Chem 1110 Lec
What is Chemistry?
study of matter
the study of the interactions of matter
study of valence electrons - since they are more unstable than innermost electrons
conservation laws
mass
energy
electrons
charges
Quantum Mechanical Model of the Atom
Schrodinger’s Cat
electrons can behave like waves and particles
Beginnings of Quantum Mechanics
until the 20th century, it was believed that all physical phenomena were deterministic
work done at the time by famous physicists discovered that for sub-atomic particle, the presnet condition does not determine the future condition
quantum mechanics forms the foundation of chemistry—periodic table, behavior of the elements in chemical bonding
Behavior of Electrons
electrons are incredible small
electron behavior determines the behavior od atoms
directly observing electrons in the atom is impossible bc observing it can change its behavior
observe electrons like particles, it will behave like one (same for waves)
A Theory that Explains Electron Behavior
quantum mechanical model explains the manner in with electrons exist and behave in atoms
helps us understand and predict the properties of atoms
Light in its Wave Nature
light is a form of electromagnetic radiation
composed of perpendicular oscillating waves, one for electric field and one for magnetic field
All electromagnetic waves move through space at the same, constant speed
3.00 × 10 to the 8th m/s
Characterizing Waves
amplitude is the height of the wave
distance from the mode to the crest
measure of how intense the light is
wavelength is a measure of the distance covered by the wave
from one crest to the other
frequency is the amount of waves that pass through a point in the given time
# of waves = # of cycles
Hertz or cycles/s
total energy is proportional to the amp and the frequency of the wave
longer the amp, more force
more frequency, more force
Relationship between Wavelength and Frequency
inversely proportional
shorter the wavelength, more time they pass
Ex 1: what is the wavelength of red light with a frequency of 4.62 × 1014 s-1?
use wavelength times frequency and convert it to nm (assume speed of light)
Color
the color of light is determined by its wavelength
or frequency
White light is a mixture of all the colors of visible light
a spectrum
ROYGBIV
When an object absorbs some of the wavelengths of white light and reflects other, it appears colored
the observed color is predominantly the colors reflected
Amp & Wavelength
different wavelength different colors
different amps, different brightness
The Electromagnetic Spectrum
shorter wavelength (high frequency) light has hiigher energy
radio wave light has the lowest energy
Gamma ray light has the highest energy
high-energy electromagnetic radiation can potentially damage biological molecules
ionizing radiation
Types of Electromagnetic Radiation
Electromagnetic waves are classified by their wavelength
as they increase in frequency, they increase in energy
Radio Signal Transmission
AM - amplitude modification
FM - frequency modification
Standing Waves
not all waves are travelling waves
standing waves remain constrained within some region of space
standing waves play an important role in our understanding of the elctronic structure of atoms and molecules
2-D Waves
vibrational patterns of a flat surface are ex of 2D standing waves
for 2d standing waves, the nodes are lines of the surface
Interference
the interaction between waves is called interference
waves in phase - constructive interference
waves out of phase - destructive interference
Diffraction
when waves encounter an obstacle or opening in a barrier that is about the same size as the wavelength, they bend around it
with the 2-slit interference, there is constructive and and destructive interference
shows the difference between wave and particle interference
waves bend around an obstacle, particles do not
Blackbody Radiation
sunlight consists of a range of broadly distributed wavelengths that form a continuous spectrum
blackbody: convenient, ideal emitter that approximates the behavior of many materials when heated
ex: metal oven that can be heated to high temps
Ultraviolet Catastrophe
maxima in blackbody curves shift to shorter wavelengths as the temp increases
theoretical expressions as functions of temp fit the observed experimental blackbody curves well at longer wavelength
but there were significant discrepancies at shorter wavelengths → uv catastrophe
Max Planck (physics??)
derived a theoretical expression for blackbody radiation that fit the experimental observations exactly
atoms vibrated at increasing frequencies (or decreasing wavelengths) as temp increases
light energy was delivered to the atoms in packets called quanta or photons
proportionality (planck’s) constant: 6.626 × 10-34 J x s
energy of a photon is directly proportional to its frequency
inversely proportional to its wavelength
Practice
By wavelength (short to long)
gamma, uv, green light, red light, microwave
By frequency (l to h)
mw, red light, green light, uv, gamma
Photoelectric Effect
Photoelectric Effect: metals emit electrons when a light shines on their surface
light energy being transferred to the elctron
if wavelength is made shorter, or the intensity is brighter, more electrons should be ejected
if a dim light were used there would be a lag time before electrons were emitted
The Problem
threshold frequency: min frequency needed before electrons would be ejected
high-frequency light from a dim source w/o lag time
Ejected Electrons
one photon at the threshold frequency gives the electrons just enough energy for it to escape the atom
binding energy
shorter wavelength photon, the electron will absorb more energy than is necessary to escape
excess energy become KE of the ejected electron
Spectra
when atoms or molecules absorb energy, that energy is released as light energy
when that light is passed through a prism, a pattern of particular wavelengths of light is seen that is unique to that type of atom or molecule
called emission spectrum
non-continuous
flame tests can identify the material
Line Spectra of Hydrogen
Balmer was able to derive an empirical equation that related the 4 visible wavelengths of light emitted by hydrogen atoms to whole integers
Rydberg’s Spectrum Analysis
Rydberg analyzed the spectrum of hydrogen from Balmer’s work and came up with a generalized empirical formula to predict all emission lines from hydrogen
n1 < n2
Rutherford’s Nuclear Model
atom contains tiny dense center called nucleus
volume of the nucleus is 1/10 trillionth volume of the atom
nucleus is essentially the entire mass of the atom
nucleus is positively charged
the positive charge is balanced with the negative charge of the electrons
electrons move around in the empty space of the atom surrounding the nucleus
Problems w/ Rutherford’s Model
electrons are moving charged particles
moving charged particles give off energy
therefore, electrons should constatnly give off energy
Bohr’s Model
nuclear model doesn’t explain the structural changes when an atom gains or loses energy
electrons travel in orbits that are a fixed distance from the nucleus
electrons emit radiation when they “jump” from an orbit with higher energy down to an orbit with lower energy
Bohr’s major idea was that the energy of the atom was quantized, and that the amount of energy in the atom was related to the electron’s position in the atom.
