AP Biology Unit 3: Cellular Energetics
3.1 & 3.2 Enzyme Structure and Catalysis
Enzyme Structure and Catalysis in Metabolism:
Introduction to Metabolism:
Definition: Metabolism encompasses all the chemical reactions that occur within an organism.
Energy and Macromolecule Requirements: Organisms constantly require energy and macromolecules to sustain their metabolic processes
Types of Metabolic Pathways:
Catabolic Pathways: these pathways break down complex molecules into simpler components, releasing energy in the process.
Anabolic Pathways: Conversely, these pathways use energy to synthesize complex molecules from simpler ones.
Roles of Enzymes in Metabolism:
Function as catalysts: enzymes are critical as they act as catalysts in metabolic pathways, significantly speeding up chemical reactions without being consumed.
Specific Example: The enzyme lactase catalyzes the hydrolysis of lactose into glucose and galactose.
Chemical Reaction Dynamics:
Bond Dynamics: all chemical reactions involve the breaking and forming of bonds, processes that inherently require energy
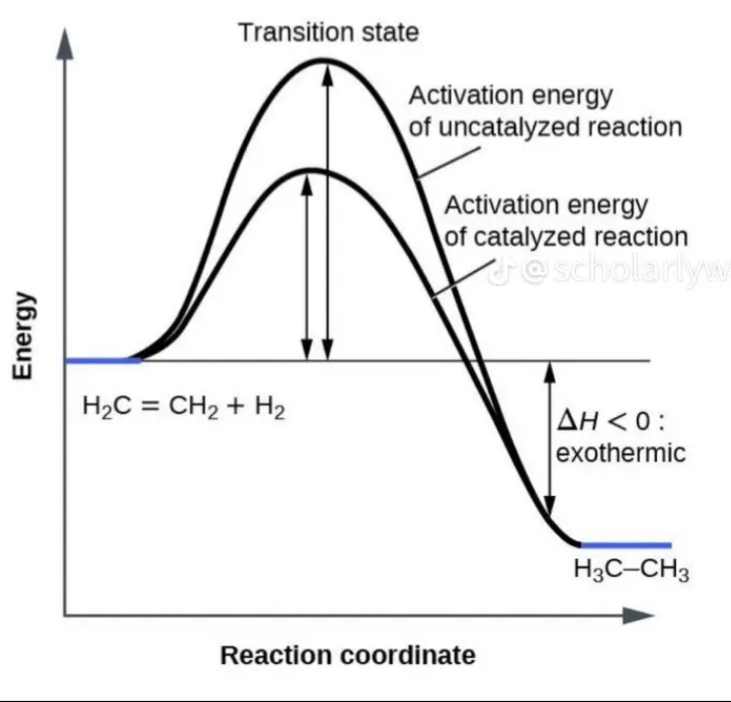
Activation Energy (EA):
Definition: activation energy is the initial energy needed to start a chemical reaction—akin to pushing a ball uphill before it can roll down
Enzyme Activity: enzymes function by lowering the activation energy required for a reaction, making it easier for the reaction to occur at a faster
Enzymatic Catalysis:
Efficiency: by reducing the activation energy, enzymes allow reactions to proceed more rapidly and at lower energy costs
Reaction Mechanism: Enzymes provide a platform where reactants can come together in an optimal orientation, which facilitates the formation of transition states and thereby lowers the activation energy needed.
Specificity and Mechanism of Enzyme Action
Enzyme Specificity:
Definition: Enzymes are highly specific catalysts, meaning they typically catalyze only one specific type of chemical reaction.
Example: Lactase is an enzyme that specifically catalyzes the breakdown of lactose into galactose and glucose.
Enzyme and Substrate Interaction:
Substrate: the substrate is the specific reactant that an enzyme acts upon. For instance, in the reaction catalyzed by lactase, lactose serves as the substrate.
Binding: enzymes are proteins that bind to their substrate at a region known as the active site.
Mechanism of Enzymatic Action:
Active Site Binding: substrates enter the enzyme’s active site and bind to it.
