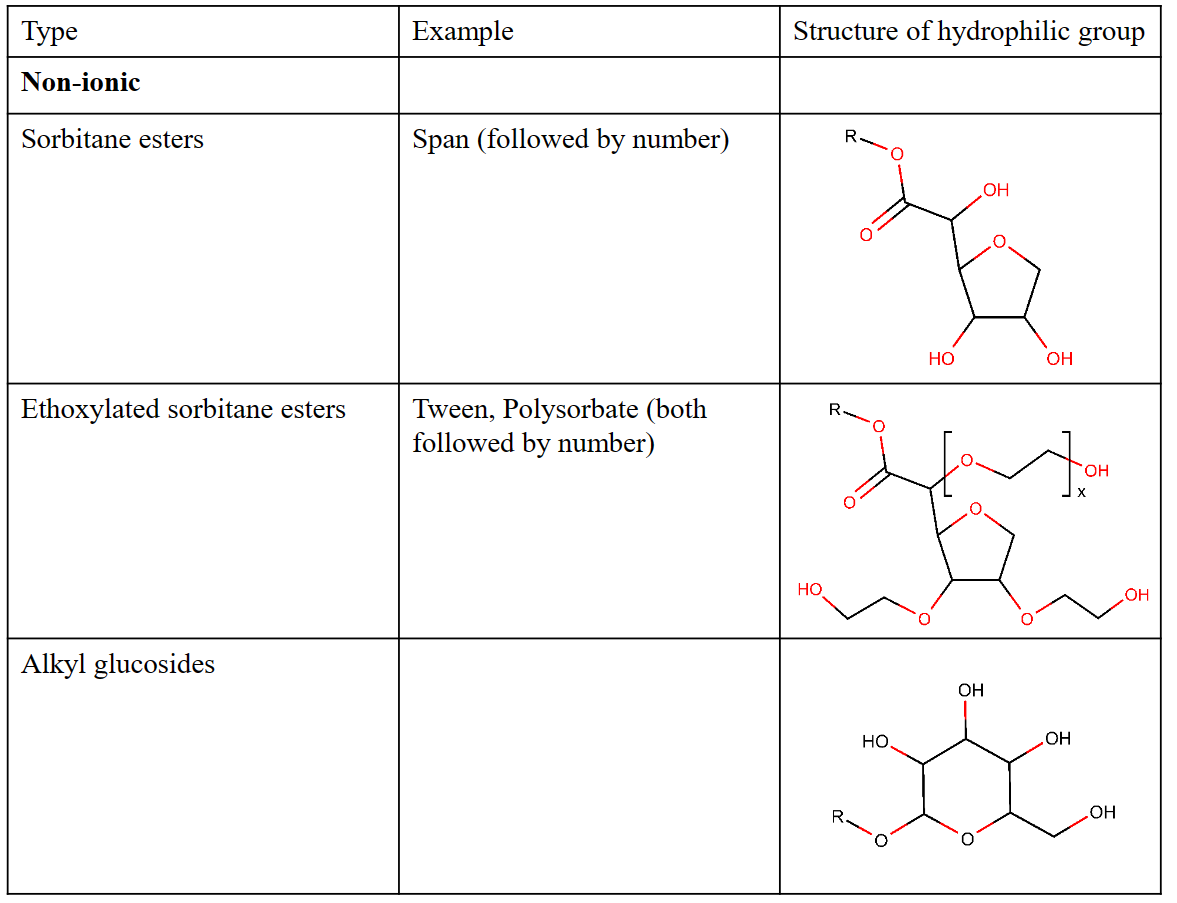
The hydrophobic tail is typically one (sometimes two) hydrocarbon chain(s) of various length
Surfactants in aqueous solution
- The polar head groups are hydrated and take part in the H-bonding structure of water
- Hydrocarbon tails do not interact with water molecules.