Quantized means that the atom could only have very specific amounts of energy
Quantum Explanation of Atomic Spectra
each wavelength in the spectrum corresponds to an electron transition between orbitals
when excited, electrons transition from an orbital of lower energy to one of higher energy
when relaxed, transitions from a higher to a lower energy level
releases a photon of light whose energy equals the energy difference between orbitals
Electron Transitions
electron must gain correct amount of energy to the difference in energy between the final and initial states
Predicting the Spectrum of Hydrogen
wavelengths of lines in the emission spectrum of hydrogen can be predicted by calculating the difference in energy between any two states
electrons in energy state n, there are (n – 1) energy states it can transition to, therefore (n – 1) lines it can generate.
used for one-electron system
Energy Transitions in Hydrogen
energy of a photon released is equal to the difference in energy between the 2 levels the electron is jumping between
calculated by subtracting the energy of the initial state from the energy of the final state
Wave Behavior of Electrons
de Broglie predicted that the wavelength of a particle is inversely proportional to its momentum
b/c its so small, the wave character of electrons is significant
Electron Diffraction
proof that the electron had wave nature came from the demonstration that a beam of electrons would produce an interference pattern as waves do
Uncertainty Principle
Heisenberg determined the fundamental limit to how accurately a particle’s position and momentum can be measured simultaneously
the more accurately the momentum is measured, the less accurately we can determine the position at that time and vice versa
Heisenberg Uncertainty Principle: It is fundamentally impossible to determine simultaneously and exactly both the momentum and the position of a particle.
Complementary Properties
when you try to observe the wave nature of the electron, you cannot observe its particle nature and vice-versa
wave nature = interference pattern
particle nature = position, which slit its passing through
wave and particle nature of the electron are complementary
as you know more about one, you know less about the other
Determinacy vs. Indeterminacy
particles move in a path determined by the particle’s velocity, position and forces acting on it
determinacy = definite, predictable future
since we can’t know both the position and velocity of an electron, we cannot predict the path it will follow
indeterminacy = indefinite future, can only predict probability
best we can do: describe the probability an electron will be found in a particular region using stats
Electron Energy
Electron energy and position are complementary since KE = ½mv2
best we can do: describe a region in the atom of high probability of finding it
Many of the properties of atoms are related to the energies of the electrons
like ionization energy, metallic energy
Schrödinger’s Equation
allows us to calculate the probability of finding an electron with a particular amount of energy at a particular location in the atom
Ĥ is the Hamiltonian operator, a set of mathematical operations representing the total energy of the quantum particle
ψ is the wavefunction of the particle
E is the total energy of the particle
Solutions to Schrödinger’s Equation produce many wave functions, ψ
A plot of distance vs. ψ2 represents an orbital, a probability distribution map of a region where the electron is likely to be found
Solutions to the Wave Function, ψ
shows the size, shape and orientation in space of an orbital are determined by 3 integer terms in the wave function called quantum numbers
principal quantum number, n (describes the size and energy of the orbital)
angular momentum quantum number, l (describes shape)
orientation quantum number, ml (describes orientation)
magnetic spin quantum number, ms (not an integer)
Principal Quantum Number, n
Characterizes the energy of the electron in a particular orbital
pertaining to Bohr’s energy level
n can be any inter >= 1
The larger the value of n, the more energy the orbital has
Energies are defined as being negative
An electron would have E = 0 when it just escapes the atom
The larger the value of n, the larger the orbital.
As n gets larger, the amount of energy between orbitals becomes smaller
Angular Momentum Quantum Number, l
determines the shape of each value of l is called by the orbital
a particular letter that designates the shape of the orbital
s orbitals are spherical (l = 0)
p orbitals are like two balloons tied at the knots (l = 1)
d orbitals are mainly like four balloons tied at the knot (l = 2)
f orbitals are mainly like eight balloons tied at the knot (l = 3)
Orientation Quantum Number, ml
specifies the orientation of the orbital
the direction in space the orbital is aligned relative to the other orbitals
Values are integers from −l to +l
including zero
gives the number of orbitals of a particular shape
when l = 2, the values of ml are −2, −1, 0, +1, +2; which means there are five orbitals with l = 2
Magnetic Spin Quantum Number, ms
indicates the spin of electrons in the orbital
has a value +1/2 or -1/2
Paramagnetic (has unpaired electrons)
substance is attracted to a magnetic field
substances with unpaired electrons are paramagnetic
Diamagnetic (has no unpaired electrons)
NOT attracted to a magnetic field
Substances have paired spins
Describing an Orbital
Each set of n, l, ml , and ms describes one orbital
Orbitals with the same value of n are in the same principal energy level
or principal shell
Orbitals with the same values of n and l are said to be in the same sublevel.
or subshell
Predicting Quantum Numbers
Determine a set of quantum numbers for an electron in a 3p orbital
n =3 for an electron in the third level
( n = 3, p = 1, l = -1, ms = -1/2)
(n = 3, p = 1, l = -1, ms = +1/2)
( n = 3, p = 1, l = 0, ms = -1/2)
( n = 3, p = 1, l = 0, ms = +1/2)
( n = 3, p = 1, l = 1, ms = -1/2)
( n = 3, p = 1, l = 1, ms = +1/2)
Probability & Radial Distribution Functions
y2 is the probability density
represents the total probability of finding an electron at a particular point in space
for s orbital maximum at the nucleus?
decreases as you move away from the nucleus
The Radial Distribution function represents the total probability of finding an electron within a thin spherical shell at a distance r from the nucleus..
the probability at a point decreases with increasing distance from the nucleus, but the volume of the spherical shell increases
Nodes in the functions are where the probability drops to 0
The Shapes of Atomic Orbitals
The l quantum number primarily determines the shape of the orbital.
l can have integer values from 0 to (n – 1).
Each value of l is called by a particular letter that designates the shape of the orbital.
s orbitals are spherical.
p orbitals are like two balloons tied at the knots.
d orbitals are mainly like four balloons tied at the knot.
f orbitals are mainly like eight balloons tied at the knot.
know s and p orbitals and be able to recognize the d orbitals
l = 0, the s Orbital
Each principal energy level has one s orbital.
Lowest energy orbital in a principal energy state
Spherical
Number of nodes = (n – 1)
l = 1, p Orbitals
Each principal energy state above n = 1 has three p orbitals.
ml = −1, 0, +1
Each of the three orbitals points along a different axis
px , py , pz
2nd lowest energy orbitals in a principal energy state
Two-lobed
One node at the nucleus, total of n nodes
l = 2, d Orbitals
Each principal energy state above n = 2 has five d orbitals.
ml = −2, − 1, 0, +1, +2
Four of the five orbitals are aligned in a different plane.
the fifth is aligned with the z axis, dz²
dxy, dyz, dxz dx² - y²
3rd lowest energy orbitals in a principal energy level
Mainly four-lobed
one is two-lobed with a toroid
Planar nodes
higher principal levels also have spherical nodes
l = 3, f Orbitals
Each principal energy state above n = 3 has seven f orbitals.
ml = −3, −2, −1, 0, +1, +2, +3
4th lowest energy orbitals in a principal energy state
Mainly eight-lobed
some two-lobed with a toroid
Planar nodes
higher principal levels also have spherical nodes.
