CHEM QUESTIONS EOY YEAR 3
Chemistry Y3 EOY
Acids, Bases, & Salts, Stoichiometry, Chemical Kinetics, Chemical Energetics, Redox, Metals
Acids, Bases, and Salts
Acids
Q1. Define ‘acid’ and state 3 physical properties. [2]
A proton donor
Acids are sour, have a pH less than 7, and turn blue litmus paper red
Q2. Explain the difference between strong and weak acids (without tests). [2]
A strong acid completely ionises in water while a weak acid partially ionises in water. A strong acid has a greater concentration of ions than a weak acid.
Q3. Explain the difference between monoprotic and diprotic acids. [2]
Monoprotic acids donate one proton per molecule in water.
Diprotic acids donate two protons per molecule in water.
Bases & Alkalis
Q4. Define ‘base’. [1]
A metal hydroxide or a metal oxide that reacts with an acid to form a salt and water only
Q5. Define ‘alkali’ and state 3 physical properties. [2]
A soluble base which dissolves to produce hydroxide ions, , in an aqueous solution.
Alkalis are bitter, have a pH of more than 7, and turn red litmus paper blue.
pH Scale
Q6. State 3 ways to measure the pH of a solution. [1]
Indicators such as methyl orange or universal indicator. A pH probe attached to a datalogger. A pH meter
Oxides
Q7. Define ‘oxide’. [1]
A compound of oxygen and another element.
Q8. Explain the 4 types of oxides. [4]
Basic oxides react with acids to form salt and water only.
Amphoteric oxides react with both acids and bases to form salt and water only.
Acidic oxides react with bases to form salt and water only.
Neutral oxides do not have acidic or basic properties.
Q9. State how to identify acidic and basic oxides. [2]
Non-metals form acidic oxides.
Metals in Groups 1 and 2 tend to form basic oxides.
Q10. Give 3 examples of amphoteric oxides and 2 examples of neutral oxides. [2]
Zinc Oxide, Aluminum Oxide, Lead (II) Oxide.
Carbon Monoxide, Nitrogen Monoxide
Salts
Q11. Define ‘salt’. [1]
Ionic compound formed by reacting acids with metals, carbonates, bases or alkalis.
Q12. Draw the solubility table of salts with their exceptions. [3]
Soluble Salts Exceptions |
Sodium, Potassium, Ammonium - |
Nitrates - |
Halides (F, Cl, Br, I) halides, halides |
Sulfates , , (sparingly soluble) |
Insoluble Salts Exceptions |
Carbonates, Phosphates, Oxides, Hydroxides Group 1 salts, Nitrates, (slightly soluble) |
Ionic Equations
Q13. Define ‘spectator ions’. [1]
Aqueous ions that do not take part in the chemical reaction.
Stoichiometric Relationships (Mole Concepts)
Relative Masses
Q24. Define ‘relative atomic mass (Ar)’. [1]
The weighted average mass of one atom of an element compared to 1/12 the mass of a carbon-12 atom.
Q25. Define ‘relative molecular mass (Mr)’. [1]
The weighted average mass of one molecule of the substance compared to 1/12 the mass of a carbon-12 atom.
Q26. Define ‘relative formula mass (Mr)’. [1]
The mass of one formula unit of an ionic compound compared to 1/12 the mass of a carbon-12 atom.
The Mole
Q27. Define ‘mole’. [1]
The unit of measurement for very small particles such as atoms, molecules, ions, and electrons.
Molar Mass of elements and substances
Q28. Define ‘molar mass’. [1]
The mass of one mole of a substance, and has the same value as relative molar mass.
Molar Volume of Gases
Q29. State the conditions at STP. [1]
One mole of any gas always occupies a volume of 22.7 at STP at 273K and 100kPa.
Concentration of a Solution
Q30. Define ‘concentration of a solution’. [1]
The amount of solute in of a solution
Empirical & Molecular Formula
Q31. Define ‘empirical’ and ‘molecular’ formulas. [2]
The simplest ratio of the atoms of different elements present in one molecule
A simple multiple of the empirical formula and shows the exact number of atoms of different elements in one molecule.
