1.2 Chemistry - Shorthand Electron Configuration
Introduction
While the electron configuration of elements is typically expressed through forms such as the main shell configuration and subshell configuration, there exists another form - shorthand electron configuration
main shell configuration
- represents the number of electrons in each energy level, or main shell of an atom.
- follows the pattern (2, 8, 8, 2), with the names of (k, l, m, n) respectively.
- once the fourth main shell is full with 2 electrons, electrons flow back into the third main shell until it reaches 18, then back to the fourth main shell until it reaches 18, etc. It eventually reaches 32.
- thus, main shell electron configuration satisfies the formula 2n^2
subshell configuration
- represents the number of electrons in each orbital, or subshell of an atom.
- subshells are located within the main shells; they are essentially different arrangements of electrons in the main shell.
- each subshell is given a name that designates the amount of electrons that it can hold; (s, p, d, f).
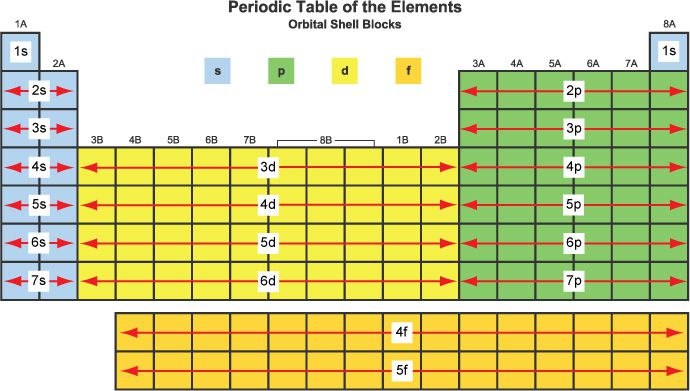
- s subshells can hold a maximum of 2 electrons
- p subshells can hold a maximum of 6 electrons
- d subshells can hold a maximum of 10 electrons
- f subshells can hold a maximum of 14 electrons
- The above chart is incredibly helpful in helping you identify what the last subshell in the subshell notation will be.
- the ‘prefix’ of the number (e.g 1s^2, 2p^6) represent the main energy level/main shell that the subshell is a part of. The greatest ‘prefix’ correlates to the period, or row that the element can be found in.
- the ‘index’ of the number (e.g 1s^2, 2p^6) represent the amount of electrons in that subshell. electrons cannot exceed the maximum amount listed above, but they can be less.
- note that if you add the subshells that begin with the same main shell #, it adds up to the amount of electrons in that main shell.
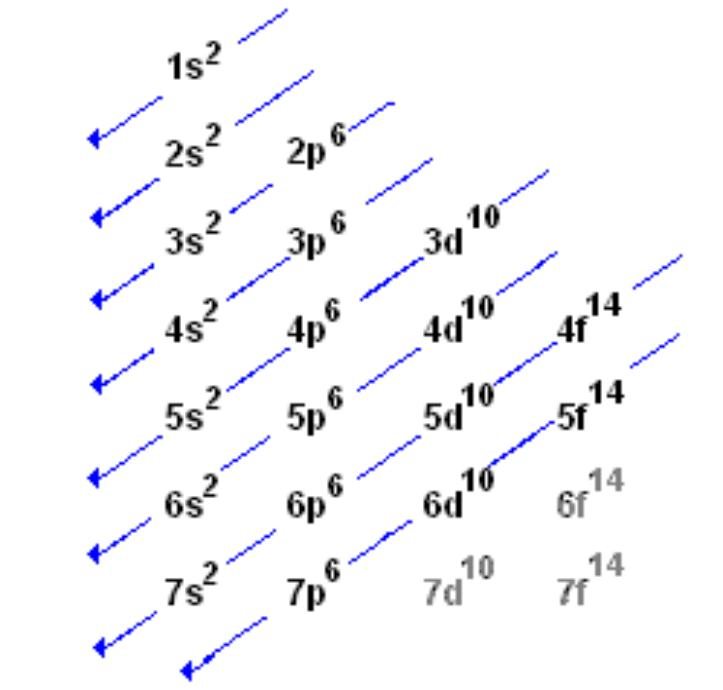
- the arrows represent which way you’re meant to go when writing the configuration of an element using subshell notation.
- for example, an element such as Fluorine (atomic number 9) can be written as:
- starting from the very top, we have 1s^2.
- we continue down the arrows to 2s^2 , 2p^6
- because 2+2+6 = 10:: 2p^6, the final subshell of Fluorine, can be written as 2p^5 instead.
- this gets complicated with elements as their electron counts increase - here’s an example with Tin (atomic number 50)
- 1s^2, 2s^2, 2p^6, 3s^2, 3p^6, 4s^2, 3d^10, 4p^6, 5s^2, 4d^10, 5p^2
Shorthand Electron Configuration
Introducing, shorthand electron configuration! This style follows up with basic subshell configuration but simplifies the process. SEC makes reference to the previous noble gas - group 8 elements, considering that they all have full outer shells.
Because of this, the subshell configuration can start on a new row and thus can begin with an ‘s’ subshell, which holds 2 electrons. Let’s use the same example of Tin as previously.
- The previous noble gas from Tin (atomic number 50) is Krypton (atomic number 36)
- We can now effectively remove all the subshell notation that makes up Krypton, and just replace it with something like this - [Kr]
- We know, after Krypton, the next value begins with an ‘s’ subshell. We also know that the prefix correlates to the period/row in which Tin is found, which is period 5. Therefore, we can append 5s^2 onto [Kr], making [Kr] 5s^2.
- All we need to do next is to follow the chart above until we reach the required amount of electrons to make up Tin. Since we already have 36 of them, there are only 14 more needed.
- [Kr] 5s^2 4d^10 5p^2 - the bolded numbers add to 14, which is the amount of electrons needed beginning from Tin, the nearest noble gas. Some people like to rearrange them to be in numerical order, which isn’t necessary, but does help people understand how subshells link with main shell configuration.
Practise
Polonium
- Polonium is #84. The nearest noble gas is Xenon, which is #54. The difference between the two is 30.
- Polonium is in period 6.
- [Xe] 6s^2, 4f^14, 5d^10, 6p^4
- [Xe] 4f^14, 5d^10, 6s^2, 6p^4 [rearranged numerically]
Yttrium
Nickel