– “Hydrophobic effect” (the segregation of water and nonpolar substances)
– Tail in solution → free energy increase
– Aggregation → minimize contact between hydrocarbon tails and water molecules
Surfactants can self-assemble
• Amphiphilicy – surfactants can form micelles and other structures
• Equilibrium structure - Thermodynamically stable
• Constant rapid exchange of individual molecules between solution and surfactants particles
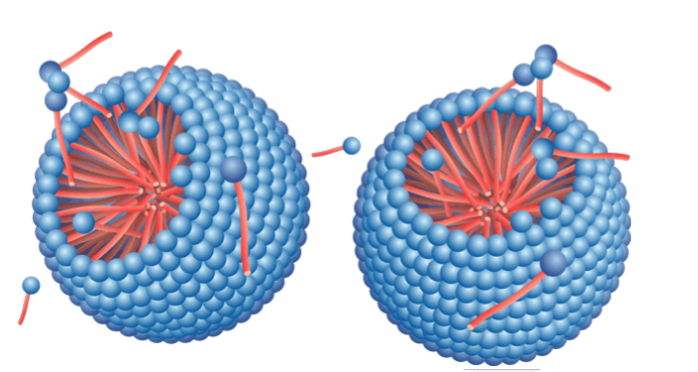
Formation of micelles
- Micelles are self-assembled equilibrium structures
- Micellar solutions are not dispersions – they are one-phase solutions!
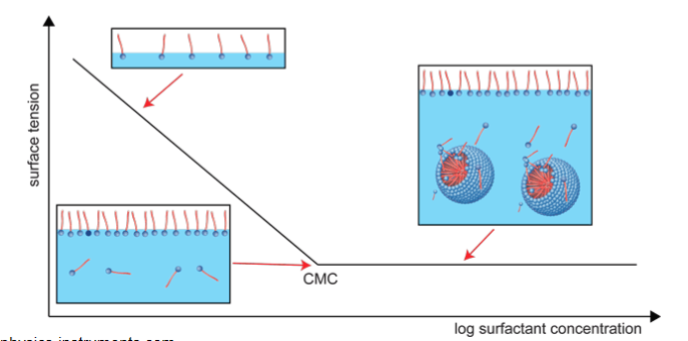
- Micelles form at the critical micelle concentration (CMC)
- Below CMC, only dissolved particles, no micelles
Micelle formation
• Micelle formation occurs as a result of interaction parameters and gives an increase in entropy.
• The standard free energy (ΔG= ΔH-TΔS) is affected by:
– Decrease
• Removal of non-polar chains from the aqueous phase.
– Increase
• Interface creation
• Repulsion between hydrophilic head groups.
• Repulsion between head groups counteracts micelle formation
– Steric (e.g large head groups → problem with packing)
– Electrostatic
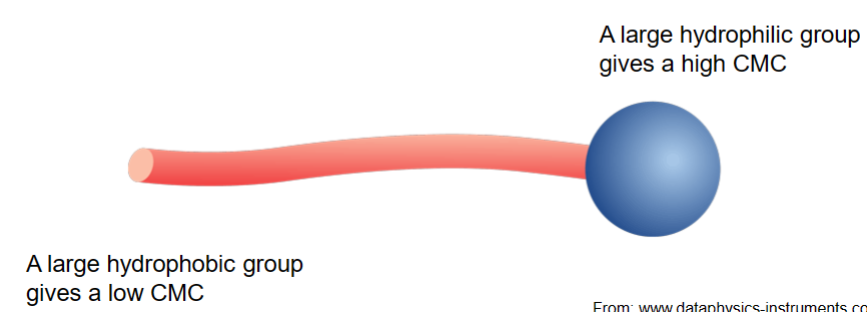
A large hydrophilic group (how long carbon chain is) gives a high CMC
A large hydrophobic group gives a low CMC
Mixed micelles
• Micelles can consist of different types of surfactants
• Non-surfactant molecules can be accommodated in micelles
– Example: fatty alcohol in fatty acid micelle (breaks up repulsion between acid groups)
• Can influence the CMC
– Example: Less repulsion between polar head groups
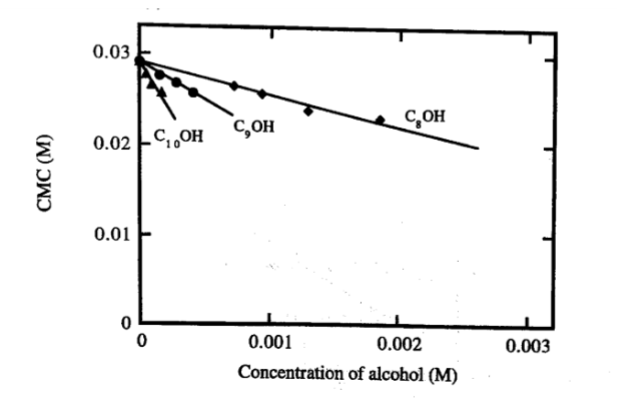