Periodicity – Periodic Table and Its Trends
History of the Periodic Table
In 1669, Hennig Brand attempted to make a Philosopher’s Stone
In 1680, Robert Boyle discovered phosphorus
in 1863, John Newlands divided and then discovered 56 elements into 11 groups based on characteristics
In 1897, English physicist J. J. Thomson first discovered electrons; small negatively charged particles in an atom. John Townsend and Robert Millikan determined their exact charge and mass. In 1900, Bequerel discovered that electrons and beta particles as identified by the Curies are the same thing.
In 1903, Rutherford announced that radioactivity is caused by the breakdown of atoms. In 1911, Rutherford and German physicist Hans Geiger discovered that electrons orbit the nucleus of an atom.
In 1913, Bohr discovered that electrons move around a nucleus in discrete energy called orbitals. Radiation is emitted during movement from one orbital to another.
Dimitri Mendeleev
Order elements by atomic mass
Observed a repeating pattern of properties
Periodic Law: when the elements are arranged in order of increasing atomic mass, certain sets of properties recur periodically
Put elements with similar properties in the same column
Used pattern to predict properties of undiscovered elements
Electron Configurations
Quantum-mechanical theory describes the behavior of electrons in atoms.
The electrons in atoms exist in orbitals.
A description of the orbitals occupied by electrons is called an electron configuration.
How Electrons Occupy Orbitals
Calculations with Schrödinger’s equation show hydrogen’s one electron occupies the lowest energy orbital in the atom.
Schrödinger’s equation calculations for multielectron atoms cannot be exactly solved.
due to additional terms added for electron-electron interactions
Approximate solutions show the orbitals to be hydrogen-like.
Two additional concepts affect multielectron atoms: electron spin and energy splitting of sublevels.
Electron Spin
Experiments by Stern and Gerlach showed a beam of silver atoms is split in two by a magnetic field.
The experiment reveals that the electrons spin on their axis.
As they spin, they generate a magnetic field.
spinning charged particles generate a magnetic field.
If there is an even number of electrons, about half the atoms will have a net magnetic field pointing “north” and the other half will have a net magnetic field pointing “south”.
The Property of Electron Spin
Spin is a fundamental property of all electrons.
All electrons have the same amount of spin.
The orientation of the electron spin is quantized, it can only be in one direction or its opposite.
spin up or spin down
The electron’s spin adds a fourth quantum number to the description of electrons in an atom, called the Spin Quantum Number, ms.
Spin Quantum Number, ms, and Orbital Diagrams
ms can have values of +½ or −½
Orbital Diagrams use a square to represent each orbital and a half-arrow to represent each electron in the orbital
A half-arrow pointing up is used to represent an electron in an orbital with spin up
Spins must cancel in an orbital
paired
We often represent an orbital as a square and the electrons in that orbital as arrows.
the direction of the arrow represents the spin of the electron.
Pauli Exclusion Principle
No two electrons in an atom may have the same set of four quantum numbers.
Therefore no orbital may have more than two electrons, and they must have with opposite spins.
Knowing the number orbitals in a sublevel allows us to determine the maximum number of electrons in the sublevel.
s sublevel has 1 orbital, therefore, it can hold 2 total electrons.
p sublevel has 3 orbitals, therefore, it can hold 6 total electrons.
d sublevel has 5 orbitals, therefore, it can hold 10 total electrons.
f sublevel has 7 orbitals, therefore, it can hold 14 total electrons.
Quantum Numbers of Helium’s Electrons
Helium has two electrons
both electrons are in the first energy level.
Both electrons are in the s orbital of the first energy level.
Because they are in the same orbital, they must have opposite spins.
first electron: n = 1, l = 0, ml = 0, ms = +1/2
second electron: n = 1, l = 0, ml = 0, ms = -1/2
Sublevel Splitting in Multielectron Atoms
The sublevels in each principal energy shell of Hydrogen all have the same energy.
or other single electron systems
Orbitals with the same energy - Degenerate
For multielectron atoms, the energies of the sublevels are split.
caused by charge interaction, shielding and penetration
The lower the value of the l quantum number, the less energy the sublevel has
Coulomb’s Law
Coulomb’s Law describes the attractions and repulsions between charged particles.
For like charges, the potential energy (E) is positive and decreases as the particles get farther apart .
For opposite charges, the potential energy is negative and becomes more negative as the particles get closer together.
The strength of the interaction increases as the size of the charges increases.
Electrons are more strongly attracted to a nucleus with a 2+ charge than a nucleus with a 1+ charge.
Effective Nuclear Charge
In a particle (atom or ion) with more than one electron, there are both repulsions (between electrons) and attractions (between electrons and nucleus) to consider.
The net positive charge from the nucleus that an electron experiences is the effective nuclear charge, Zeff.
A valence electron experiences a Zeff that is lower than the actual nuclear charge, Z (number of protons). 𝑍eff<𝑍
Some of the nuclear charge is shielded by the core (inner) electrons.
𝑍eff=𝑍−𝑆, where S is the shielding.
Shielding of Nuclear Charge
Sodium has 1 valence electron in the 3s orbital and 10 core electrons, shielding it from the +11 charge of the nucleus.
has an overall charge of +1 since 11 - 10
Magnesium has 2 valence electrons in the 3s orbital and the same 10 core electrons, shielding them from the +12 charge of the nucleus.
has an overall charge of +2 since 12 - 10
Each valence electron shields the other to a small extent, but core electrons are more effective at shielding than valence electrons.
Therefore, magnesium experiences a higher Zeff than sodium
**Slater’s Rules for Calculating Shielding, S**
Valence electrons (in n energy level) contribute a value of 0.35 toward the shielding.
Core or inner electrons (in any energy level less than n) contribute a value of 0.85 toward the shielding.
𝑆 = # valence electrons (0.35) + # core electrons (0.85)
For sodium :
Z (amt of protons) = 11
S= 0(.35) + 10(.85) = 8.5
Zeff = Z - S = 11 - 8.5 = +2.5
having a higher effective nuclear charge makes it easier to take valence electrons away from an atom
Penetration
The closer an electron is to the nucleus, the more attraction it experiences.
The better an outer electron is at penetrating through the electron cloud of inner electrons, the more attraction it will have for the nucleus.
The degree of penetration is related to the orbital’s radial distribution function.
In particular, the distance the maxima of the function are from the nucleus.
Penetration and Shielding
The radial distribution function shows that the 2s orbital penetrates more deeply into the 1s orbital than does the 2p.