Q32. An acid contains 40.0% carbon, 6.67% hydrogen, and 53.3% oxygen. If the relative molecular mass of the acid is 60, deduce the empirical and molecular formula. [3]
Element C H O |
Mass in 100g / g 40.0 6.67 53.3 |
Molar mass / g 12 1 16 |
Amount / mol 3.33 6.67 3.33 |
Mole ratio 1 2 1 |
∴ The empirical formula is
Let the molecular formula be
of = 60
= 60
n = 2
∴ The molecular formula is
Limiting Reagent
Q33. Define ‘limiting reagent’. [1]
The reactant that is completely used up when the reaction is completed.
Q34. 6.50g of Zn are added to 50.0 of 0.450 . Find the volume of produced at STP. [2]
x
Since
Hence, is in excess, and is the limiting reagent
Since
x
x
Chemical Kinetics
Collision Theory
Q35. State the collision theory. [2]
The rate of a chemical reaction is directly proportional to the frequency of effective collisions.
Measuring Rate of Reaction
Q36. Draw a set-up to measure the change in mass of a reaction. [3]
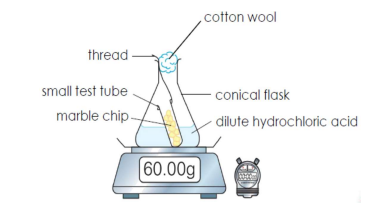
Q37. State and explain when the rate of reaction is the fastest, decreases, and zero. [3]
It is the fastest in the beginning because the concentration of the reactants is the greatest.
It decreases as the reaction progresses because the concentration of reactants decreases.
It is zero when the limiting reactant is used up.
Q38. Draw a set-up to measure the change in volume of a reaction. [3]

Factors Affecting Rate of Reaction
Q39. Explain how concentration affects the rate of reaction. [2]
When the concentration of solutions increases, the number of reactant particles per unit volume increases, increasing the frequency of effective collisions between the reactant particles. Hence, the rate of reaction increases.
Q40. Explain how pressure affects the rate of reaction. [2]
As the pressure of a gas increases, reactant gas particles are forced closer, increasing the number of gaseous particles per unit volume, increasing the frequency of effective collisions between the gaseous particles. Hence, the rate of reaction increases.
Q41. Explain how the size of particles affects the rate of reaction. [2]
As particle size decreases, there is a greater total exposed surface area for reacting particles to collide increasing the frequency of effective collisions between reactant particles. Hence, the rate of reaction increases.
Q42. Explain how temperature affects the rate of reaction. [2]
As temperature increases, particles gain kinetic energy and move faster. There will be a higher proportion of particles having energy greater than or equal to activation energy, increasing the frequency of effective collisions between particles. Hence, the rate of reaction increases.
Q43. Define ‘catalyst’ and explain how the presence of one affects the rate of reaction. [3]
A catalyst is a substance which increases the rate of reaction of a chemical reaction itself remaining chemically unchanged at the end of the reaction and provides an alternative reaction pathway of lower activation energy.
There will be a greater proportion of particles having energy greater than or equal to activation energy, increasing the frequency of effective collisions between the reactant particles. Hence, the rate of reaction increases.
Chemical Energetics
Enthalpy Change in a Reaction
Q44. Define ‘enthalpy’ and ‘enthalpy change’. [2]
Energy content that is stored in a substance.
The difference in energy content of the reactants and products.
Exothermic Reactions
Q45. Define ‘exothermic’ and draw a temperature-time graph for it. [3]
A reaction in which heat is released to the surroundings
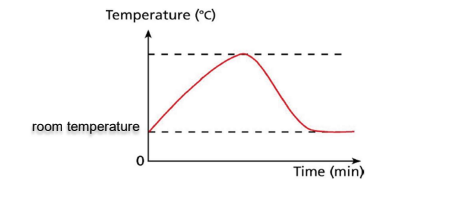
Q46. Draw and explain an exothermic energy level diagram. [3]

Endothermic Reactions
Q47. Define ‘endothermic’ and draw a temperature-time graph for it. [3]
A reaction in which heat is absorbed from the surroundings
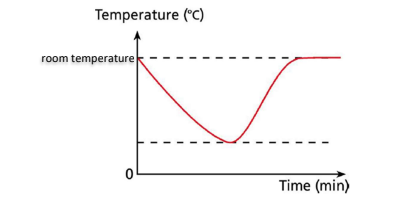
Q48. Draw and explain an endothermic energy level diagram. [3]

Bond Breaking & Forming
Q49. State the relationship between the type of reactions and the energy released. [2]
Exothermic: More energy is released in forming bonds than is absorbed in breaking bonds.
Endothermic: More energy is absorbed in breaking bonds than is released in forming bonds.