Decrease in CMC for potassium octanoate upon addition of fatty alcohols
• Decrease depends on alkyl chain length
• Decrease is practically linear with alcohol concentration
Functional properties of surfactants determined by CMC- Most functional properties of surfactants are determined by the CMC
- Washing – detergency
- Foaming
- Emulsification
- Solubilization of hydrophobic compounds
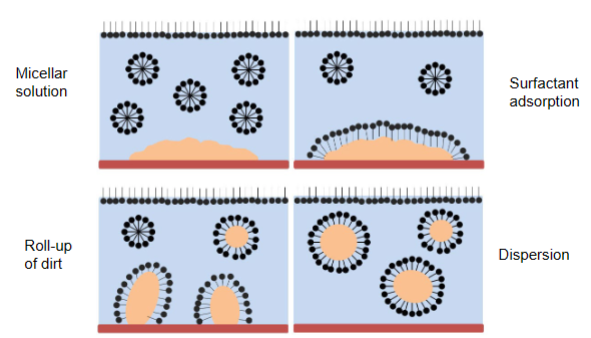
Detergency
- Micellar solution- Above CMC
- Surfactant adsorb to the dirt
- Roll-up of dirt
Can either occur:
- Spontaneously
- Mechanical force (rubing etc) - most common
- Dispersion (Dirt inside micelle)
Micelle formation- Micelles are dynamic, short lifespan meaning that reform equally fast. They are equilibrium surfactant unimers (individual molecules). They interact with the solvent through the hydrophilic surface and have a hydrophobic inner domain.
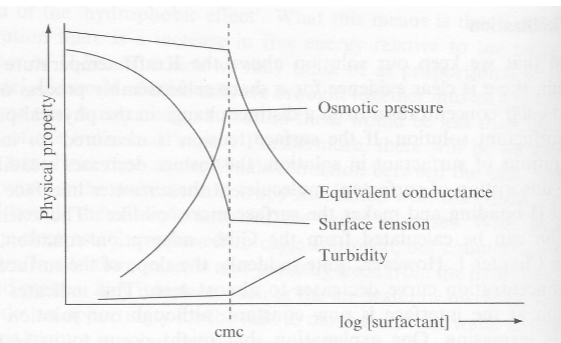
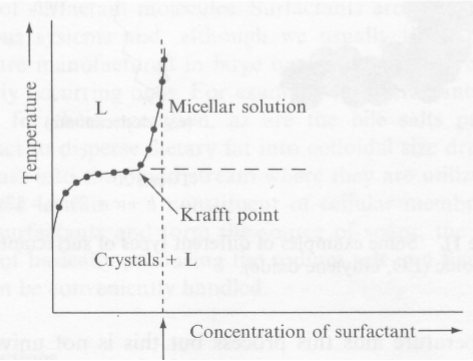
Physical properties change at the CMC
Krafft point- The Krafft point is the temperature surfactant solubility increases drastically.
- Below Krafft temperature is the solubility of the surfactant is low and micelles can not be formed.
- It is a typical property for ionic surfactants.
Wetting- The ability of a liquid to maintain contact with a solid surface. Adsorption of surfactants changes the properties of the surface
Clean glass: High energy surface – often slightly anionic
Water on a surfactant monolayer of hexadecyltrimethylammonium bromide (cationic):
Low energy surface
No wetting of surface by water
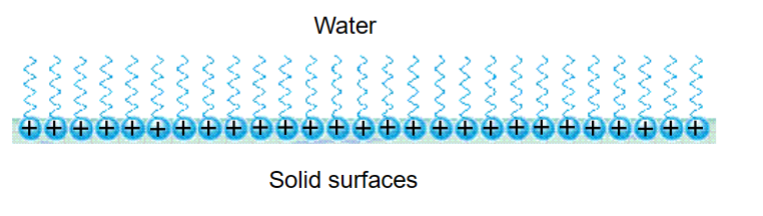
Adsorption of surfactants
• Wetting can be restored by increasing the surfactant concentration to the CMC.
– Bilayer formation.