Induced Fit Model: upon substrate binding, the enzyme changes its shape slightly to better enfold and hold the substrate, a process known as “induced fit”
Conversion to Products: while bound to the enzyme, the substrate is transformed into the product(s) of the reaction.
Release of Products: once the conversion is complete, the products are released from the active site.
Reusability of the Enzyme: after the products are released, the active site becomes available again for new substrate molecules to bind and undergo the same reaction.
Diagram of an Enzymatic reaction:
Reaction sequence: this can be generalized as follows:
E + S ➡︎ ES ➡︎ EP ➡︎ E + P
◼︎ E: Enzyme
◼︎ S: Substrate
◼︎ ES: Enzyme-Substrate complex
◼︎ EP: Enzyme-product complex
◼︎ P: Product
3.3 Environmental Impacts on Enzyme Function
Environmental Impacts on Enzyme Function
Influence of Environmental Factors:
Enzyme activity is influenced by several environmental conditions, including temperature, pH, substrate concentration, and the presence of inhibitors.
Temperature Effects:
Reaction Rate: Increasing the temperature generally increases the rate of enzymatic reactions because substrates collide with active sites more frequently,
Denaturation: However, excessively high temperatures can disrupt the weak chemical bonds that maintain the protein’s structure, leading to denaturation, where the enzyme loses its functional shape and becomes inactive. This process is sometimes reversible.
pH effects:
Optimal pH Range: Enzymes function within specific pH ranges. Deviations from this range can also lead to denaturation, as excessive [H+] ions can alter the protein’s shape through disruption in hydrogen bonding.
Examples:
◼︎ Amylase gas an optimal pH of 7
◼︎ Pepsin functions best at a pH of 2
◼︎ Trypsin is most effective at a pH of 8
Substrate Concentration:
Reaction Rate Increase: Higher substrate concentrations typically increase the reaction rate up to a point. This is because mroe molecules are availible to collide with and react at the ezyme’s active sites.
Saturation Point: The reaction rate plateaus when all enzyme active sites are occupied, a state known as saturation
Enzyme Concentration:
Reaction Rate Limitation: An increase in enzyme concentration will also increase the reactioni rate, provided there is enough substrate present to bind to the available active sites.
Effects of Inhibition:
Types of inhibition:
Competitive Inhibitors: these molecules compete with the substrate for binding at the enzymes active site, effectively blocking the substrate from binding.
Noncompetitive Inhibitors: These bind to a diffrent part of the enzyme, cause a change in the enzymes shape that makes the active site less effevtive or unable to bind the substrate.
Regulatory Role: Inhibitors play crucial roles inr egulating which enzymative reactions occur within a cell, preventing all reations from happening simultaneously.
Allosteric regulation:
this form of regulation involves molecules binding to sites other than the active site (allosteric sites), which affects the enzyme’s function by inducing changes in its conformation.
3.4 Cellular Energy
Fundamental Concepts of Energy in Cells:
Energy Dynamics: life processes require a continuous input of energy, where energy input generally exceeds energy output. This constant flow is essential for sustaining life.
Transformation and Conservation of Energy: In biological systems, energy is converted from one form to another but is never destroyed, adhering to the first law of thermodynamics, which states that energy cannot be created or destroyed.
Energy and Thermodynamics:
Non-Recyclability of Energy: although materials like carbon can be recycled, energy itself, once used, is often released as heat and cannot be reused by the organism.
Thermodynamic Laws: Life processes do not violate the laws of thermodynamics; instead, they are exemplary models of these principles in action
Types of Biochemical Reactions:
Exergonic Reactions: these reactions result in a net release of energy, making them spontaneous. The energy released from exergonic reactions is often harnessed to power other cellular processes.
Endergonic Reactions: conversely, these reactions absorb free energy from their surroundings and thus require an input of energy to proceed, making them non-spontaneous.
Energy Coupling:
Definition: This is the process by which the energy released from exergonic reactions is used to drive endergonic reactions, a critical aspect of metabolic pathways.
Controlled Energy Transfers: Cellular pathways are carefully sequenced to ensure that energy transfers are controlled and efficiant. The product of one reaction often serves as the reactant for the next, facilitating a continous flow of energy through various biochemical pathways.