Bond Energy or Enthalpy
Q50. Define ‘bond enthalpy’. [1]
The average amount of heat absorbed to break one mole of that particular bond in a particular compound in the gaseous state.
Q51. Given that one H-H bond, Cl-Cl bond, and H-Cl bond have the bond enthalpy of 436, 242, and 431 kJ/mol respectively, calculate the total enthalpy change for if it is an exothermic process. [2]
436 + 242 = 678
2(431)=862
678 - 862= -184
kJ/mol
Progress of Reaction
Q52. Define ‘activation energy’. [1]
The minimum energy that is needed by the reactant particles for a chemical reaction to occur.
Energy Profile Diagram
Q53. Define ‘energy profile diagram’. [1]
Represents the energy change involved during a reaction, including the activation energy.
Q54. Draw an energy profile diagram for an exothermic and endothermic reaction. [6]
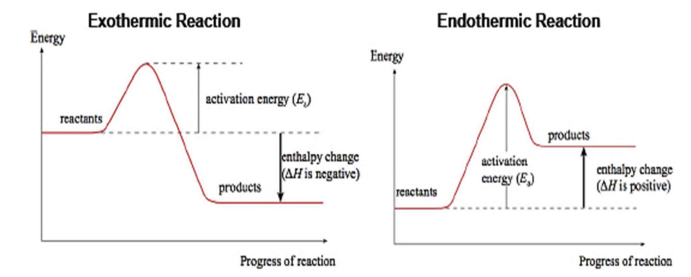
Redox
Oxidation & Reduction
Q55. Define ‘redox reaction’. [2]
A chemical reaction involving both oxidation and reduction
Q56. Given , explain and identify the substance that is being oxidised and reduced in terms of oxygen. [2]
is oxidised as it gains oxygen to form
is reduced as it loses oxygen to form
Q57. Given , explain and identify the substance that is being oxidised in terms of hydrogen. [1]
is oxidised as it loses hydrogen to form
Q58. Given , explain and identify the substance that is being oxidised and reduced in terms of electron transfer. [2]
is oxidised as it loses electrons to form
is reduced as it gains electrons to form
Q59. Define ‘oxidation state’. [1]
A number that is assigned to an element in a substance to show its degree of oxidation
Q60. Given , explain and identify the substance that is being oxidised and reduced in terms of oxidation state. [2]
is oxidised as the oxidation state of increased from +4 in to +6 in
is reduced as the oxidation state of decreased from +7 in to +2 in
Oxidising & Reducing Agents
Q61. Given , identify the oxidising and reducing agent and explain in terms of oxidation and reduction. [2]
is the oxidising agent. oxidises as the oxidation state of increases from -1 in to 0 in .
itself is reduced as the oxidation state of chlorine decreases from 0 in to -1 in
is the reducing agent. reduces as the oxidation state of decreases from 0 in to -1 in
itself is oxidised as the oxidation state of increases from -1 in to 0 in
Q62. Fill in the observations for the following common oxidising agents. [3]
Oxidising Agents Observations |
Purple to colourless |
Orange to green |
Remains colourless |
Greenish-yellow to colourless |
Brown to colourless |
Yellow to light green |
Q63. Fill in the observations for the following common reducing agents. [3]
Reducing Agents Observations |
Colourless to brown |
Effervescence of colourless, odourless gas which relights glowing splint (oxygen) |
Remains colourless |
Light green to yellow |
Remains colourless |
Metals
Structure of Metals
Q64. Explain the structure of metals. [2]
All metals have giant metallic lattice structures with strong electrostatic forces of attraction between the layers of metal cations and the sea of delocalised and mobile electrons.
Physical Properties of Metals
Q65. State 5 and explain the physical properties of metals. [4]
Good conductors of heat and electricity. The sea of delocalised electrons acts as mobile charge carriers and conducts heat.
High melting points and boiling points. The strong electrostatic forces of attraction require a large amount of energy to overcome.
High densities. Metal ions are closely packed in the solid state.
Malleable & Ductile. Atoms are orderly arranged in layers and these layers of atoms can slide over each other easily when a force is applied.
Alloys
Q66. Define ‘alloy’. [1]
A mixture of a metal with other elements.
Q67. Explain why alloys are stronger than metals. [2]
Alloys are made up of atoms of different sizes. This disrupts the orderly arrangement of atoms, making it hard for the layers of atoms to slide over one another when a force is applied.