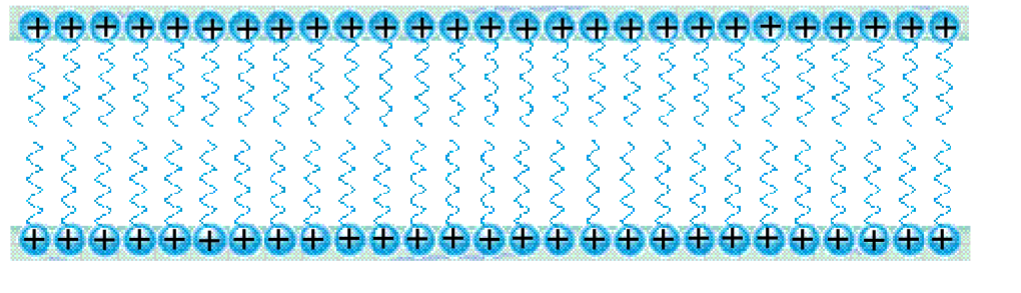
Wetting phenomena and surfactants in applications
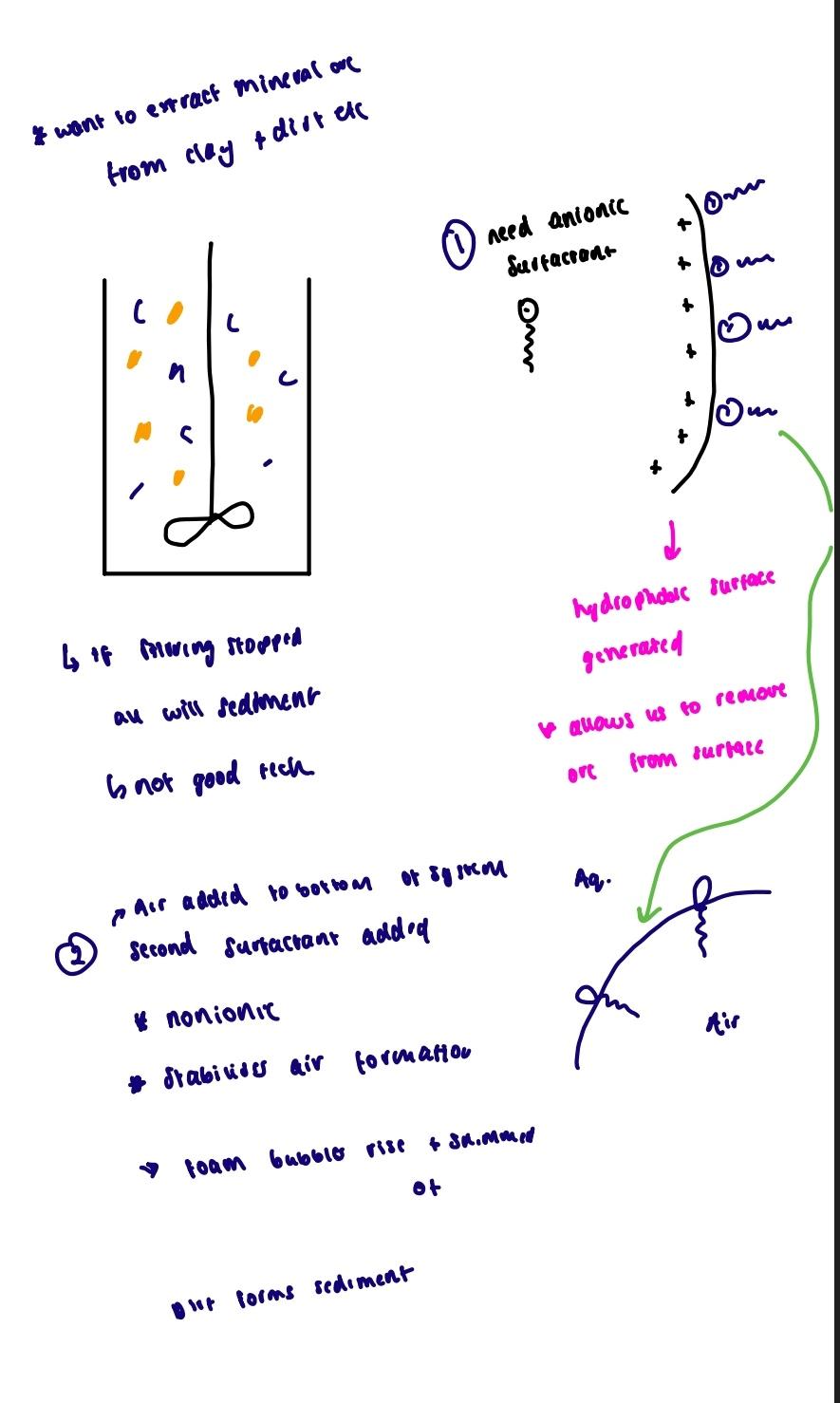
Mineral ore flotation
Want to extract mineral ore from clay and dirt etc.