The weaker penetration of the 2p sublevel means that electrons in the 2p sublevel experience more repulsive force, they are more shielded from the attractive force of the nucleus.
The deeper penetration of the 2s electrons means electrons in the 2s sublevel experience a greater attractive force to the nucleus and are not shielded as effectively.
Effect of Penetration and Shielding
Penetration causes the energies of sublevels in the same principal level to not be degenerate.
In the fourth and fifth principal levels, the effects of penetration become so important that the s orbital actually lies lower in energy than the d orbitals of the previous principal level.
The energy separations between one set of orbitals and the next become smaller beyond the 4s.
The ordering can therefore vary among elements.
causing variations in the electron configurations of the transition metals and their ions
Rules in Filling in the Orbitals
Energy levels and sublevels fill from lowest energy to highest.
s → p → d → f
Aufbau Principle
Orbitals that are in the same sublevel have the same energy.
No more than two electrons per orbital
Pauli Exclusion Principle
When filling orbitals that have the same energy, place one electron in each before completing pairs.
Hund’s Rule
Ground State Electron Configuration
The electron configuration is a listing of the sublevels in order of filling with the number of electrons in that sublevel written as a superscript.
Kr = 36 electrons = 1s22s22p63s23p64s23d104p6
A short-hand way of writing an electron configuration is to use the symbol of the previous noble gas in [ ] to represent all the inner electrons, then just write the last set.
Rb = [Kr]5s1
Order of Sublevel Filling
Start by drawing a diagram putting each energy shell on a row and listing the sublevels, (s, p, d, f), for that shell in order of energy (left-to right).
Next, draw arrows through the diagonals, looping back to the next diagonal each time
ex: Mn 1s22s22p63s23p64s23d5
practice: full ground state orbital diagram and electron configuration of potassium
1s22s22p63s23p64s1
Valence Electrons
The electrons in all the sublevels with the highest principal energy shell are called the valence electrons.
Electrons in lower energy shells are called core electrons.
Chemists have observed that one of the most important factors in the way an atom behaves, both chemically and physically, is the number of valence electrons.
Electron Configuration & the Periodic Table
The Group number corresponds to the number of valence electrons.
The length of each “block” is the maximum number of electrons the sublevel can hold.
The Period number corresponds to the principal energy level of the valence electrons.
Transition Elements
For the d block metals, the principal energy level is one less than valence shell.
one less than the Period number
sometimes s electrons are “promoted” to d sublevel
For the f block metals, the principal energy level is two less than valence shell.
two less than the Period number they really belong to
sometimes d electron in configuration
Irregular Electron Configurations
Because of sublevel splitting, the 4s sublevel is lower in energy than the 3d; and therefore the 4s fills before the 3d.
ex: Cr = [Ar] 4s13d5
Cu = [Ar] 4s13d10
Mo = [Kr] 5s14d5
Ru = [Kr] 5s14d7
Pd = [Kr] 5s04d10
However, the difference in energy small.
Some of the transition metals have irregular electron configurations in which the ns only partially fills before the (n−1)d or doesn’t fill at all.
Therefore, their electron configuration must be found experimentally.
Properties & Electron Configuration
The properties of the elements follow a periodic pattern.
Elements in the same column have similar properties.
Elements in a period show a pattern that repeats.
The quantum-mechanical model explains this because the number of valence electrons and the types of orbitals they occupy are also periodic.
The Noble Gas Electron Configuration
The noble gases have eight valence electrons.
except for He, which has only two electrons
The noble gases are especially non-reactive.
He and Ne are practically inert
The reason the noble gases are so non-reactive is that the electron configuration of the noble gases is especially stable
ex: Na : [Ne] 3s1 (one unpaired e- so it is paramagnetic)
ex: Te : [Kr] 5s24d105p4 (2 unpaired e- so paramagnetic)
ex: Tc : [Kr] 5s24d5 (5 unpaired e- so paramagnetic)
The Alkali Metals
The alkali metals have one more electron than the previous noble gas.
In their reactions, the alkali metals tend to lose one electron, resulting in the same electron configuration as a noble gas.
forming a cation with a 1+ charge
The Halogens
The electron configurations of the halogens all have one fewer electron than the next noble gas.
In their reactions with metals, the halogens tend to gain an electron and attain the electron configuration of the next noble gas.
forming an anion with charge 1−
In their reactions with nonmetals, they tend to share electrons with the other nonmetal so that each attains the electron configuration of a noble gas.
Eight Valence Electrons
Quantum mechanical calculations show that eight valence electrons should result in a very unreactive atom.
an atom that is very stable
Noble gases have eight valence electrons and are all very stable and unreactive.
Note: Helium only has two valence electrons, but that fills its valence shell.
Conversely, elements that have either one more or one less electron should be very reactive.
Halogen atoms have seven valence electrons and are, in general, the most reactive nonmetals .
Alkali metals have one more electron than a noble gas atom and are, in general, the most reactive metals
Electron Configuration & Ion Charge
Many metals and nonmetals form one ion, and that the charge on that ion is predictable based on its position on the Periodic Table.
Group 1A = 1+
Group 2A = 2+
Group 7A = 1−
Group 6A = 2−
etc.
These atoms form ions that will result in an electron configuration that is the same as the nearest noble gas.
they are isoelectronic to their closest noble gases
Ground State Electronic Configuration of Anions
Anions are formed when nonmetal atoms gain enough electrons to have eight valence electrons.
filling the s and p sublevels of the valence shell
The sulfur atom has six valence electrons.
S atom = 1s22s22p63s23p4
In order to have eight valence electrons, sulfur must gain two more.
S2- anion = 1s22s22p63s23p6
Ground State Electronic Configuration of Cations
Cations are formed when a metal atom loses all its valence electrons.
This results in a new lower energy level valence shell.
However, the process is always endothermic.
The magnesium atom has two valence electrons.
Mg atom = 1s22s22p63s2
When magnesium forms a cation, it loses its valence electrons to produce a stable noble-gas configuration.
Mg2+ cation = 1s22s22p6
Trend in Atomic Radius - Main Group
There are several methods for measuring the radius of an atom, and they give slightly different numbers.
van der Waals radius = nonbonding
covalent radius = bonding radius
Atomic radius is an average radius of an atom based on measuring large numbers of elements and compounds.
Atomic Radius increases down a group.
The valence shell is farther from the nucleus.
Effective nuclear charge is fairly close.
Atomic Radius decreases across period (left to right).
Electrons are added to the same valence shell.
Effective nuclear charge increases.
The valence shell is held closer by the nucleus.
Quantum-Mechanical Explanation for the Group Trend in Atomic Radius
The size of an atom is related to the distance the valence electrons are from the nucleus.
the larger the orbital an electron is in, the farther its most probable distance will be from the nucleus and the less attraction it will have for the nucleus.