Reactivity Series
Q68. Draw the reactivity series table for the reaction with cold water, steam, and dilute hydrochloric acid. Include the magnitude of the reaction, explanations, and colour change (if any) [8]
1: darts around and sizzles
2: Effervescence of colourless, odourless gas that extinguishes lighted splint with a ‘pop’ sound.
Metal With cold water, With steam, With dilute |
Potassium, K Very Violently Potassium 1, 2 Explosively Explosively, 2 |
Sodium, Na Violently Sodium 1, 2 |
Violently, 2 |
Calcium, Ca Readily, 2 |
Magnesium, Mg Very Slowly, 2 Violently Bright white glow produced. Grey solid turns white Rapidly, 2 |
Aluminium, No visible reaction |
Carbon, C Reference point (non-metal) |
Zinc, Zn No Reaction Readily Hot grey Zn forms a yellow solid that turns white when cooled Moderately Fast, 2 |
Iron, Fe Slowly Grey Fe turns red before forming a black solid Slowly, 2 |
Lead, Pb No Reaction No Reaction |
Hydrogen, H Reference point (non-metal) |
Copper, Cu No Reaction No Reaction No Reaction |
Silver, Ag |
Gold, Au |
Displacement Reaction
Q69. Explain what happens when copper is added to silver nitrate solution. [2]
Colourless solution turns blue, and the silvery solid is deposited. Copper is more reactive than silver and so it loses electrons more readily. Hence, copper will displace silver from the silver nitrate solution to form copper (II) nitrate and silver.
Action of Heat on Carbonates
Q70. Draw the action of heat on carbonates of the reactivity series. [6]
Metal Carbonate Observation |
Do not decompose on heating |
Decomposes into metal oxide and carbon dioxide on heating |
Decomposes into silver and carbon dioxide on heating |
Reduction of Metal Oxides
Q71. Explain the reaction between a metal and the oxide of another metal. [2]
A more reactive metal can reduce a less reactive metal from its oxide.
Q72. Draw the diagram of reduction with carbon and hydrogen. [6]
Carbon Hydrogen
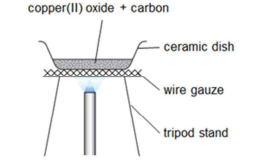
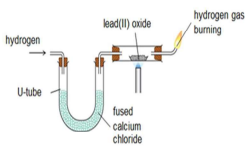
Q73. Draw a table for the reduction of metal oxides with carbon and hydrogen. [4]
Metal Oxide Reduction with Carbon |
Cannot be reduced by carbon |
Reduced by carbon to form metal + carbon dioxide |
Oxide can be decomposed by heating alone without any reducing agent. |
Metal Oxide Reduction with Hydrogen |
Cannot be reduced by Hydrogen |
Reduced by hydrogen to form metal + steam |
Oxide can be decomposed by heating alone without any reducing agent. |
Extraction of Metals
Q74. Draw a table for the methods of extraction of the reactivity series. [4]
Metal Method of Extraction |
K, Na, Ca, Mg, Electrolysis |
C Metals below can be extracted by reduction with C |
Zn, Fe, Pb, Cu, Ag Reduction of metal oxides with carbon (coke) |
Au Exists as pure gold |
Application of Steel
Q75. State the carbon composition, properties, and uses for the 3 types of steel. [3]
Mild Steel: 0.25% Carbon. Malleable but softer than high-carbon steel. Car bodies and machinery
High Carbon Steel: 0.45-1.5% Carbon. Stronger but brittle. Knives and hammers.
Stainless Steel: Iron, carbon, chromium, and nickel. Durable, and highly resistant to corrosion. Cutlery and surgical instruments
Rusting of Iron
Q76. Define ‘rusting’. [1]
The gradual oxidation of iron to form hydrated iron (III) oxide.
Q77. Explain 2 methods of preventing rust. [4]
Use a protective layer. Provides a barrier for iron or steel and prevents it from coming in contact with oxygen and water.
Sacrifices Protection. Uses a more reactive metal to protect a less reactive metal
Recycling of Metals
Q78. State 2 advantages and disadvantages of recycling metals. [4]
Prevents pollution of land and water from the extraction of metals by mining. Conserve fossil fuels used during mining to extract the metal from its ores.
Recycling can be costly due to the processing from the collection, transportation, cleaning, and melting of the scrap metals.
Harmful gases may be produced in the melting of scrap metals during recycling causing air pollution.