- Anionic surfactants
Hydrophobic surface generated which allos us to remove ore from surface
- Air is added to the bottom of system and second non-ionic surfactant is added
The surfactants stabilize air formation and foam bubbles rise and dirt forms sediment
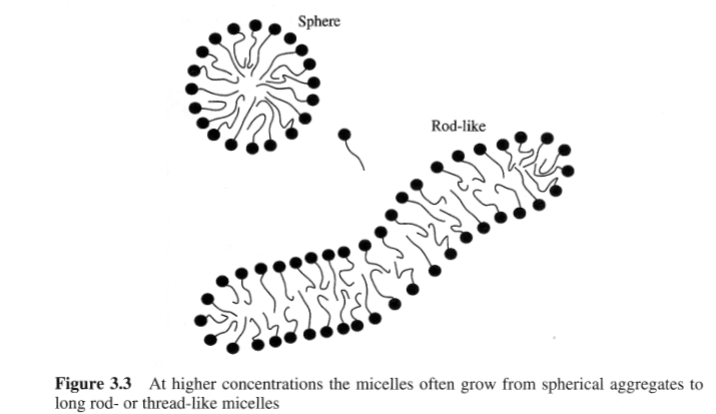
Surfactant self-assembly
• Concentration above CMC additional self-assembled structures start to form (affects viscosity)
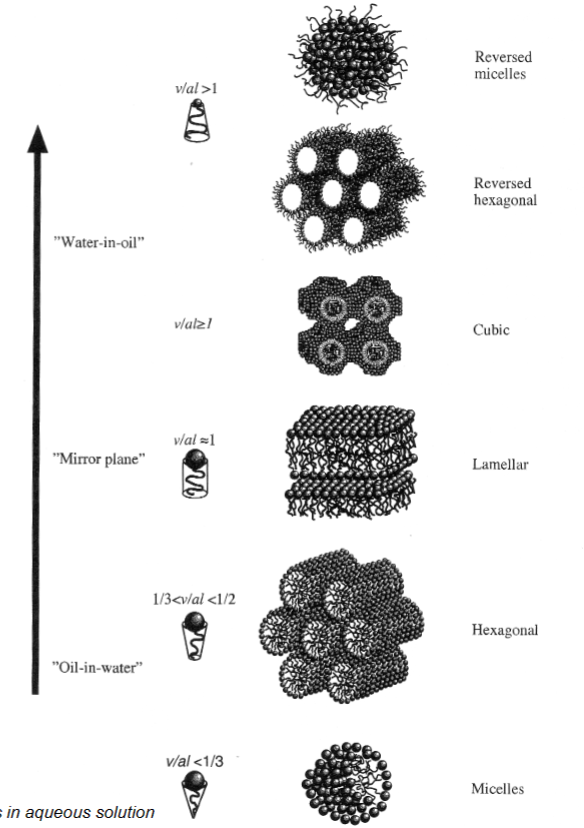
Reversed hexagonal:
- Water inside
Hexagonal:
- Water is in between tubes
- Inside is hydrophobic
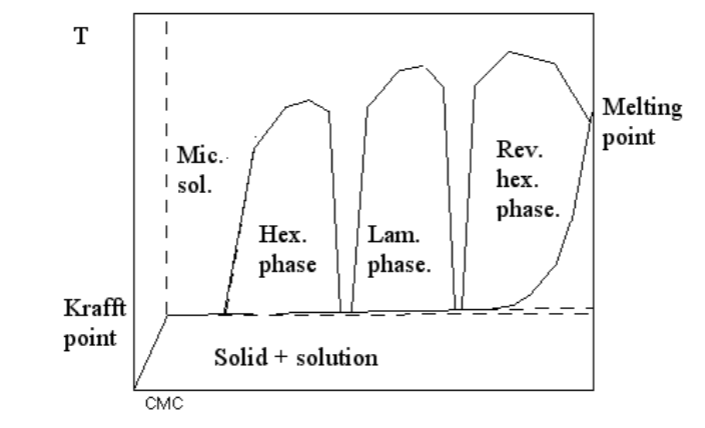
Surfactant phase behavoiur
Detergents are a mixture of ionic and non-ionic surfactant
- Low temperature is good ethoxylated surfactants → no Krafft point, low CMC
- High temperature is required for ionic surfactants
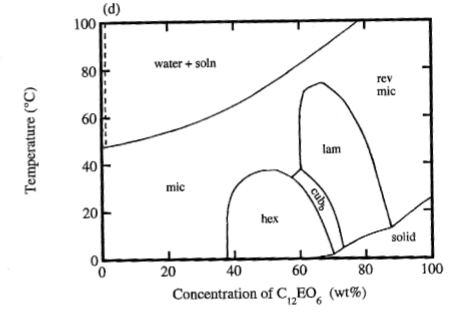
Typical phase diagram for an ionic surfactant
- Between phases, it be can different types of sub-phase
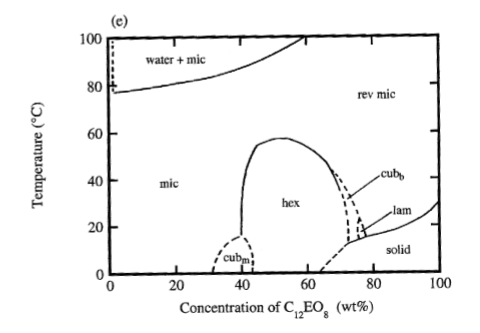
Ethoxylated surfactants