Traversing down a group adds a principal energy level.
The larger the principal energy level an orbital is in, the larger its volume.
therefore, quantum-mechanics predicts the atoms should get larger down a column.
Quantum-Mechanical Explanation for the Period Trend in Atomic Radius
The larger the effective nuclear charge an electron experiences, the stronger the attraction it will have for the nucleus.
The stronger the attraction the valence electrons have for the nucleus, the closer their average distance will be to the nucleus.
Traversing across a period increases the effective nuclear charge on the valence electrons.
therefore, quantum-mechanics predicts the atoms should get smaller across a period.
Trends in Atomic Radius - Transition Metals
Atoms in the same group increase in size down the column.
Atomic radii of transition metals roughly the same size across the d block.
much less difference than across main group elements
valence shell ns2, not the (n−1)d electrons
effective nuclear charge on the ns2 electrons approximately the same
Electron Configurations of Main Group Cations
Cations form when the atom loses electrons from the valence shell.
Electron Configurations of Transition Metal Cations
When transition metals form cations, the first electrons removed are the valence electrons, even though other electrons were added after.
Electrons may also be removed from the sublevel closest to the valence shell after the valence electrons.
The iron atom has two valence electrons.
Fe atom = 1s22s22p63s23p64s23d6
When iron forms a cation, it first loses its valence electrons.
Fe2+ cation = 1s22s22p63s23p63d6
It can then lose 3d electrons.
Fe3+ cation = 1s22s22p63s23p63d5
Magnetic Properties of Transition Metal Atoms & Ions
Electron configurations that result in unpaired electrons mean that the atom or ion will have a net magnetic field – this is called paramagnetism.
will be attracted to a magnetic field
Electron configurations that result in all paired electrons mean that the atom or ion will have no magnetic field – this is called diamagnetism.
slightly repelled by a magnetic field
Transition Metal Atoms and Ions: Electron Configuration & Magnetic Properties
Both Zn atoms and Zn2+ ions are diamagnetic.
showing that the two 4s electrons are lost before the 3d
Zn atoms [Ar]4s23d10
Zn2+ ions [Ar]4s03d10
Ag forms both Ag+ ions and, rarely, Ag2+.
Ag atoms [Kr]5s14d10 are paramagnetic
Ag+ ions [Kr]4d10 are diamagnetic
Ag2+ ions [Kr]4d9 are paramagnetic
Trends in Ionic Radius
Ions in same group have same charge.
Ion size increases down the column.
higher valence shell, larger
Cations are smaller than neutral atoms.
Anions are larger than neutral atoms.
Cations are smaller than anions.
except Rb+ & Cs+ bigger or same size as F− and O2−
Larger positive charge = smaller cation
for isoelectronic species
isoelectronic = same electron configuration
Larger negative charge = larger anion
for isoelectronic species
Quantum-Mechanical Explanation for the Trends in Cation Radius
When atoms form cations, the valence electrons are removed.
The farthest electrons from the nucleus then are the p or d electrons in the (n − 1) energy level.
This results in the cation being smaller than the atom.
These “new valence electrons” also experience a larger effective nuclear charge than the “old valence electrons,” shrinking the ion even more.
Traversing down a group increases the (n − 1) level, causing the cations to get larger.
Traversing to the right across a period increases the effective nuclear charge for isoelectronic cations, causing the cations to get smaller.
Quantum-Mechanical Explanation for the Trends in Anion Radius
When atoms form anions, electrons are added to the valence shell.
These “new valence electrons” experience a smaller effective nuclear charge than the “old valence electrons,” increasing the size.
The result is that the anion is larger than the atom.
Traversing down a group increases the n level, causing the anions to get larger.
Traversing to the right across a period increases the effective nuclear charge for isoelectronic anions, causing the anions to get smaller.
Ionization Energy
Minimum energy needed to remove the outmost electron from an atom or ion
gas state
endothermic process
valence electron easiest to remove, lowest IE
M(g) + IE1 → M1+(g) + 1 e-
M1+(g) + IE2 → M2+(g) + 1 e-
1st ionization energy (IE) = energy to remove electron from neutral atom
2nd IE = energy to remove from 1+ ion; etc.
General Trends in 1st Ionization Energy
The larger the effective nuclear charge on the electron, the more energy it takes to remove it.
The farther the most probable distance the electron is from the nucleus, the less energy it takes to remove it.
1st IE decreases down the group.
The valence electron is farther from the nucleus.
1st IE generally increases across the period.
Effective nuclear charge increases.
doesn’t change much for transition elements since they all have the same amounts of valence electrons
Quantum-Mechanical Explanation for the Trends in First Ionization Energy
The strength of attraction is related to the most probable distance the valence electrons are from the nucleus and the effective nuclear charge the valence electrons experience.
The larger the orbital an electron is in, the farther its most probable distance will be from the nucleus and the less attraction it will have for the nucleus
therefore quantum-mechanics predicts the atom’s first ionization energy should get lower down a column.
Traversing across a period increases the effective nuclear charge on the valence electrons.
therefore quantum-mechanics predicts the atom’s first ionization energy should get larger across a period.
Exceptions in the 1st IE Trends
generally increases from left to right across a period
except from 2A to 3A, 5A to 6A
To ionize beryllium, a full sublevel must be broken up, resulting in extra energy
When boron is ionized, a full sublevel is the formed, resulting in less energy
To ionize nitrogen, a half-full sublevel must be broken up, resulting in extra energy
When oxygen is ionized, a half-full sublevel is formed, resulting in less energy
Trends in Successive Ionization Energies
Removal of each successive electron costs more energy
Shrinkage in size is due to having more protons than electrons
Outer electrons are closer to the nucleus; therefore, they are harder to remove
Regular increase in energy for each successive valence electron
Large increase in energy when start removing core electrons
Second and Third Ionization Energies
The second ionization energy of an atom is the energy required to remove the second electron from the +1 gaseous ion.
The third ionization energy is the energy required to remove the third electron from the +2 ion.
Electron Affinity
Energy is released when an neutral atom gains an electron
gas state
M(g) + 1 e- → M1-(g) + EA
EA is defined as exothermic (−), but may actually be endothermic (+).
Some alkali earth metals & all noble gases are endothermic. Why?
