Sterility Preservatives
Sterility of and Preservatives in Ocular Drugs
Lecture Overview
Key Topics: Manufacture and Sterility, Preservatives, D Value, Classes, Examples, Effects on Ocular Surface, Other Issues
Learning Outcomes:
Understanding the sterile conditions required to manufacture eye drops and the potential impact of contamination.
Comprehension of preservatives used in ophthalmic products and associated risks.
Manufacture of Ophthalmic Drugs
Sterile Manufacturing Procedures:
HVAC System: Maintains temperature, controls airborne particles using HEPA filters, manages room pressure, and limits humidity.
Sterilised Containers: Used in aseptic environments, applying aseptic filling and capping methods.
Sterility of Ophthalmic Solutions
Importance of Sterility: Prevents the introduction of pathogens to the eye.
Production Methods:
Autoclaving: Uses pressure at 121°C for 15 minutes to destroy microorganisms but may decompose some drugs.
Filtration: Aseptically filters using porous membranes (~0.22µm) to prevent bacteria (~ 0.5 um) passage but does not filter viruses.
Tests of Sterility
Conduct under aseptic conditions to prevent contamination.
Culture Media Preparation:
Fluid thioglycollate for anaerobic bacteria; detects aerobic bacteria.
Soya-bean casein digest medium for fungi and aerobic bacteria.
Testing Procedure:
Transfer contents to be tested, filter, and transfer to culture medium followed by 14 days of incubation.
Check for microbial growth; lack of growth indicates compliance.
Importance of Drug Sterility
Case Study: Framingham pharmacy linked to 377 fungal infections and 28 deaths due to poorly prepared steroid injections.
Recalls and Issues:
Ameridose LLC recalls products due to sterility testing failures, contamination risks, and shipping issues before sterility results are available.
Recalls of Ophthalmic Drugs
Notable Recalls:
Zovirax Ointment: Recalled Oct 2014 due to metal particles, risk of eye damage, causing product shortage.
Diamox Tablets: Recalled Feb 2016 for potential fungal contamination.
Requirement of Preservatives in Multi-Use Preparations
Essential to maintain sterility post-opening.
Risks of Preservatives:
Can cause corneal toxicity and allergic reactions, disrupting the tear film.
Issues more common in patients with pre-existing tear film anomalies or prolonged use.
Problems typically resolve after stopping treatment or switching to preservative-free products.
Ideal Criteria of Preservatives
Non-irritating yet effective.
Non-sensitising
possess anti-bacterial and anti-fungal properties.
render sterility over a reasonable period of time
chemical stability.
Should not interfere with primary ingredient activity.
Categories of Preservatives
Bacteriostatic: Inhibits bacterial growth.
Bactericidal: Destroys bacteria.
Fungistatic: Inhibits fungal growth.
Fungicidal: Destroys fungi.
Typically, preservatives used in ocular tissues show bacteriostatic properties.
Rate of Destruction of Bacteria
Factors influencing effectiveness include:
Nature of preservative, organism type, dispersion degree, organic matter amount, temperature, pH, viscosity agents, and solution age.
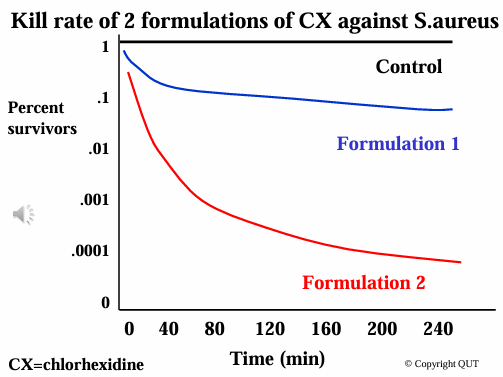
D-value
Ability of a preservative to kill organisms
Defines the time to decrease microorganism counts by 90% (1 log unit).
A lower D-value indicates a stronger antimicrobial effect, with bacteria being killed more rapidly than fungi.