- Strong temperature dependence (good at low temperatures)
- Above the Krafft point, temperature does not influence phase behaviour greatly
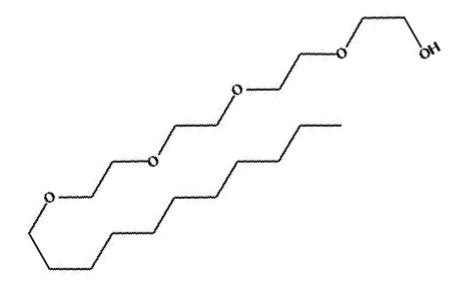
Lecithin-water
No Krafft point → amorphous so don’t form crystallised structures
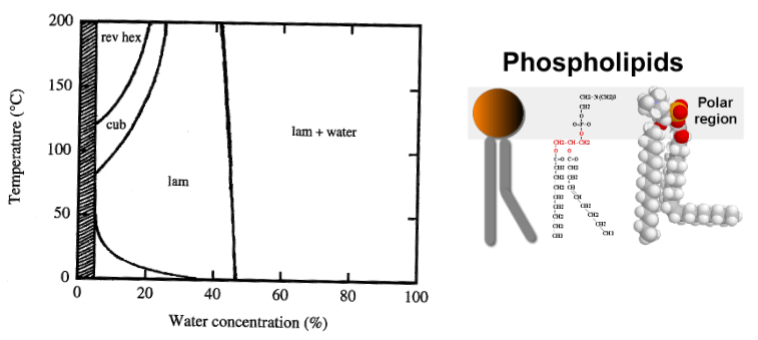
Lamellar phase and vesicles (liposomes)
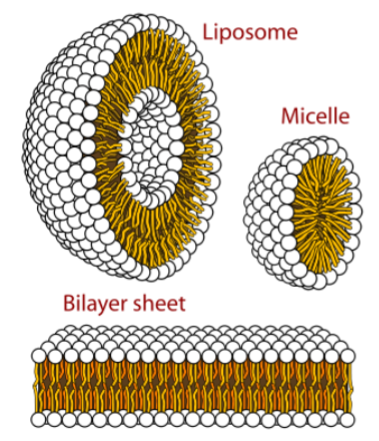
Examples of liposomes
Polymer prevents unwanted interaction
Ligands used to direct the drug
Examples of self-assembled surfactant (polar lipid) structures for drug delivery
RNA-based vaccines for COVID-19
Highly schematic
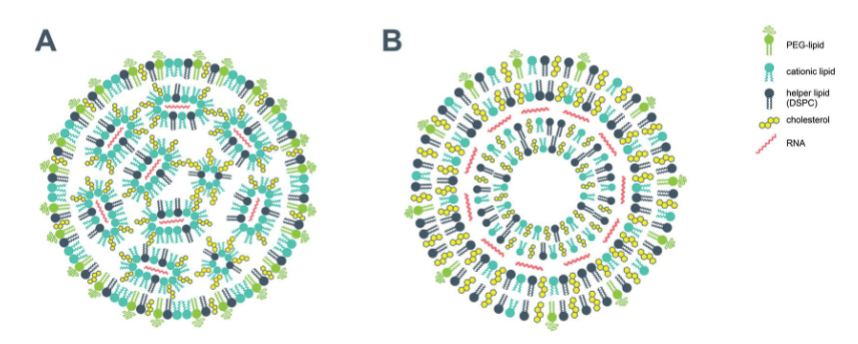
B) Cationic surfactant: it will not be charged in tissue, charged and de-charged
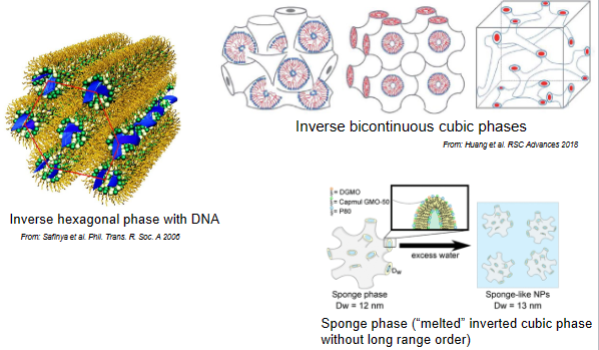
Examples of self-assembled inverse architectures for encapsulation/drug delivery
Encapsulating for example enzyme
Sponge phase: channels are not straight, instead curvy. For example slow release
Ternary phase diagram- Three component system.
