Class notes.docx
NATS1516 \n Winter semester 2023
INSTRUCTOR: DR. Stephany
Unit 1: Fundamental Concepts
Impacts of mercury moving through the environment
- Water pollution affecting humans and animals
- Toxicity to plants
- Air pollution
Fundamental concepts
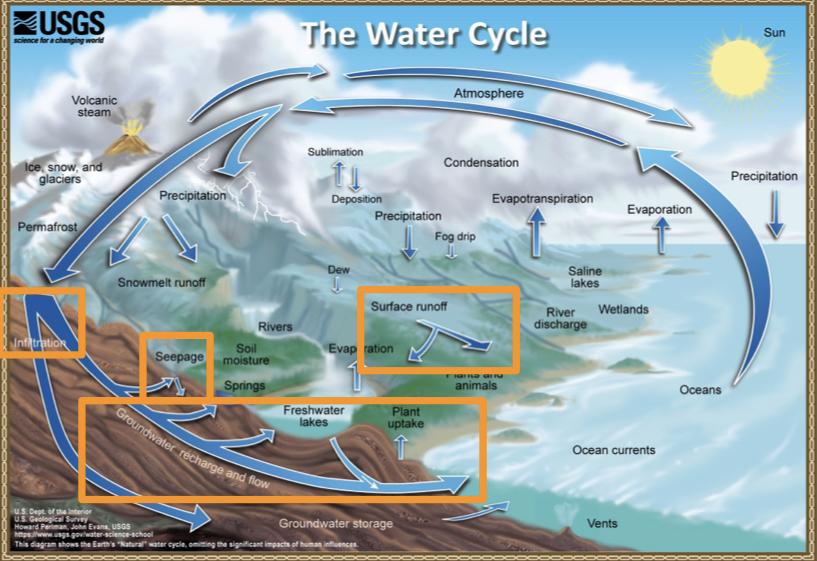
- The water cycle
- Food chain theory and magnification
- Food web complexity
Mercury dumped into a river could:
- Travel down the river into another body of water
- Seep into groundwater
- Evaporate into the atmosphere
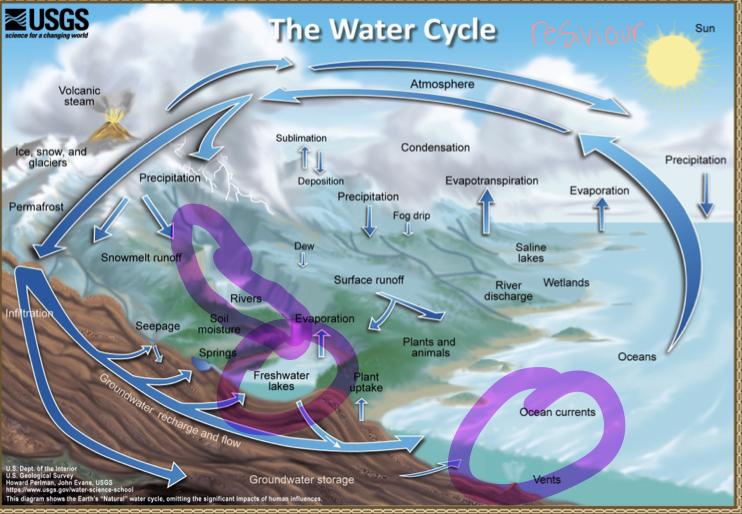
The water cycle
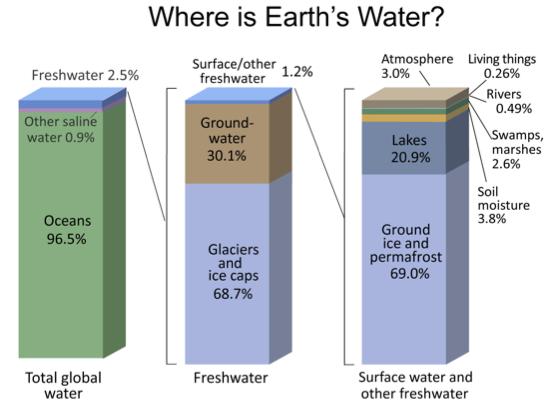
Reservoirs
- A reservoir is a location where water is stored
- Major water reservoirs:
- Oceans 96.5%
- Fresh water 2.5%
- Ground water
- Glaciers
- Surface waters (lakes, rivers, soil, moisture, etc)
- Atmosphere
Iclicker question >
The water cycle: Transport processes
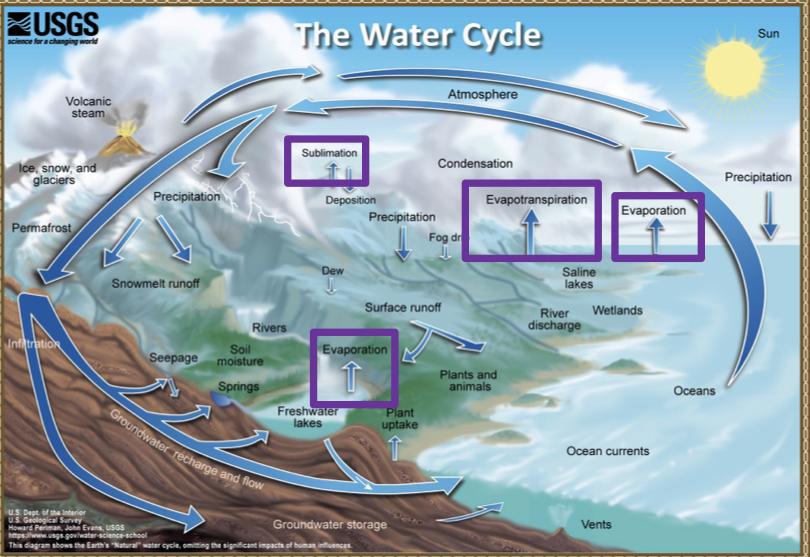
Processes that transport water TO the atmosphere:
- Evaporation: As the sun heats up water ( eg in the ocean) it changes from a liquid to a gas (vapor), transporting the water to the atmosphere
- Sublimation: Ice and snow can sublimate directly from plants vapor (solid water -> gaseous water)
- Evapotranspiration: evaporated water specifically from plants and soils
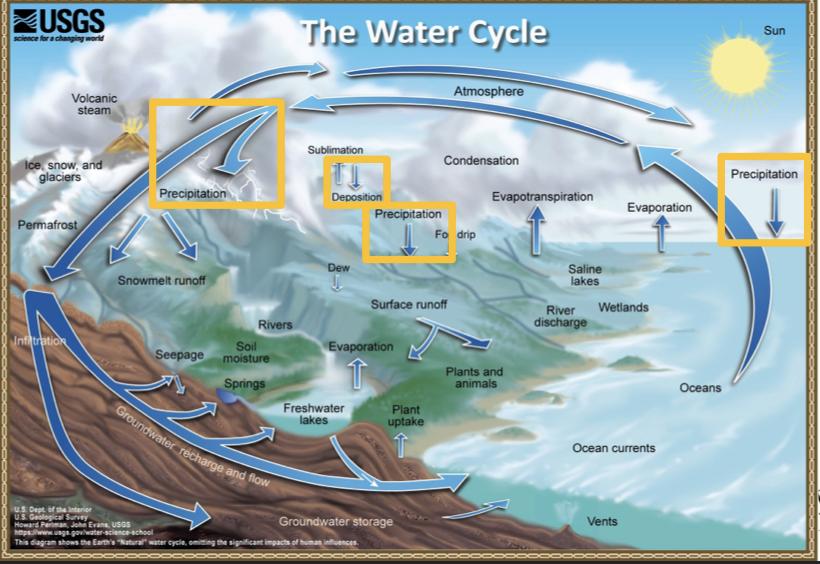
Processes that transported water FROM the atmosphere:
- Condensation: water vapor in the atmosphere cools and condenses into clouds. Gas->liquid
- Precipitation: if cloud particles grow large enough, they can fall out of the sky as precipitation (as either snow, rain, hail, etc) (the fall)
- Deposition: transfer of water vapor directly to solid water (ice/snow). Gas->solid
Processes that transport water ALONG the earth’s surface:
- Surface runoff: due to gravity, precipitation flows over the ground until it ends up in a river/stream (which eventually will flow into a lake/ocean). Having water running off into a body of water.
- Infiltration: due to gravity, precipitation can leak into the groundwater and eventually recharge into a lake/ocean.
The water cycle:
- Pollutants can move throughout the entire world via water cycle…
- E.g Mercury emitted in China can evaporate, travel through the atmosphere to the US, precipitation down in the rain and collect in a river/lake and cause harmful impacts to organisms there.