The more energy that is released, the larger the electron affinity.
the more negative the number, the larger the EA
Trends in Electron Affinity
Alkali metals decrease electron affinity down the column.
but not all groups do
generally irregular increase in EA from 2nd period to 3rd period
“Generally” increases across period
becomes more negative from left to right
not absolute
Group 5A generally lower EA than expected because extra electron must pair
Group 2A and 8A generally very low EA because added electron goes into higher energy level or sublevel
Highest EA in any period = halogen
Properties of Metals & Nonmetals
Metals
malleable & ductile
shiny, lusterous, reflect light
conduct heat and electricity
most oxides basic and ionic
form cations in solution
lose electrons in reactions – oxidized
Nonmetals
brittle in solid state
dull, non-reflective solid surface
electrical and thermal insulators
most oxides are acidic and molecular
form anions and polyatomic anions
gain electrons in reactions – reduced
Metallic Character
Metallic character is how closely an element’s properties match the ideal properties of a metal
more malleable and ductile, better conductors, and easier to ionize
Metallic character decreases left-to-right across a period
metals are found at the left of the period and nonmetals are to the right
Metallic character increases down the column
nonmetals are found at the top of the middle Main Group elements and metals are found at the bottom
Quantum-Mechanical Explanation for the Trends in Metallic Character
Metals generally have smaller first ionization energies and nonmetals generally have larger electron affinities.
except for the noble gases
therefore, quantum-mechanics predicts the atom’s metallic character should increase down a column because the valence electrons are not held as strongly
therefore, quantum-mechanics predicts the atom’s metallic character should decrease across a period because the valence electrons are held more strongly and the electron affinity increases.
Chemical Bonding and Lewis Structures
Bonding Theories
Explain how and why atoms attach together to form molecules.
Explain why some combinations of atoms are stable and others are not.
why is water H2O, not HO or H3O
Use to predict the shapes of molecules.
Use to predict the chemical and physical properties of compounds.
Lewis Bonding Theory
One of the simplest bonding theories is called Lewis Theory
Lewis Theory emphasizes valence electrons to explain bonding
Using Lewis theory, models can be drawn based on Lewis structures.
aka Electron Dot Structures
Many properties of molecules can be predicted by using Lewis structures.
such as molecular stability, shape, size, polarity
Why Do Atoms Bond?
Chemical bonds form because they lower the potential energy between the charged particles that make up the atoms.
A chemical bond forms when the potential energy of the bonded atoms is less than the potential energy of the separate atoms.
To calculate this potential energy, the following interactions need to be considered:
nucleus–to–nucleus repulsions
electron–to–electron repulsions
nucleus–to–electron attractions
Ionic Bonds
When a metal atom loses electrons, it becomes a cation.
Metals have low ionization energies, making it relatively easy to remove electrons from them.
When a nonmetal atom gains electrons, it becomes an anion.
Nonmetals have high electron affinities, making it advantageous to add electrons to these atoms.
The oppositely charged ions are then attracted to each other, resulting in an ionic bond. Ionic bonds are electrostatic in nature.
Covalent Bonds
Nonmetal atoms have relatively high ionization energies, so it is difficult to remove electrons from them.
When nonmetals bond together, it is better in terms of potential energy for the atoms to share valence electrons.
Potential energy is lowest when the electrons are between the nuclei.
Shared electrons hold the atoms together by attracting nuclei of both atoms
Metallic Bonds
The relatively low ionization energy of metals allows them to lose electrons easily.
The simplest theory of metallic bonding involves the metal atoms releasing their valence electrons to be shared as a pool by all the atoms/ions in the metal.
It resembles an organization of metal cation islands that are separated in a sea of electrons.
Electrons are delocalized throughout the metal structure.
Bonding results from attraction of cation for the delocalized electrons
Valence Electrons & Bonding
Valence electrons are held most loosely.
Chemical bonding involves the transfer or sharing of electrons between two or more atoms.
Valence electrons are most important in bonding.
Lewis theory focuses on the behavior of the valence electrons
Determining the Number of Valence Electrons in an Atom
the column number on the periodic table indicates how many valence electrons a main group atom has
most transition elements have 2 valence electrons
Lewis Structures of Atoms
in a Lewis structure, the valence electrons of main-group elements are represented as dots surrounding the symbol for the element
aka electron dot structures
the symbol of element is used to represent the nucleus and the inner electrons
dots around the symbol are used to represent valence electrons
pair the first 2 dots for the s orbital electrons
put one dot on each open side for first three p electrons
pair the rest of dots for the remaining p electrons
Stable Electron Arrangement and Ion Change
metals form cations by losing enough electrons to get the same electron configuration as the previous noble gas
nonmetals form anions by gaining enough electrons to get the same electron configuration as the next noble gas
the noble gas electron configuration must be very stable
Lewis Bonding Theory
atoms form bonds bc bonding results in a more stable electron config.
more stable = lower PE
atoms bond tg by either transferring or sharing outer electrons
this results in most atoms obtaining an outer shell with 8 electrons
octet rule
some exceptions
Octet Rule
ns2np6
noble gas configuration
exceptions:
H, Li, Be, B : attain an electron config like He
helium: two valence electrons, a duet
lithium: loses its one valence electron
hydrogen: shares or gains one electron
usually loses to become H+
beryllium: loses two electrons to become Be+
usually shares its 2 electrons in covalent bonds, resulting in 4 valence electrons
boron: loses 3 electrons to become B+
usually shares its 3 electrons in covalent bonds, resulting in 6 valence electrons
expanded octets: often occur for elements in period 3 or below
usually by empty valence d orbitals
Ionic Bonds
when a metal loses electrons, it becomes a cation
metals have low ionization energies, making it easy to remove electrons from them
when a nonmetal atom gains electrons it becomes an anion
nonmetals have high electron affinities, making it beneficial for them to add electrons to these atoms
oppositely charged ions are attracted to each other, resulting in an ionic bond which are electrostatic in nature
Electron Transfer to Form Compounds
metal atoms can transfer electrons to nonmetal atoms to form ions
these ions attract each other due to opposite charges forming ionic bonds
when forming ionic compounds, atoms tend to accept or donate electrons to achieve the electronic structure of the nearest noble gas
Ionic Lattice
individual pairs of ions do not bond together, rather huge numbers of both types of ions form a 3D lattice
the chemical formula of an ionic compound indicates the simplest ratio of the types of ions in the lattice
the lattice is held together by electrostatic attraction s between the many oppositely charged cations and anions
very different from the bonding of discrete particles
Ionic Bonding and the Crystal Lattice
the extra energy released comes from the formation of a strucutre in which every cation is surrounded by anions
called a crystal lattice
held tg by electrostatic attractions of the cations for all surrounding anions
c.l maximizes the attractions between cations and anions which leads to the most stable arrangement
Crystal Lattice
electrostatic attraction is non-directional
no direct anion-cation pair
therefore, there is no ionic molecule
the chemical formula is an empirical formula by giving the ratio of ions based on charge balance
Lattice Energy
the extra stability that accompanies the formation of the crystal lattice is measured as the lattice energy
the lattice energy is the energy released when the solid crystal forms from separate ions in the gaseous state
always exothermic
hard to measure directly, but can be calculated from knowledge of other process
born-haber cycle
depends directly on size of charges and inversely on distance between ions
∆𝐸=1/(4𝜋𝜀o ) (𝑞1𝑞2)/𝑟
Trends in Lattice Energy Ion Size
force of attraction between charged particles is inversely proportional to the distance between them
larger ions mean the center of positive charge (nucleus of the cation) is farther away form the negative charge (electrons of the anion)
larger ion = weaker attraction
weaker attraction = smaller lattice energy
Trends in Lattice Energy Ion charge
the force of attraction between oppositely charged particles is directly proportional to the product of the charges
larger charge means the ions are more strongly attracted to each other
larger charge = stronger attraction
stronger attraction = larger lattice energy
of the two factors, charge is more important
Ionic Bonding: Model vs IRL
Lewis theory implies that the attractions between ions are strong
Lewis theory predicts ionic compounds should have high melting points and boiling points because breaking down the crystal should require a lot of energy
the stronger the attraction (larger the lattice energy), the higher the melting point
Ionic compounds do have high melting points and boiling points
MP generally > 300 °C
All ionic compounds are solids at room temperature.