- Every corner represents a pure condition
- Each side of the triangle represents all possible binary combinations of the three components. On any of those sides, the fraction of the third component is zero (0%).
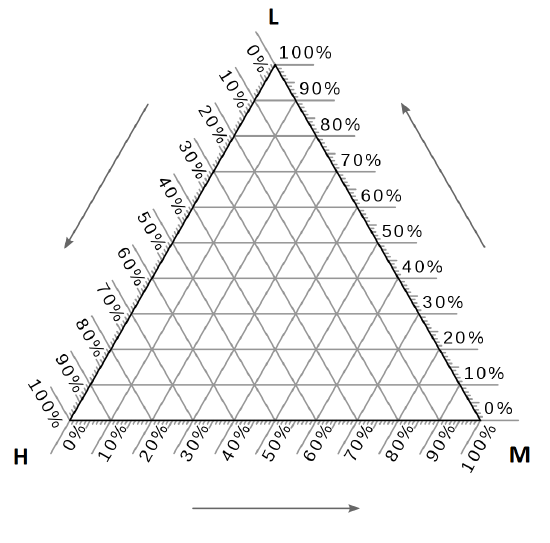
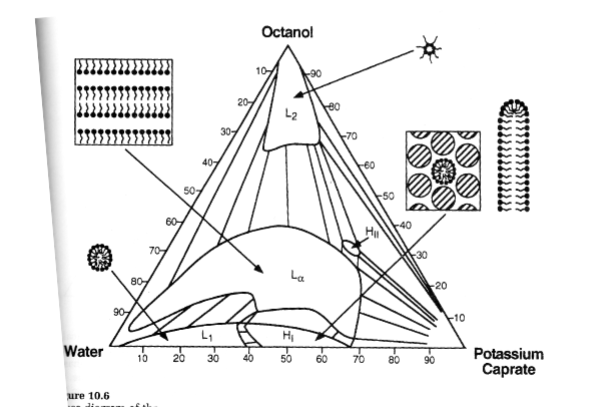
Ternary phase diagrams for surfactants
L1=miceller phase
Lα=lameller phase
Octanol is the corresponding alcohol to surfactant (potassium caprate)
Non-ionic ethoxylated surfactants- Increasing temperature leads to two phases. This is an exception.
Cloud point temperature at which a surfactant solution leads to a two phase transition, typically from a clear solution to a cloudy state due to phase separation (the point where precipitation starts).
- Functionality lost above cloud point (solubility is lost)
- Balancing of hydrophobic effects and head-group hydration (temperture might influence orientation of hydrophobic chain → making it less hydrophobic)
- Very low Krafft point (not relevant for applications) - we consider it not be have a Krafft point
Applications of anionic surfactants- Used in soaps, detergents and as stabilizers.
- Form stable films (good foaming agents)
- Sensitive to high ionic strengths (since they are charged)
- Sulphate/Sulphonate surfactants are less sensitive to Ca2+ than the fatty acids are. If we have hard water it will be less sufficient, forming an insoluble salt of calcium ions and two surfactants
- However, irritant to the skin.
- ”Bath tub ring”
Applications of cationic surfactants-
- Cationic surfactants interact strongly with biological membranes → toxic.