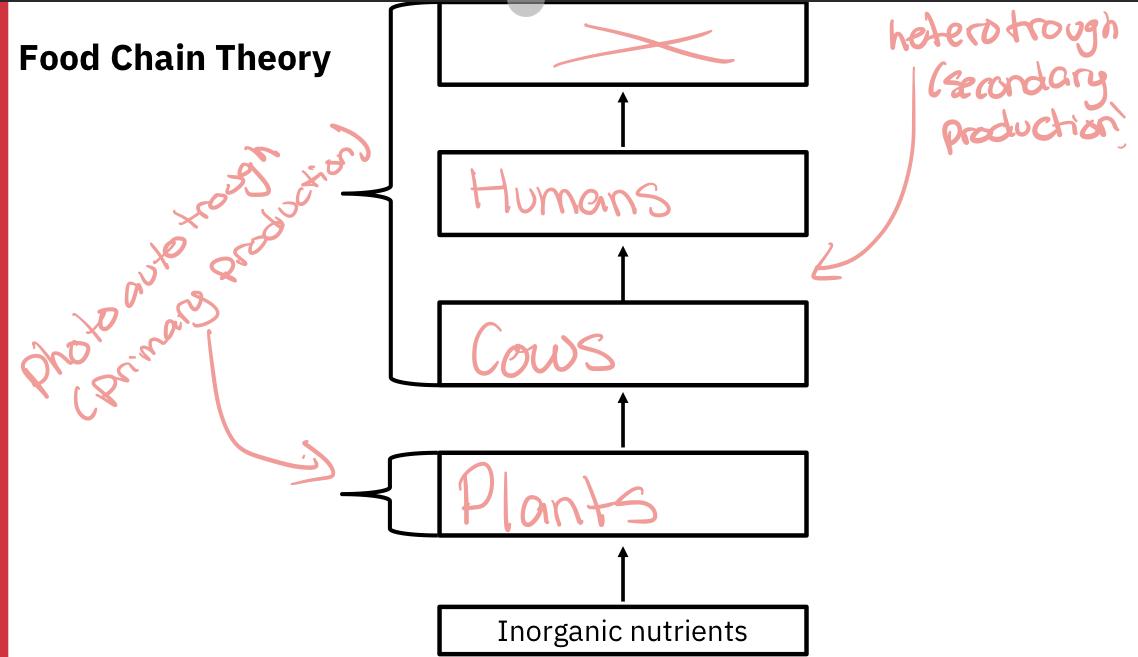
Food chain theory
- Respiration
- Sunlight + CO2 (carbon dioxide)
- Photosynthesis
- Organic compound +O2 (glucose) (oxygen)
- All animals have the ability to transform organic compounds from one form to another (think of a human converting their lunch into necessary fat and protein molecules)
- However, only plants (and certain bacteria) have the ability to manufacture organic high-energy compounds (such as glucose) from inorganic low-energy constituents (carbon dioxide). This transformation is referred to as primary production.
- If the source of energy driving this conversion is light, this process is called photosynthesis.
- If the source of energy is from a chemical reaction, this process is called chemosynthesis (only bacteria and fungi).
Iclicker question
Is the following true or false?
Every photosynthesis reaction can be called a primary production transformation, but not every primary production transformation can be called a photosynthesis reaction.
Answer: True
- Primary production
- Photosynthesis (light)
- Chemosynthesis (chemical reaction)
- All living organisms depend either directly or indirectly on primary producers as a source of food.
- Organisms that produce most or all of the substances they need from inorganic compounds are called photoautotrophs to chemoautotrophs (depending whether the energy needed comes from the light or a chemical reaction, respectively)
- Example of photoautotroph:
- Any plant (ex seaweed)
- Organisms that lack autotrophic abilities are called heterotrophs. Heterotrophs produce their biomass via the conversion of organic matter (food) into living biomass, and this is called secondary production.
- Example of heterotrophs:
- Humans, animals
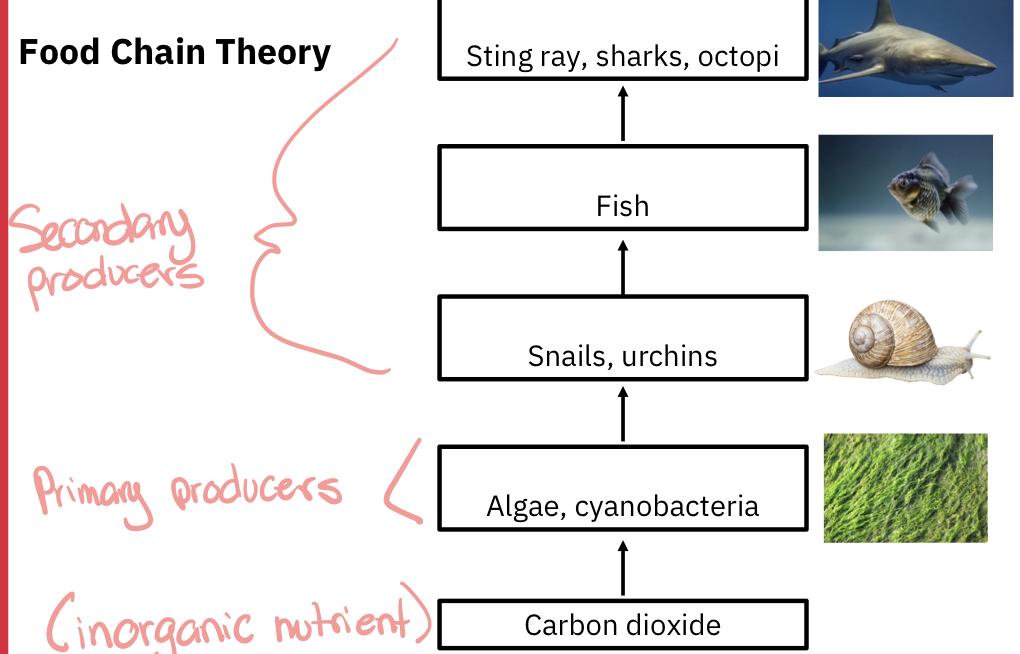
Food chain example:
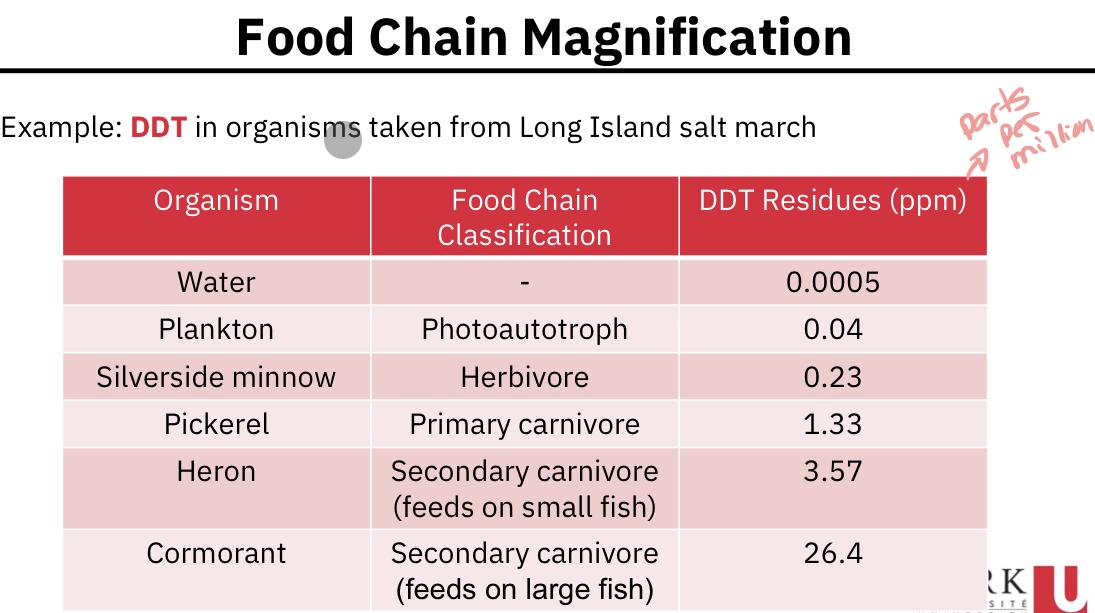
Food chain magnification:
What does all this discussion of food chains have to do with aquatic pollution?
- If a water pollutant is not biodegradable (broken down inside an individual organism), it may be passed from prey to predator and in this way, spread through the entire food chain.
- There has been considerable research performed to examine the distribution of pollutant concentration among the trophic levels in a food chain. Often we see a steady increase in concentration as the trophic level increases.
How do we explain the increase in DDT up the food chain?
- Certain pollutants (such as DDT) once ingested are not as effectively respired or excreted as is the remainder of the food.
- A pollutant (or a metabolite of a pollutant) if resistant to biological breakdown can be stored in an organism’s fatty tissues rather than being directly excreted.
- Once stored, if that organism is consumed by another, the pollutant is transferred to the predator.
- Food chain magnification = biomagnification
Bioaccumulation:
- Increase in concentration of a pollutant in a single organism over its lifetime.
Biomagnification:
- Increase in concentration of a pollutant up the food chain.
Iclicker question:
- When an osprey consumes fish, it accumulates 300 ug of DDT per day into its body. If the osprey consumed seal instead of fish, would you expect the osprey accumulation of DDT per day to be higher or lower than when consuming fish?
- Answer: Higher
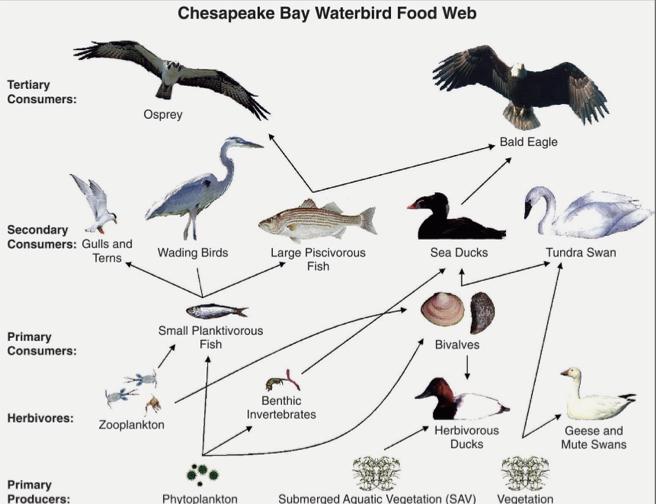
Food Web Complexity:
- Many animals can be assigned to multiple trophic levels.