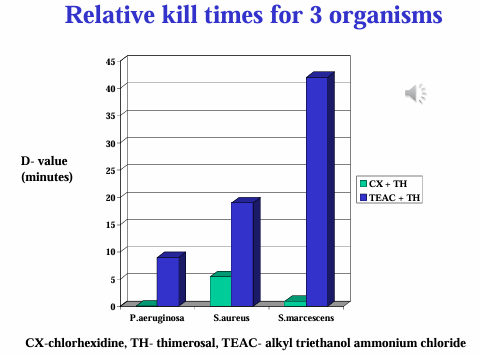
Types of Preservatives in Ocular Drugs
Surfactants:
Detergents and ionically charged molecules that alter interface relationships.
Disrupt plasma membranes and improve drug penetration.
Usually have bactericidal properties.
They have a "toxicity" effect, disturbing tears and corneal health.
Can be inactivated by organic matter but are stable.
Examples: CX, BAK.
Chemical Toxins:
Block normal metabolic processes of cells.
Act non-selectively on all cells and may cause allergic reactions.
Do not affect tear production.
Examples: Thimerosal (TH), iodine, alcohols.
Oxidative Preservatives:
Penetrate cell membranes/walls and interfere with essential cellular functions.
Example: Hydrogen peroxide.
Common Preservatives
Benzalkonium Chloride: Effective but can disrupt tear film.
Chlorhexidine: Effective against various bacteria; less corneal toxicity compared to BAK.
Thimerosal: Banned in many contexts due to mercury concerns; can cause hypersensitivity.
Sodium Perborate: Converts to hydrogen peroxide in the eye; utilized in lubricant eye drops.
SofZia: Reduces epithelial cell damage in glaucoma medications.
EDTA: Chelating agent that enhances preservative action; low toxicity.
Benzalkonium Chloride (BKC/BAK/BAC)
Type: Cationic detergent that acts on membrane permeability, extremely effective.
Combination: Often combined with EDTA.
Functionality: Antibacterial and antifungal properties.
Applications: Used for preservation of eye drops, instrument sterilization, cleaning of skin, and improving corneal penetration of drugs.
Stability: Stable with a long shelf life.
Concentration: Typical concentration is 0.01%, with a range of 0.004% to 0.02% found in glaucoma medications.
Side Effects: Disrupts tear film and can cause corneal toxicity.
Usage Restriction: Not for intraocular use.
Chlorhexidine
Type: Disinfectant used in skin/hand cleaners (e.g., Hibitane 5%) and eye drops (0.02%) - may have a role in treating acanthamoeba keratitis.
Surfactant Properties: The positively charged chlorhexidine molecule binds to the negatively charged bacterial cell wall, rupturing the cell membranes.
Effectiveness: Effective against bacteria (both gram-positive and gram-negative) and yeasts (broad spectrum).
Compatibility: Not used in conjunction with sulfates of atropine or neomycin (precipitates).
Corneal Effects: Less corneal epithelial effect than BAK.
Sensitivity: Some individuals may develop sensitivity to chlorhexidine.
Oral Use Caution: In some mouthwash formulations; if used too often, may damage the mouth’s healthy bacteria.
Cetrimide
Type: Antiseptic.
Combination: Chlorhexidine increases effectiveness (e.g., SAVLON contains both cetrimide and chlorhexidine).
Usage: Popular in the UK, with concentrations ranging from 0.005% to 0.02% for eye drops.
Side Effects: After prolonged application, may induce toxic epitheliopathy.

Polyquaternium-1 (Polyquad)
Type: Detergent-type preservative derived from BAK.
Composition: Polymeric quaternary ammonium antimicrobial preservative.
Mechanism: Bacterial cells attract Polyquad, yet human corneal epithelial cells tend to repel the compound, leading to less toxicity.
Effect on Goblet Cells: Can decrease numbers of conjunctival goblet cells and affect mucous production in tears.
Contact Lenses: Does not concentrate in contact lenses.