Covalent Bonding
Lewis Theory of Covalent Bonding
implies that an alternative way atoms can achieve an octet of valence electrons is to share their valence electrons with other atoms
the shared electrons would then count towards each atom’s octet
sharing of e- is covalent bonding
Covalent Bonding: Model vs Reality
Lewis theory implies that some combinations should be stable, whereas others should not.
Because the stable combinations result in “octets”
Using these ideas, Lewis theory allows us to predict the formulas of molecules of covalently bonded substances.
Hydrogen and the halogens are all diatomic molecular elements, as predicted by Lewis theory.
Oxygen generally forms either two single bonds or a double bond in its molecular compounds, as predicted by Lewis theory.
There are some stable compounds in which oxygen has one single bond and another where it has a triple bond, but it still has an octet.
Lewis theory of covalent bonding implies that the attractions between atoms are directional.
The shared electrons are most stable between the bonding atoms.
Therefore, Lewis theory predicts that covalently bonded compounds will be found as individual molecules.
rather than an array like ionic compounds
Compounds of nonmetals are made of individual molecule units.
Lewis theory predicts the melting and boiling points of molecular compounds should be relatively low.
It involves breaking the attractions between the molecules, but not the bonds between the atoms.
The covalent bonds are strong, but the attractions between the molecules are generally weak.
Molecular compounds have low melting points and boiling points.
MP generally < 300 °C.
Molecular compounds are found in all three states at room temperature.
Lewis theory predicts that the hardness and brittleness of molecular compounds should vary depending on the strength of intermolecular attractive forces.
The type and the strength of the intermolecular attractions vary based on many factors.
Some molecular solids are brittle and hard, but many are soft and waxy.
Lewis theory predicts that neither molecular solids nor liquids should conduct electricity.
There are no charged particles around to allow the material to conduct.
Molecular compounds DO NOT conduct electricity in the solid or liquid state .
Molecular acids conduct electricity when dissolved in water because they are IONIZED by water.
Lewis theory predicts that the more electrons two atoms share, the stronger the bond should be.
Bond strength is measured by how much energy must be added into the bond to break it in half.
In general, triple bonds are stronger than double bonds, and double bonds are stronger than single bonds.
however, Lewis theory would predict double bonds are twice as strong as single bonds, but the reality is that they are less than twice as strong.
Lewis theory predicts that the more electrons two atoms share, the shorter the bond should be.
when comparing bonds to like atoms
Bond length is determined by measuring the distance between the nuclei of bonded atoms.
In general, triple bonds are shorter than double bonds, and double bonds are shorter than single bonds.
Polar Covalent Bonding
Covalent bonding between unlike atoms results in unequal sharing of the electrons.
One atom pulls the electrons in the bond closer to its side.
One end of the bond has larger electron density than the other.
The result is a polar covalent bond.
bond polarity
The end with the larger electron density gets a partial negative charge.
The end that is electron deficient gets a partial positive charge.
Bond Polarity
Most bonds have some degree of sharing and some degree of ion formation to them.
Bonds are classified as covalent if the amount of electron transfer is insufficient (less than 50%) for the material to display the classic properties of ionic compounds.
If the sharing is unequal enough to produce a dipole in the bond, the bond is classified as polar covalent.
Electronegativity
The ability of an atom to attract bonding electrons to itself is called electronegativity.
Increases across period (left to right).
Decreases down group (top to bottom).
Fluorine is the MOST electronegative element.
Francium is the LEAST electronegative element.
Noble gas atoms are not assigned values.
Opposite of atomic size trend
The larger the difference in electronegativity, the more polar the bond.
The negative end is toward the more electronegative atom.
The Effect of Delta EN on Bond Type
Delta EN < .5 (non-polar covalent bond)
.5 < Delta EN < 1.9 (polar covalent bond)
Delta EN > 1.9 (ionic)
Bond Dipole Moments
Dipole moment, m, is a measure of bond polarity.
A dipole is a material with a “+” and “−” end.
It is directly proportional to the size of the partial charges and directly proportional to the distance between them.
m = (q)(r)
not Coulomb’s Law
measured in Debyes, D
Generally, the more electrons two atoms share and the larger the atoms are, the larger the dipole moment.
Percent Ionic Character
The percent ionic character is the percentage of a bond’s measured dipole moment compared to what it would be if the electrons were completely transferred.
The percent ionic character indicates the degree to which the electron is transferred.
Partial Charge on Dipole Moment
If the % character is 50% or greater, the bond is considered ionic.
If it is less than 50%, it is considered a polar covalent bond with a dipole moment.
For example, if a diatomic molecule (XY) has a % ionic character of 15%. This indicates that the electron has been transferred 15% from X to Y.
Therefore, the partial charge on elements X and Y would be
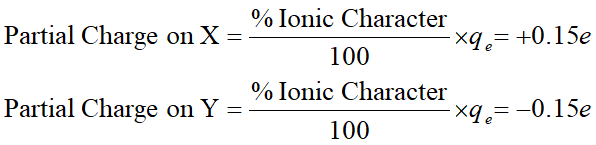
Lewis Structures of Molecules
Lewis theory allows us to predict the distribution of valence electrons in a molecule.
It is useful for the understanding the bonding in many compounds.
Allows us to predict shapes of molecules.
Allows us to predict properties of molecules and how they will interact together.
Lewis Structures
In general, follow the common bonding patterns.