- It is complete and diverse depending on where it goes.
- Grassy Narrows, ON
- In 1962, Dryden Chemical company began operating a chloralkali process plant in Dryden, Ontario (it produced sodium hydroxide and chlorine that were used for bleaching paper).
- From 1962-1970, it was estimated that 9000 kb of Mercury (Hg) had been dumped by the company into the Wabigoon-English river system.
- Airborne emissions of Mercury (Hg) continued until 1975 until the company stopped using Mercury (Hg) in their processing.
- Fish contained high levels of Mercury, impacting cognitive function, reproduction, etc.
- Mercury does not biodegrade which means it bioaccumulates up the food chain.
- Commercial and tourist fishing was stopped.
- Surrounding soil continues to have high levels - negatively impacting land, animals and plants.
- Loss of fish as a food source.
- Numerous health impacts as a result of Mercury poisoning.
Unit 2: Sources of water pollution: Nonpoint source
Nonpoint source pollution:
- Input does not occur at the end of a pipe or a man-made wastewater outfall but in a continuous manner along a shoreline and/or has a diffuse source.
- Problems are associated with inputs of:
- Sediment
- Nutrients
- Oxygen-consuming wastes
- Pathogens
- Toxic substances (e.g. pesticides and heavy metals)
- Two important types of Nonpoint source pollution are:
- Runoff from agricultural land
- Runoff from urban land
- In both cases, the most significant water quality problem is frequently the introduction of sediment due to soil erosion.
Runoff from agricultural land
- The second most significant water quality problem:
- Cultural eutrophication caused by nutrient input
- Eutrophication is arguably the most widespread Nonpoint source pollution problem globally
- Case location: Mississippi River & Gulf of Mexico:
- The Mississippi River is the largest river basin in North America, the third largest in the world.
- It drains more than 40% of the land area of US, 58% of which is productive farmland
- As fertilizer use has increased since the 1950’s, so has the nutrient concentrations in the lower Mississippi River.
- When the nutrient rich water reaches the Gulf of Mexico, it fertilizes the marine phytoplankton (algae) -cultural eutrophication occurs and a hypoxic zone is created.
- Strategies to reduce nutrient loading in the Gulf of Mexico:
- Nutrient management: varying the amount, timing and method by which fertilizers are applied to crop lands.
- Cover crops: Planting certain grasses, grains or clovers can recycle excess nutrients and reduce soil erosion, keeping the nutrients out of the waterways.
- Buffers: Planting trees, shrubs, and grass around fields, especially those that border bodies of water, can absorb and filter out nutrients before they reach the body of water.
- Conservation Tillage: reducing how often fields are tiled reduces soil erosion and soil compaction, reducing runoff.
- Managing livestock waste: keeping animals and their waste out of streams, rivers and lakes reduces nutrient concentrations.
- Draining water management: reducing nutrient loading that drains from agricultural fields.
Cultural eutrophication
- Nutrients such as nitrogen (N) and phosphorus (P) are naturally occurring in our environment but excessive inputs of N and P into the water systems is a direct result of human activities.
- Primary sources of nutrient pollution are:
- Fertilizers
- Manure/ animal byproduct
- Sewage pipes/ human feces
- Detergents
- Iclicker Question
- Prior to human existence eutrophication of aquatic systems did not occur.
- Answer: False, because eutrophication has been around due to natural things like, animal by product, humans are just worsening it with other add on’s.
- Nutrient input into a body of water.
- Algae at the surface of the water system feed on nutrients and flourish and block sunlight from entering the ecosystem.
- Eventually algae die and drift to the bottom of the water system, providing food for decomposers.
- As decomposers breakdown algae, they remove oxygen from water, limiting the amount available to fish and plants.
- With a hypoxic zone( Low/no oxygen - dead zone) created, most plants and fish die.
- There are instances when cultural eutrophication may be beneficial to an aquatic ecosystem.
- The deliberate fertilization of ponds or similar enclosed systems is a basic technique used in aquaculture to produce large crops of fish or shellfish - there is nothing wrong with stimulating production.
- The problem comes about if the system is not properly managed.
- What are some problems associated with cultural eutrophication?
- Lack of food source
- Food chain issues
- Loss of biodiversity
- Mass extinction
- Changes in the water Ph
- Problematic impacts of eutrophication
- The organisms that thrive from eutrophication (e.g. Cyanobacteria, certain fish populations, etc.) are frequently associated with undesirable water quality condititions and their value (monetary, aesthetic and scientific) is often lower than the original ecosystem.
- E.g. In Kaneohe Bay ( Hawaii, USA), a virtually worthless form of algae grew over and destroyed once-healthy coral reefs.
- Oxygen concentrations in a highly eutrophication system generally fluctuate over a much wider range than other systems
- It is impossible for some organisms to function efficiently unless the oxygen concentration in the water is near saturation.
- Large-scale fish kills and the elimination of desirable species as a result of oxygen depletion are both serious problems.
- Problematic ecosystem
- Fluctuations of different areas (oxygen rich/ lack of).
- Excessive amounts of phytoplankton and/or macroscopic plants in the water create aesthetic problems and reduce the value of the body of water as a recreational resource.
- The diversity of organisms is frequently much lower in a eutrophic system than in an oligotrophic system (one that is not eutrophied)
- For certain purposes, it may be desirable to have organisms in a system be dominated by a few species (an aquaculturist designs and manages a “farm” system that produces a few species of fish or shellfish).
- Iclicker question:
- What is a way we can reduce/eliminate the occurrence of eutrophication?
- Answer: Natural pesticides, Use a buffer system, Use phosphorus free detergent, localize the dumping to an enclosed area.
Runoff from urban land
- Urban runoff, comprising stormwater and snowmelt (in regions with snow), is a major transport vector of pollutants released in the urban environment, and therefore a significant contributor to the deterioration of water quality.
- Storm water: rain and melted snow flowing along the streets into storm drains, eventually flowing into an aquatic community.
- General composition of the urban runoff:
- Sediments
- Oxygen consuming wastes
- Nutrients (nitrogen and phosphorus-leading to eutrophication)
- Metals and toxic chemicals (vehicle oil, industrial pollutants, etc).
- Sediments
- Some of the sediments are large particles (such as pebbles) that are too heavy to be suspended and thus are transported along the bottom of the stream or storm sewer.
- The rest of the suspended load is determined from the concentration of suspended solids (SS) ( those that ate smaller sands, silt and clay particles that remain suspended in the water column)
- Oxygen consuming wastes
- Measuring using two metrics:
- Biological oxygen demand (BOD): the amount of oxygen consumed in a sample of water incubated in the dark at 20 degrees celsius for 5 days
- More critters will appear as the oxygen levels decrease because they are consuming it.
- The sample must be kept in the dark to prevent photosynthesis (plants producing O2)
- Oxygen levels decrease, that indicates there are organisms in the runoff that respiring (breathing) and thus taking up oxygen.
- Chemical oxygen demand (COD): the amount of oxygen consumed as a result of a chemical oxidation reaction (such as by iron)
Urban runoff: Composition:
Nutrients (N and P)
- Primarily from atmospheric (deposited from the ground from the atmosphere) deposition, fertilizers, pet waste, automotive detergents and biogenic materials (lawn clippings and leaves)
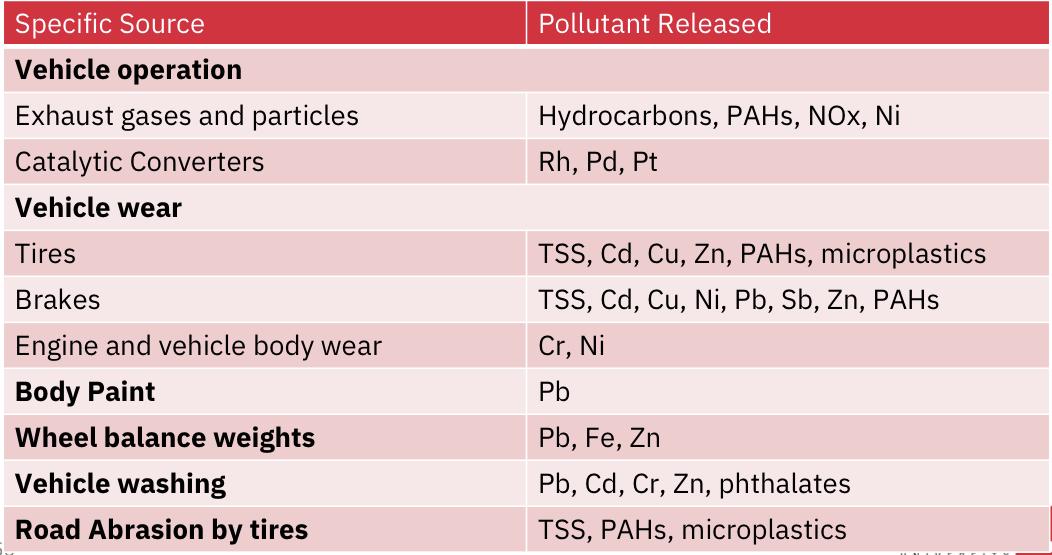
Trace metals
- Mainly: Cd (cadmium), Cr (chromium), Cu (copper), Ni (nickel), Pb (lead) and Zn (Zinc)
- Primarily from industries, buildings, roofs with metal elements, vehicle parts, fuel and oils and roads metallic structures.