Chlorobutanol
Type: Alcohol-based chemical toxin formed by simple nucleophilic addition of chloroform and acetone.
Action: Acts via cell lysis, disrupting microbial cell membrane lipid configuration.
Effectiveness: Bacteriostatic (effective against gram-positive and gram-negative bacteria) and fungistatic.
Concentration: Typically used in concentrations ranging from 0.03% to 0.5%.
Combination: Enhances actions when combined with BAK and also used with EDTA.
Irritation: Relatively non-irritating compared to BAK.
Usage Restriction: Not for use with soft contact lenses.
Stability: A volatile compound that can diffuse through plastic bottles during prolonged storage and is unstable at room temperature.
Thimerosal (Thiomersal)
Type: Organomercury compound (chemical toxin); was in many multidose vaccines but recently banned to fulfill the aim of reducing mercury exposure (replaced by single dose vials in developed countries).
Popularity: Once very popular; 0.005% concentration in all contact lens solutions.
Allergic Reactions: Can cause red, irritated eyes with epithelial changes, photophobia, and lacrimation.
Hypersensitivity: 25 to 50% of people develop a delayed hypersensitivity follicular reaction – increases IgG tear antibodies.
Effectiveness: Not as effective as many other preservatives, such as chlorhexidine and chlorobutanol.
Stabilised Oxychloro Complex (Purite)
Type: Oxidative preservative (chloride/oxygen compound).
Decomposition: Converts to water & NaCl, neutralized by human cells, doesn’t accumulate.
Effectiveness: Effective against microorganisms – even at low concentrations (0.005%).
Toxicity: Reduced toxicity, improved tolerability profile; well tolerated when administered frequently.
Applications: Found in Alphagan P (brimonidine tartrate for glaucoma).
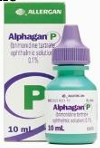
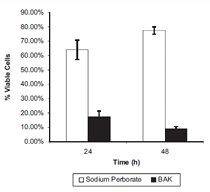
Sodium Perborate
Type: Oxidative preservative (0.005% - 0.01%).
Conversion: Converts to hydrogen peroxide when combined with water.
Decomposition: Decomposed to water and oxygen in the eye.
Effectiveness: Broad spectrum activity against bacteria and Aspergillus (fungus).
Applications: Found in Genteal lubricant eye drops.
SofZia
Type: Unique ionic buffer containing borate, sorbitol, propylene glycol, and zinc.
Mechanism: Inactivated by cations in the tear film; results in less ocular surface cytotoxicity.
Applications: Used in travoprost (Travatan Z, Alcon, glaucoma medication; earlier formulation contained BAK).
Benefits: Switching from a glaucoma medication with BAK to one with SofZia results in reduced SPK (less corneal epithelial cell damage).
The rapid and complete reduction of all microbial challenges demonstrates that antimicrobial activity of latanoprost with 0.02% BAK exceeds that of travoprost with SofZia preservative system
Sorbate (Sorbic Acid)
Limited Anti-Microbial Activity: Exhibits weak antimicrobial properties.
Mechanism of Action: Inhibits growth of cells primarily through acidification.
Chemical Reactions: Can react with other compounds to form derivatives that possess enhanced preservative effects.
Energy Utilization: Causes waste of cellular energy stores.
Safety Profile: Often promoted as safe for sensitive eyes and contact lens wearers due to its low toxicity.
Additional Uses: Also utilized as a food preservative.
EDTA (Edetate Disodium)
Chelating Agent: Binds metals which aids preservative action and can treat calcium deposits in the eye.
Preservative Enhancement: Assists the action of preservatives such as thimerosal and BAK.
Toxicity: Low-toxicity but also aids in detoxification.
Common Applications: Found in many glaucoma medications like Acular and Betagan.
Additional Uses: Also present in conditioners, cleansers, moisturizers, aftershaves, deodorants, and mouthwashes.
Water Softening: Softens water.
Side Effects: Can cause contact dermatitis.
 Knowt
Knowt