– Pesticides and bactericides
- Mineral ore flotation
- Asphalt – (positive charged bitumen emulsions and negative gravel)
- Hair conditioners – reducing static electricity and frizziness (hair is negatively charged → frizzy)
- Anti-caking agents – preventing liquid bridges in powders through water-wetting prevention (make cationic → hydrophobic)
- Anti-corrosion agents – preventing water wetting of steel surfaces
- Drug delivery/gene therapy – form complexes with anionic macromolecules
Applications of nonionic surfactants- As stabilizers in many applications
- Less sensitive to high ionic strength and pH
- Less efficient at high T
- Often efficient at lower T (compared to anionic surfactants)
- Form less rigid films than ionic surfactants (lack of electrostatic repulsion)
- Useful for low foaming applications.
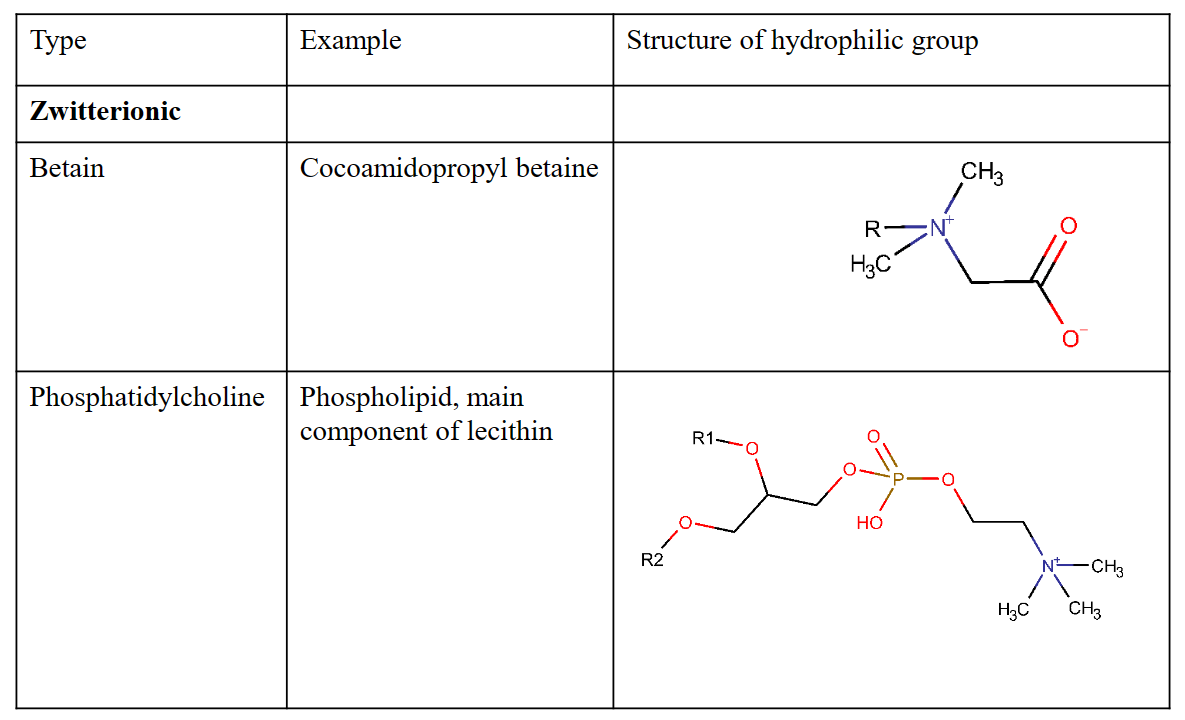
Applications of zwitterionic surfactants
– Betain
• Low irritant on skin
• Personal-care products
– Schampoos
– Phospholipids – ”lecithin”
• Biological material
• Food and Pharmaceutical applications
Environmental issues
• Came to attention in the 1960s and 1970s.
• Some surfactants are slowly degraded.
• High toxicity for aquatic organisms.
– Ex. ethoxylated nonyl and octyl phenols (Triton).
– To decrease toxicity the apolar chain was changed to linear.
Aerosol OT ( try to remove release of oil in sea) → sank to the bottom of sea
• Increase biodegradability:
– Introduction of cleavable surfactants (ester linkage etc.) between head and tail- tend to hydrolysis (not so stable)
• Increased interest in surfactants from renewable resources.
– Alkyl glucosides to substitute ethoxylated surfactants