Important sources of urban pollution:
- Atmospheric Deposition
- Drainage Surfaces
- Anthropogenic activities
- Urban drainage systems
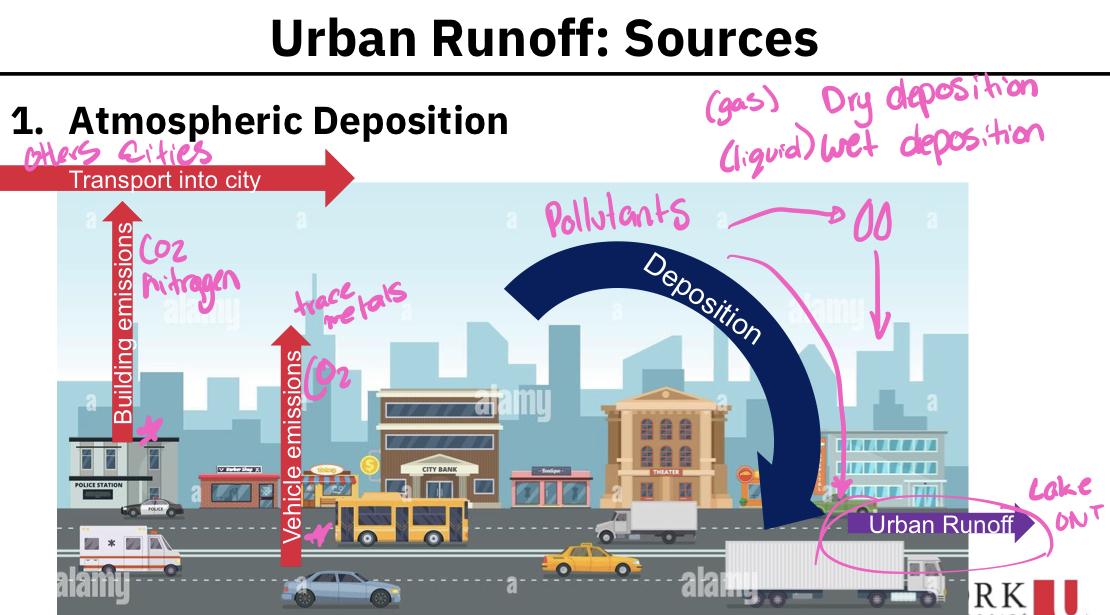
Atmospheric deposition:
- Serves as a transport pathway, rather than a source of pollution
- Pollutants are brought into the urban environment by emissions and transport from local, regional and remote sources
- Because there so many sources of emissions, the atmospheric deposition of pollutants is highly variable
- Primary pollutants that are delivered by atmospheric deposition:
- SS (suspended soils)
- Nutrients (N and P)
- Metals associated with traffic and industries (e.g. Cr, Cu, Ni, Pb)
- Polycyclic aromatic hydrocarbons (PAHs) - Cancer causing
- Pesticides
Drainage surfaces:
- Roads and impermeable paved surfaces can contribute to urban pollution via:
- Mechanical wear of pavement surface by vehicle tires
- Dissolution of chemicals into water running along the pavement surface
- E.g. sealants used to enhance asphalt driveways were found to be a major source of PAHs in urban stormwater
- Building materials and structure surfaces
- Any surface in contact with rainwater or surface runoff can affect water quality, such as: buildings, sculptures, lamp poles, etc.
- E.g. corrosion of metal structures (like metal roofs) are a major source of Cu and Zn in runoff
- E.g. buildings are often also coated in pesticides, and these are also in contact with rainwater and can runoff
- Green areas (parks, lawn, urban forests and sport facilities)
- Toronto has 10.2 million trees in its urban area
- Green areas release dissolved organic matter (DOM), which has a string binding affinity for heavy metals (Cd, Cu, Ni, and Pb) and hence influence their transport and transformation
- Green areas are also hot spots for nutrients like N and P
Anthropogenic (human-caused) activities:
- Vehicular transportation
- Main Pollution sources identified as: vehicle operation, automotive fluid leakages, vehicle washing and road abrasion.
Iclicker question:
- What is the most significant pollutant a vehicle emits?
- Answer: NOx (nitrogen oxides)
Road maintenance
- In climates with snow, snow clearance and de-icing practices are used to maintain road safety
- Estimated Canada uses 5 million tonnes of road salt annually (US is estimated at 18 tonnes)
- Common road salts contain: Chloride (60% by weight in NaCl), anti-clumping agents, ferrocyanide, chromate or phosphate.
Iclicker question:
- Provide one impact of road salt on the nearby aquatic ecosystem.
- Answer: Increases the salinity of freshwater ecosystems.
- Chemicals effect of road salt are two-fold:
- Direct source of pollution (i.e. chrlorde pollution - salinity)
- Agents increasing the dissolve phase of metals
- Washing of road structures also can contribute to road runoff pollution
Industrial activities
- Recognized as a source of pollutants in 2 ways:
- Pollutant emissions into the atmosphere(deposition)
- By direct surface runoff from industrial land
- Contribution of pollution depends on the type of industrial activity
- E.g. industrial activity that produces microplastic pellets used in personal care products will likely be a large source of microplastics in stormwater.
Construction
- Known to contribute significant amounts of sediment and soil particles to runoff
- Generated by soil erosion
- Source of nutrients (N and P) trace metals and PAHs attached to soils, pesticides, etc.
Littering, illicit dumping, household waste disposal
- Seasonal roadway deposition of litter varying from 14.3 kg/km/day into winter to 24.8 kg/km/day in the summer.
- Of particular importance is plastic litter (e.g. bottles, bags, etc.) that can degrade into microplastics in the marine environment
- Plastic litter can also contain an carry absorbed trace metals and organic pollutants that may be release to surroundings and runoff
- Illicit dumping can also be a large source of pollution to runoff
- Common example: fast food operations dumping of fryer oils into parking lot storm sewers
- Proper disposal of fryer oil is to let it harden and throw away in the green bin
Gardening
Iclicker question:
- Based on what we have learned so far, how do you think gardening can contribute to water pollution?
- Answer: soil erosion, nitrogen and phosphorus, suspended soils, fertilizers, manure, pesticides.
- In heavy thunderstorms, gardens can contribute suspended soi;s and/or nutrients originating from fertilizers into stormwater
- Pesticides used in gardening to control insects, weeds and fungi can also potentially affect stormwater runoff
Pets and wildlife
- Dropping from pets and birds are sources of nutrients and fecal microorganisms in urban stormwater runoff
- Study done by Hobbie et al. (2017) that names household pet waste (particularly dog waste) as one of the top inputs of N and P to urban watersheds in St. Paul (Minnesota)
- Pet waste is the 3rd largest source of N (after residential fertilizer and atmospheric deposition)
- Pet waste is the greatest source of P
- Generally assumed that N comes from animal urine and P from feces
Iclicker question:
- Would it be easier to reduce the N (ur nine) or P (poop) pollution from pet waste
- Answer: Phosphorus
- Animal waste is also a source of bacteria that can infect humans
- A study done in Toronto beaches indicated that in shallow water, the dominant fecal indicator bacteria (FIB) (e.g. E.coli) were from bird droppings stored in wet beach sand
Washing of buildings and structure surfaces
- Building roofs and facades and other structures collect deposited pollutants, may suffer from moss or algae, and can be subject to application of protective or biocidal chemicals
- When these surfaces are washed, transporting these components into storm water runoff
- Graffiti is washed off of building walls constantly and it could lead to pollution of stormwater runoff because metals are what give paint colour
Urban drainage systems
- Green infrastructure and stormwater control
- Green infrastructure and stormwater control are designed to reduce runoff volumes and improve stormwater quality- it is therefore counterintuitive to consider them as a source of pollution
- GI prompted the increase of permeable pavements in as urban are- these are reported to significantly increase suspended soils concentration in runoff, as well as carry particle-bound metals and other substances
- Green roofs may act as a source of nutrients, pesticides, COD (chemical or biological oxygen demand) and some metals
- Misconnection
- Intentional and unintentional cross-connection of wastewater and stormwater sewers
- Includes household, industrial and commercial wastewater into storm sewers
- Pipe materials
- Quality and composition of stormwater may change during its transport by storm sewers to receive waters based on pipe material
- Studies have investigated PVC vs concrete pipes and have shown concrete pipes can affect water quality significantly, mailing by increasing its pH
- Flint water quality event (water lead contamination)
Putting it all together:
- Runoff from agricultural land
- This delivers N and P to aquatic systems which creates cultural eutrophication
- Runoff from urban land
- Composition: sediments, oxygen consuming wastes, nutrients, metals
- Sources: atmospheric deposition, drainage surfaces, anthropogenic activities and urban drainage systems
Unit 3: Sources of water pollution: Sewage treatment
Sewage treatment
- Every day, millions of cubic meters of wastewater are discharged from homes, businesses etc. into sewer systems
- Municipal wastewater is one of the largest sources of pollution to surface water in Canada
- From 2013-2017, approximately 86% of wastewater from Canada's population is treated in plants POTWs
- That means approximately 14% are serviced by septic tanks or cesspools
- Septic tank
- An under group chamber of various building material (e.g. concrete, fiberglass or plastic) through which domestic wastewater flows for basic sewage treatment
- Settling and anaerobic digestion reduce soils and organic waste, but treatment efficiently is minimal
- “Treated” water is then drained into a septic drain field
- Popular in rural areas
- Iclicker question:
- What do you think is likely the biggest problem associated with septic tank systems?
- Answer: Groundwater pollution, nitrogen pollution, dumping in lakes.
- Cesspool
- Perforated concrete block that is buried underground to hold wastewater
- Concrete block has holes so that water can escape to surrounding soil areas
- Sludge needs to be pumped regularly
- Tank needs to be removed once solid is so saturated that wastewater is pooling at the surface
- Septic tanks and cesspools
- Both septic tanks and cesspools are older techniques of wastewater treatment and not widely used today
- The discharge of sewage can create serious water pollution problems
- Sewage has high concentration of:
- Nitrogen and phosphorus
- E. Coli ( and other pathogens)
- Ammonia
- Suspended soils (SS)
- Sewage treatment
- Treatment of sewage occurs in wastewater treatment plants, often referred to as publicly owned treatment works (POTWs)
- POTWs remove most of the suspended soils, biochemical oxygen demand and pathogens but less than half if the nitrogen and phosphorus
- This is a cause for concern because it can damage aquatic systems, bioaccumulation can occur and cultural eutrophication
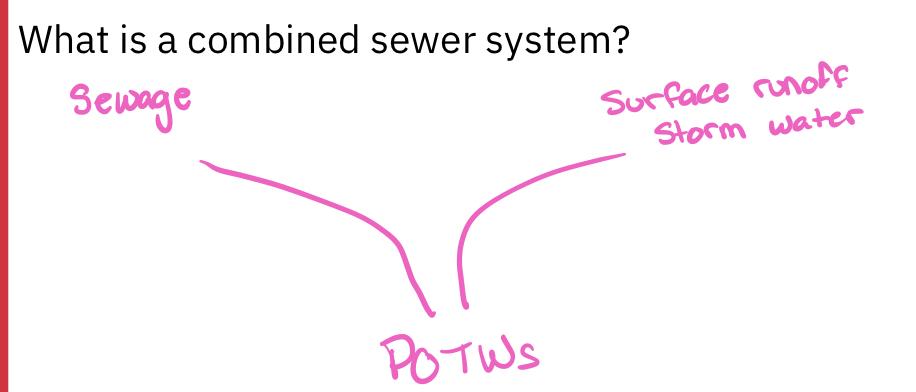
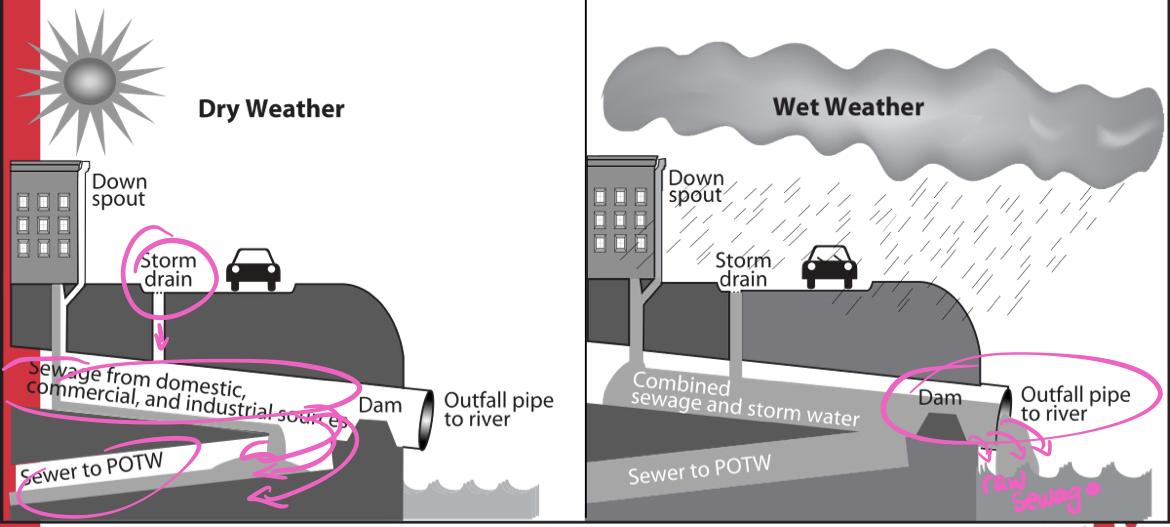
- What problem is associated with combined sewers?
- Frequent rain=large water pollution events
- Ph changes
- Illnesses of humans and wildlife
- POTWs (publicly owned treatment works)
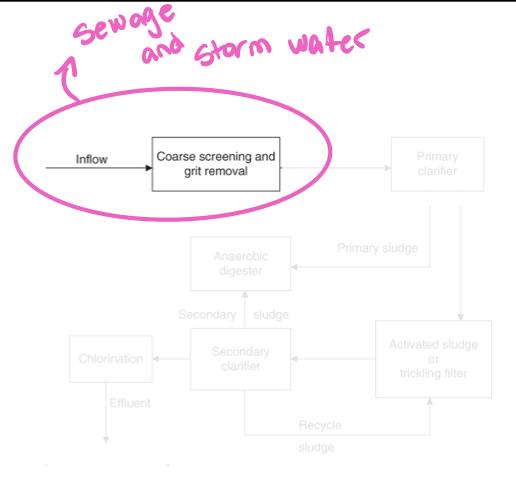
- Because of combined sewer systems, POTWs need to process both sanitary sewage and street runoff simultaneously during storms
- Household sewage composition is well defined, but industrial sewage is highly variable and can contain toxic substances, heavy metals etc.
- cesspool pumping is another factor of POTWs need to deal with
- Once pumped, the contents are directly injected into the sanitary sewer line
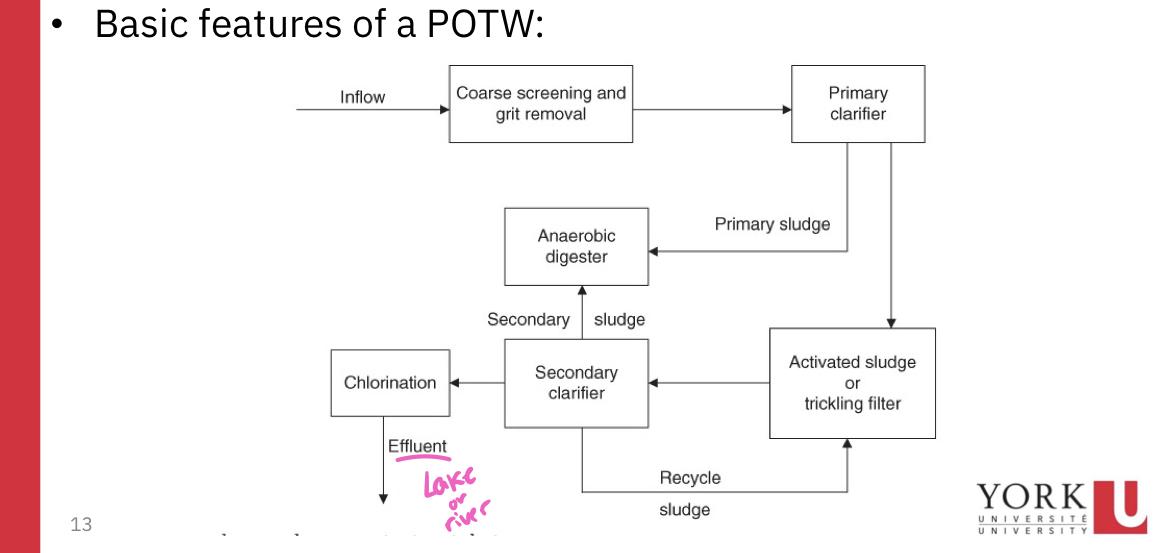
- Screening process:
- Removes large objects that may damage POTW
- May be nothing more than metal bars that are spaced 5-10 cm apart
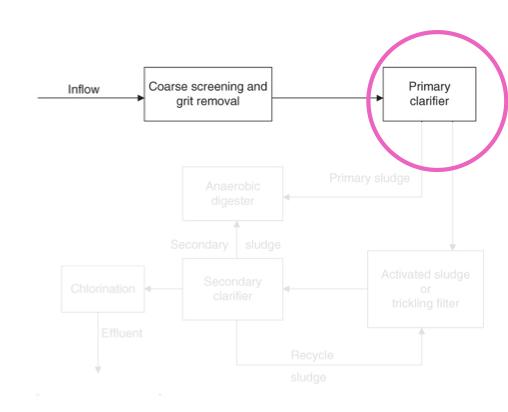
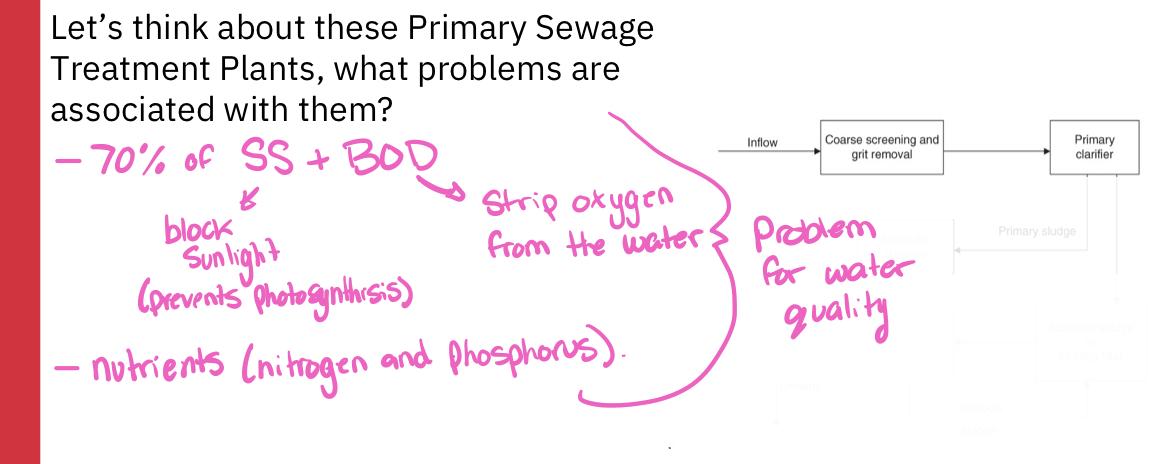
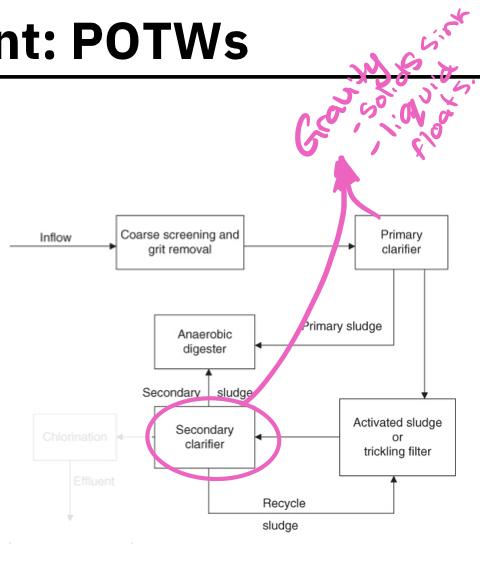
- Primary clarifier:
- A large tank in which settleable or floatable solids are removed from the sewage
- Soil sewage remains at the bottom of the tank, floatable materials are skimmed off
- Primary sludge:
- The solid material that is skimmed from the top of the clarifier and the sludge that remains at the bottom are referred to as primary sludge
- If the treatment process stops, the treatment process is termed primary sewage treatment
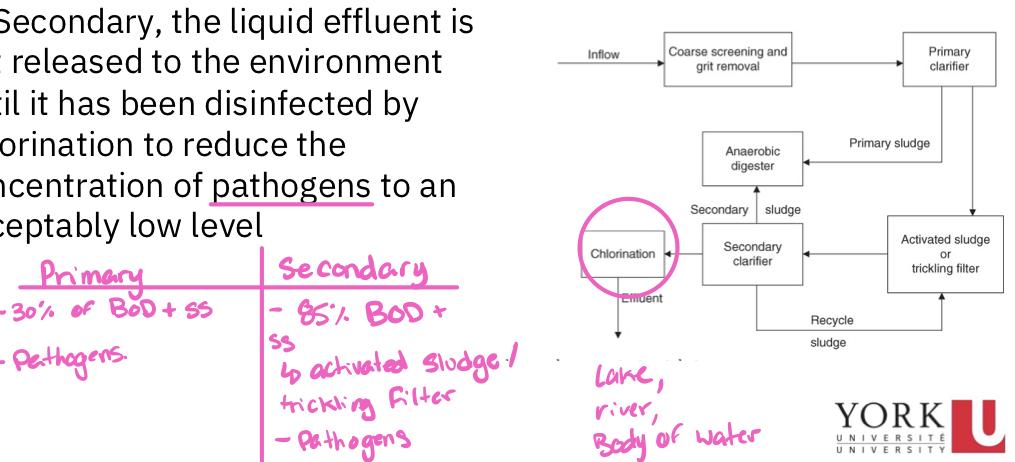
- A primary sewage treatment must remove at least 30% of the biochemical oxygen demand and suspended soils from the sewage
- A primary sewage treatment must remove at least 30% of the biochemical oxygen demand and suspended soils from the sewage
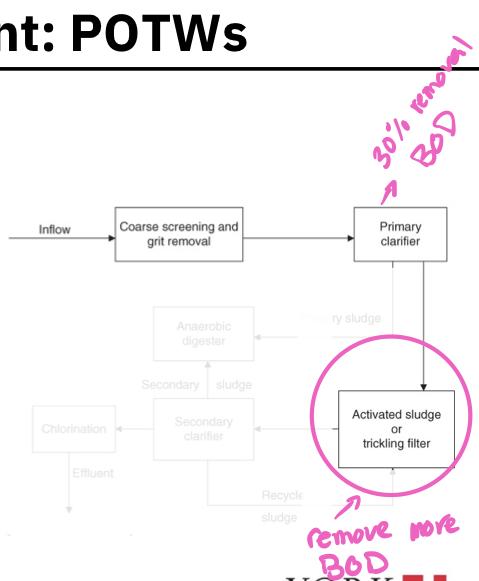
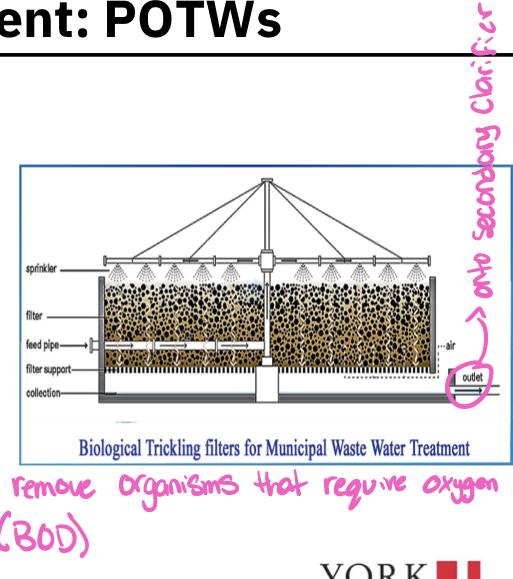
- If the POTW is a secondary treatment plant, the primary effluent flows into the second tank where biological processes are used to remove much of the BOD (activated sludge or trickling filter)
- Organisms living in the tank are allowed to consume the organic substances in the primary effluent
- Secondary clarifier:
- The biomass of these consumer organisms are removed from the secondary sludge by passing it into a secondary clarifier
- The biomass settles to the bottom in the form of a floc or sludge and is removed
- A portion of the secondary sludge is pumped to the anaerobic digester while the remainder is recycled
- The secondary treatment must remove at least 85% of suspended soils and biochemical oxygen demand from raw sewage and reduce SS and BOD concentration to less than 30 mg/L
- Chlorination
- Regardless if the POTW is primary or secondary, the liquid effluent os not released to the environment until it has been disinfected by chlorination to reduce the concentration of pathogens to acceptably low level
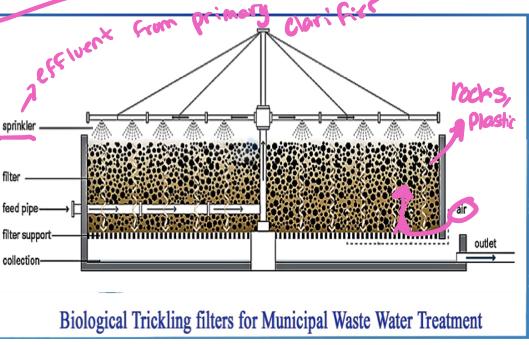
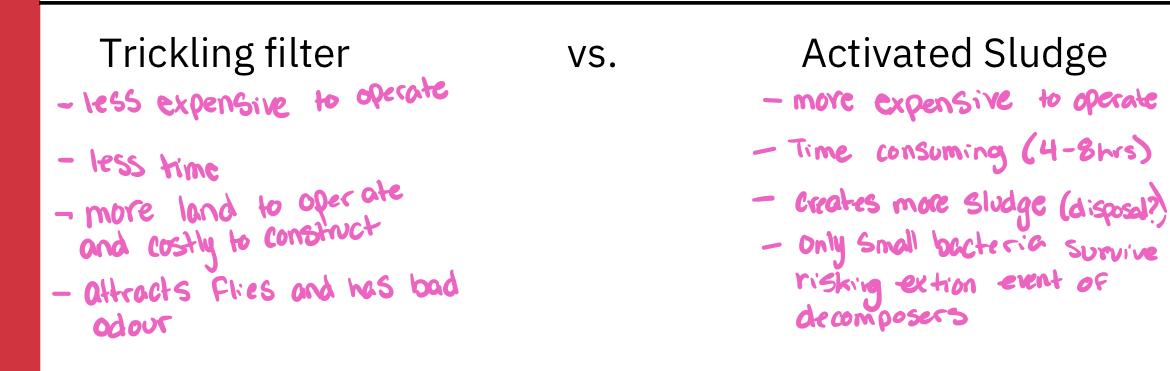
- Trickling filter
- Consists of a cylindrical tank 2-3 meters in depth filled with inert (unreactive) substances ( such a as rocks or, more recently porous arrangement of modular plastic media)
- Media are loosely packed to allow downward flow of sewage and upwards flow of air through the system
- Effluent from primary clarifier is sprinkled down over the bed of media
- As sewage trickles down the plastic media, bacteria and fungi that grow on the surface of the media ingest the dissolved organic substances in the water
- The bacteria and fungi are in turn fed on by a variety of higher trophic level organisms (Protozoa, worms, insects, etc.)
- About 70% of organic waste in the primary clarifier effluent are consumed-another 15-20% are converted to organism biomass, which is collected at the bottom and sent to secondary clarifier for removal
- Part of the effluent is recycled back to the trickling filter to maintain population of decomposers organisms in the filter
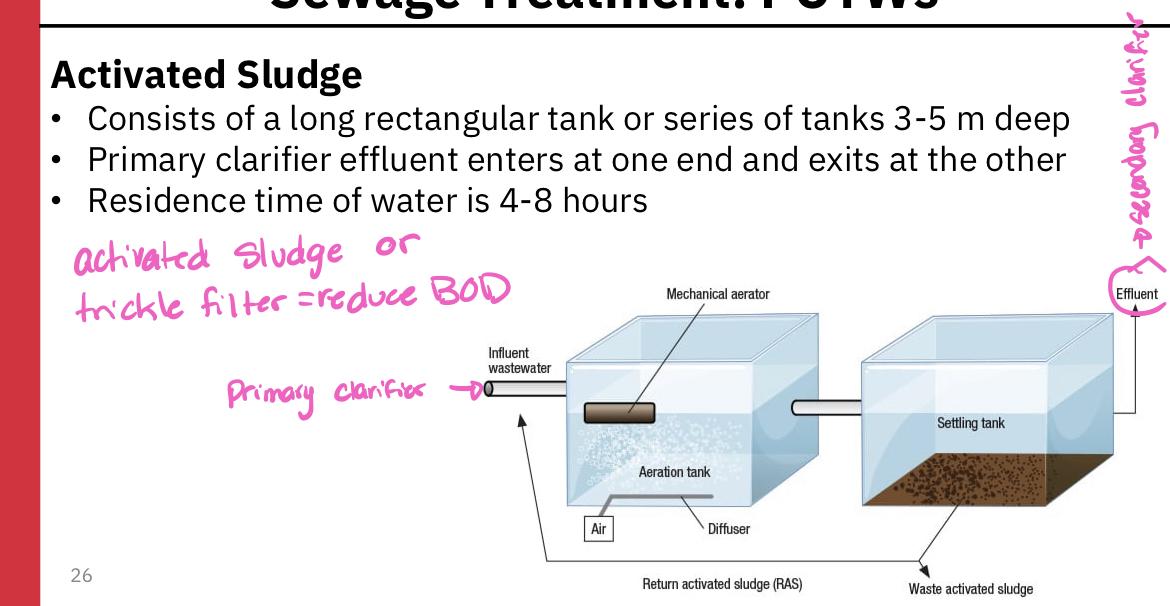
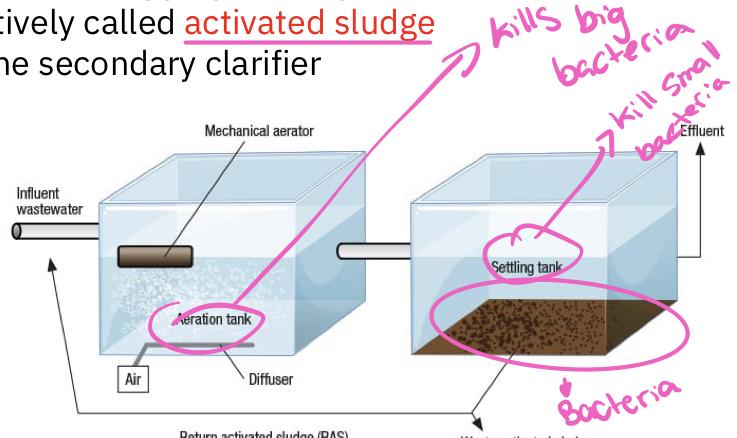
- Activated sludge
- Consists of a long rectangular tank or sierpes of tanks 3-5 meters deep
- Primary clarifier effluent enters one and exists at the other
- Residence time of water is 4-8 hours
- During its passage through the tank(s), the sewage is vigorously mixed from below with bubbles of air that keep the contents in a steamer of turbulence
- This mixing and aeration are necessary to prevent oxygen concentration in the water from dropping low, otherwise respiratory activity of organisms is likely to be slowed
- Because of the vigorous mixing that occurs, only small aquatic organisms can survive, and thus bacteria are the principal decomposer organisms
- Most of the bacteria are found aggregated together in flocculent masses and are collectively called activated sludge
- Sludge is removed in the secondary clarifier
- Iclicker question:
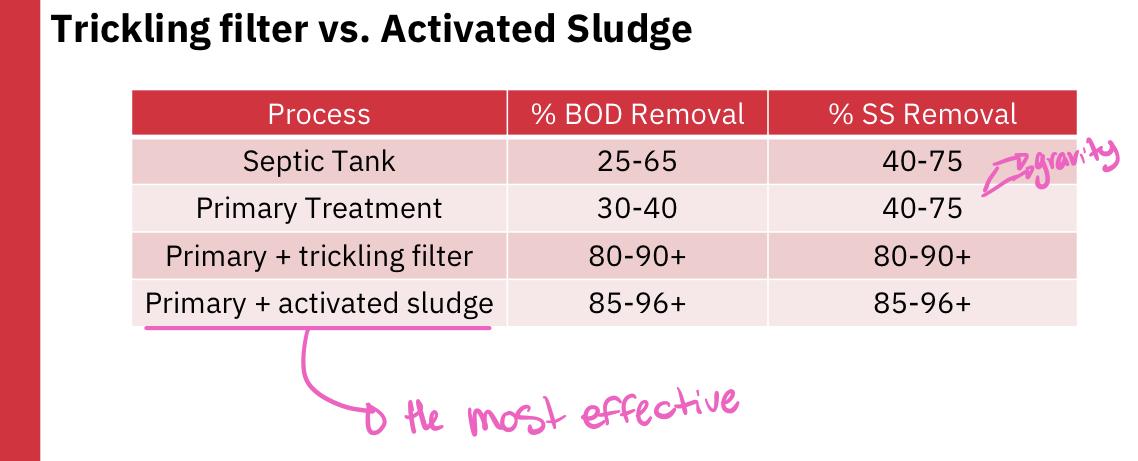
- Is activated sludge or trickling filter more effective at reducing BOD and SS?
- Answer: Activated sludge
- Answer: Activated sludge
- Iclicker question:
- What is the drawback associated with activated sludge?
- Answer: nitrogen and phosphorus
- Answer: nitrogen and phosphorus
- Iclicker question:
- A POTW often treats wastewater from and industrial site where accidents or shutdowns can lead to sudden changes in the characteristics of sewage. In this POTW, would it be advantageous to have a trickling filter or activated sludge system in place?
- Answer: trickling filter because a large variety of decomposers means ability to deal with changes in composition.
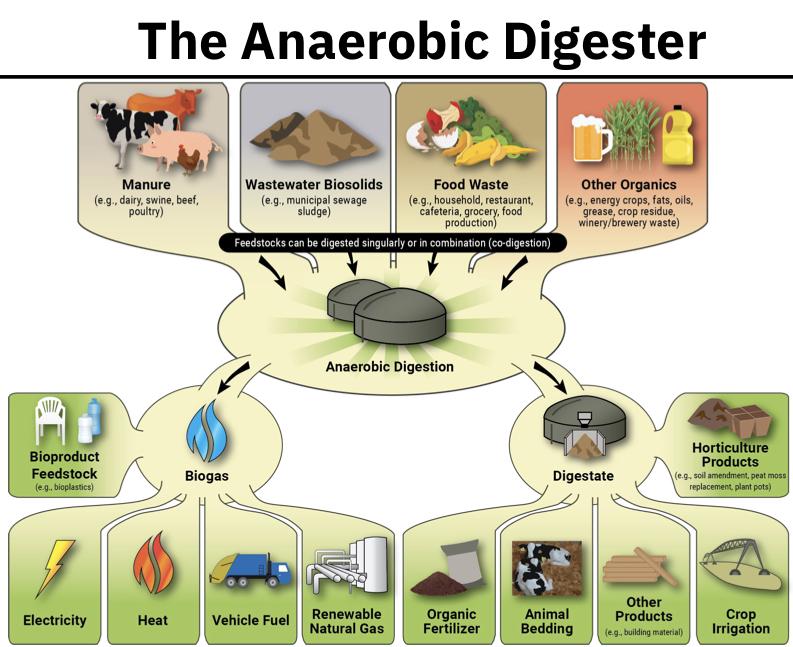
- Anaerobic (no oxygen) digester
- The sludge collected from primary and secondary clarifiers cannot be deposited in a landfill (since it contains putrescible organic biomass)
- in the past, sludge was deposited in the ocean (but in 1981 this was banned
- One option: burn it and completely oxidize the sludge
- Currently 20% of sludge in the USA is incinerated
- Problems with sewer sludge incineration is the smell and air pollution that comes with it
- Alternatives to incineration are: deposition in a sanitary landfill or some form of land application
- Before either of this can occur, the putrescible nature of sludge must be eliminated
- The sludge is pumped into a closed cylindrical tank where it provides food for a special class of microorganisms that carry on their metabolic activities in the absence of oxygen (i.e. anaerobically )
- The products of the anaerobic digester are: digested sludge, a supernatant fluid and gasses (e.g. CH4) - methane
- Volume of digested sludge is ⅓ of that of primary and secondary sludge and it is more stable and less offensive (not as smelly)
- Volume of digested sludge is ⅓ of that of primary and secondary sludge and it is more stable and less offensive (not as smelly)
- Tertiary Treatment
- Additional processing of secondary effluents undertaken to improve their quality.
- In some places, tertiary treatment means further removal of SS and BOD.
- In USA and Canada, tertiary treatment means removing substances of specific concern (primary nutrients and other contaminants)
- Primary treatment plants removal of 5-15% of nutrients
- Secondary treatment plants remove 30-50% of activated sludge.
- Tertiary treatment: Phosphorus removal
- Of the two nutrients, phosphorus is easy to remove because it forms insoluble compounds with calcium, aluminum and iron.
- To remove phosphorus: add a compound (e.g. Lime (CaO)) that when it reacts with the phosphorus in the wastewater, it creates a solid which can settle out.
- The phosphorus recovered can be used as a fertilizer.
- Phosphorus removal efficiency is using this technique is 88-95%
- Other techniques exist but will not be considered in this course.
- Tertiary treatment: Nitrogen removal
- At the pH of typical sewage, nitrogen is in the form of ammonium (NH4+)
- A common method for removing nitrogen is to convert NH4+ to ammonia (NH3) gas- at pH> 11, 98% of the NH4+ is converted to NH3
- Since NH3 is a gas, it can be removed by vigorous aeration
- Nitrogen removal efficiency using this technique is - 90%
- Tertiary treatment: PPCP removal
- Sources of pharmaceuticals and personal care products to wastewater:
- Most PPCPs are removed via traditional secondary treatment with activated sludge (90% efficient)
- Further treatment such as ozónate on, advanced oxidation, activated carbon, etc, can remove more then 90% of PPCPs
- Example impact of PPCPs: Feminization of male-fish
- Over the past decade, 37 species of feminized male fish have been found in lakes and rivers across North America
- The culprit: chemicals that activate the vertebrate estrogen receptor, such as ethinylestradiol, a synthetic estrogen used as a pharmaceutical in humans
- Although concentrations are not high enough to be dangerous to humans, the problem is significant for aquatic organisms
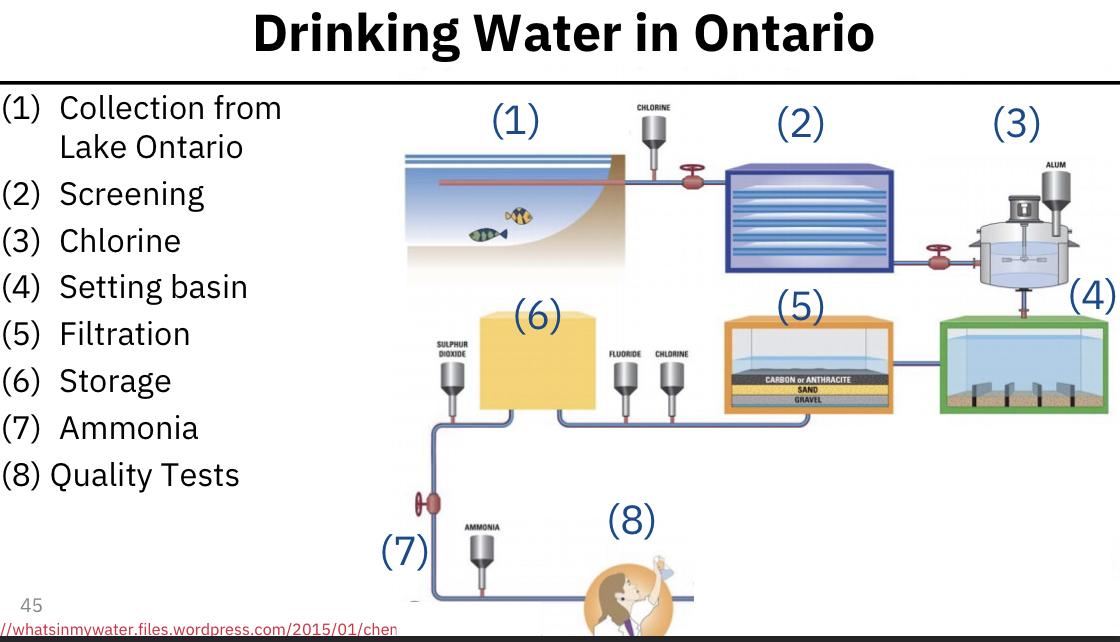
- Drinking water in Ontario
- Land application of sewage effluent
- Sewage effluent is potentially a valuable resource, for 2 main reasons:
- The high nutrient concentrations in sewage can stimulate plant growth (and while we don’t want to stimulate this growth in aquatic ecosystems, sewage could be applied to fields to stimulate crop growth, just like a High-nutrient fertilizer does)
- Use of sewage effluent for purposes such as groundwater recharge, irrigation, toilet flushing and cooling (or any other process that doesn’t require potable water) can be a effective wait to increase freshwater drinking supplies
- Land application of sewage effluent
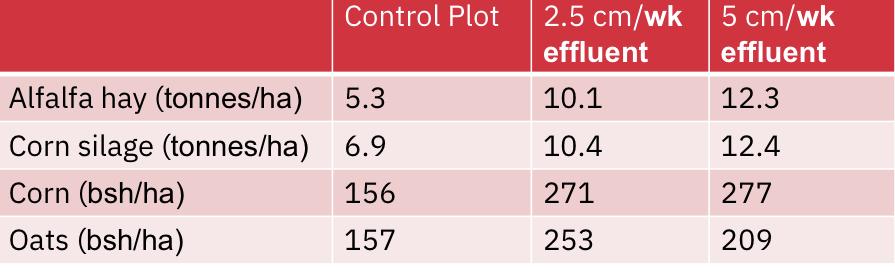
- One of the first experiments spaying sewage effluent on crop lands was done in 1963 in Pennsylvania where effluent was applied to experimental crops from April to November each year for a period of 5 years.
- Results were compared to a control plot (treated with commercial fertilizer)
- Land application of sewage effluent: use of effluent to recharge groundwater
- Studies have shown that when effluent is sprayed on land (and allowed to percolate to the groundwater), this percolation through 1m of well-aerated soil is an efficient mechanism for removing sewage pathogens (they do not survive well in soil)
- Caveat: sewage effluent contains bacteria, helminths (parasitic worms), Protozoa and viruses. Chlorination at POTW does a good job of killing bacteria and helminths, and soil percolation can effectively remove Protozoa… but viruses are of concern
- Studies have shown that although 100,000-folds reduction of viruses was possible after standard secondary treatment followed by percolation through soil for 10 days, detectable viruses were still present in the groundwater
- In addition to liquid effluent from a POTW, the soil sludge can also be put to good use in land application
- Sludge typically contains 3.2% nitrogen, 1.8% phosphorus and 0.3% potassium, 30-50% organic matter.
- Sewage sludge is an excellent source of N and P and because the elements are bound to organic matter, they are slowly released following land application
- Experiments have shown that a single application of sludge can provide N and P requirements for terrestrial planets for as long as 3-5 years.
- Benefits of the land application of sewage sludge:
- The ocean dumping Ban Act of 1988 prohibits ocean dumping of sludge after December 21, 1991 (so this provides an alternative disposal method)
- Sanitary landfills are filling up rapidly
- Incinerating sludge produces air quality issues
- In the USA, 3.4 million ha of land is bare due to strip mining (could benefit from this fertilizer)
- It is easier to transport solid sludge than wastewater
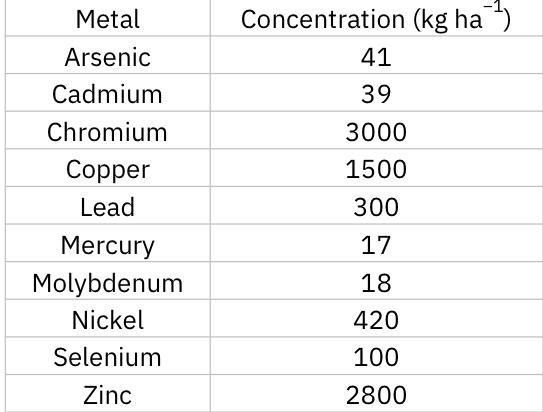
- Major drawback: must consider the metal concentration in sludge
- Maximum permissible cumulative amounts of metals associated with application of sewage sludge to agricultural land.
- Maximum permissible cumulative amounts of metals associated with application of sewage sludge to agricultural land.
- Phosphates in wastewater
- Detergents that contain phosphates have been (and continue to be) an important source of phosphates to water bodies, and can be a leading contributor to eutrophication development
- In the 1970’s, an experiments was conducted in Lake 226 (Canadian experimental lakes area) where the lake was divided into two halves with a plastic curtain
- Carbon and nitrogen were added to one half whereas carbon, nitrogen and phosphates were added to the other half
- One half: carbon + nitrogen fertilization
- Other half: carbon + nitrogen + phosphorus Fertilization
- Detergent phosphates
- Created in 1930, sodium tripolyphosphate (STP) is used as a water softening agent in laundry and dishwashing detergents
- By the 1960’s, as much as 50% or more of the phosphorus in municipal wastewater came from phosphates used in these detergents
- Because of the results Lake 226 experiment, legislation was passed to control phosphorus in sewage and remove phosphates from laundry detergent
- Currently, all laundry and dishwasher detergents sold in Canada contain no more than 0.5% phosphorus by weight (previous restrictions were 2.2%)