C = 4 bonds & 0 lone pairs; N = 3 bonds & 1 lone pair, O= 2 bonds & 2 lone pairs; H and halogen = 1 bond; Be = 2 bonds & 0 lone pairs; B = 3 bonds & 0 lone pairs
Lewis structures with line bonds often have the lone pairs left off.
Their presence is assumed from common bonding patterns.
Structures that result in bonding patterns different from the common patterns may have formal charges.
Formal Charge
The charge that is assigned to an atom in a molecule, assuming that electrons in all chemical bonds are shared equally between atoms, regardless of relative electronegativity.
The BEST Lewis structure for a molecule is chosen such that the formal charge on each of the atoms is as close to zero as possible.
FC = valence e− − nonbonding e− − ½ bonding e−
Sum of all the formal charges in a molecule is zero.
In an ion, the total of the formal charges equals the overall charge.
Exceptions to the Octet Rule
Expanded octets
Elements with empty d orbitals can have more than eight electrons.
Odd number electron species e.g., NO
will have one unpaired electron
free-radical
very reactive
Incomplete octets
B, Al
Resonance
Lewis theory localizes the electrons between the atoms that are bonding together.
Extensions of Lewis theory suggest that there is some degree of delocalization of the electrons – resonance.
Delocalization of charge helps to stabilize the entire molecule
Resonance Structures
When there is more than one Lewis structure for a molecule that differ only in the position of the electrons, they are called resonance structures.
The actual molecule is a combination of the resonance forms – a resonance hybrid.
The molecule does not resonate between the two forms, even though we often draw it that way.
Look for multiple bonds or lone pairs.
Rules of Resonance Structures
Resonance structures must have the same connectivity.
Only electron positions can change.
Resonance structures must have the same number of electrons.
Second row elements have a maximum of eight electrons.
bonding and nonbonding
Third row can have expanded octet.
Formal charges must total same.
Evaluating Resonance Structures
Better structures have fewer formal charges.
Better structures have smaller formal charges.
Better structures have the negative formal charge on the more electronegative atom
Bond Energies
Chemical reactions involve breaking bonds in reactant molecules and making new bonds to create the products.
The delta H°reaction can be estimated by comparing the cost of breaking old bonds to the income from making new bonds.
The amount of energy it takes to break one mole of a bond in a compound is called the bond energy.
in the gas state
each atom gets ½ bonding electrons
bonds broken → endothermic reactions
bonds formed → exothermic reactions
Trends in Bond Energies
In general, the more electrons two atoms share, the stronger the covalent bond.
must be comparing bonds between like atoms
C≡C (837 kJ) > C=C (611 kJ) > C−C (347 kJ)
C≡N (891 kJ) > C=N (615 kJ) > C−N (305 kJ)
In general, the shorter the covalent bond, the stronger the bond.
must be comparing similar types of bonds
Br−F (237 kJ) > Br−Cl (218 kJ) > Br−Br (193 kJ)
Bonds get weaker down the column.
Bonds get stronger across the period.
Bond Lengths
The distance between the nuclei of bonded atoms is called the bond length.
Because the actual bond length depends on the other atoms around the bond we often use the average bond length.
It is averaged for similar bonds from many compounds.
Shapes and Bonding Theories
Structure Determines Properties!
Properties of molecular substances depend on the structure of the molecule.
The structure includes many factors, such as:
the skeletal arrangement of the atoms
the kind of bonding between the atoms
ionic, polar covalent, or covalent
the shape of the molecule
So far, Lewis dot structure only reveals electron sharing in two dimensions.
Therefore, Lewis bonding theory needs to be modified in order to allow for the prediction of molecular shapes.
Molecular Geometry
Molecules are 3-dimensional objects.
The shapes of a molecule can be related to different geometric figures.
These geometric shapes have characteristic “corners” that indicate the positions of the surrounding atoms around a central atom in the center of the geometric shape.
The geometric figures also have characteristic angles -bond angles.
Lewis Theory Predicts Electron Groups
Lewis theory predicts that there are different regions (domains) of electrons in an atom.
Some regions result from placing shared pairs of valence electrons between bonding nuclei.
Other regions result from placing unshared valence electrons on a single nuclei.
Using Lewis Theory to Predict Molecular Shapes
Lewis theory says that these regions of electron groups/domains should repel each other.
This idea can then be extended to predict the shapes of molecules.
The position of atoms surrounding the central atom will be determined by where the bonding electron groups are located – electron group geometry.
The positions of the electron groups will be determined by trying to minimize repulsions among them.
VSEPR Theory
Electron groups around the central atom will be most stable when they are as far apart from each other as possible. This is called the Valence Shell Electron Pair Repulsion theory.
Because electrons are negatively charged, they should be most stable when they are separated from each other as much as possible.
The resulting geometric arrangement allows for the prediction of the shapes and bond angles in a molecule
Electron Groups/Domains
Lewis dot structure predicts the number of valence electron pairs around the central atom(s) .
Each lone pair of electrons constitutes one electron group on a central atom.
Each bond constitutes one electron group on a central atom.
regardless of whether it is single, double, or triple!!!
Electron Group Geometry
There are five basic arrangements of electron groups around a central atom.
based on a maximum of six bonding electron groups
Each of these five basic arrangements results in five different basic electron geometries.
In order for the molecular shape and bond angles to be a “perfect” geometric figure, all the electron groups must be the same, bonded groups or lone pairs.
For molecules that exhibit resonance, it doesn’t matter which resonance form you use – the electron geometry will be the same.
Linear Electron Geometry
When there are two electron groups around the central atom, they will occupy positions on opposite sides of the central atom.
This results in the electron groups taking a linear geometry.
The bond angle is 180°.
Trigonal Planar Electron Geometry
When there are three electron groups around the central atom, they will occupy positions in the shape of a triangle around the central atom.
This results in the electron groups taking a trigonal planar geometry.
The bond angle is 120°.
Tetrahedral Electron Geometry
When there are four electron groups around the central atom, they will occupy positions in the shape of a tetrahedron around the central atom.
This results in the electron groups taking a tetrahedral geometry.
The bond angle is 109.5°.
Trigonal Bipyramidal Electron Geometry
When there are five electron groups around the central atom, they will occupy positions in the shape of two tetrahedrals that are base-to-base with the central atom in the center of the shared bases.
This results in the electron groups taking a trigonal bipyramidal geometry.
The positions above and below the central atom are called the axial positions.
The positions in the same base plane as the central atom are called the equatorial positions.
The bond angle between equatorial positions is 120°.
The bond angle between axial and equatorial positions is 90°.
The bond angle between the two axial positions is 180°.
Octahedral Electron Geometry
When there are six electron groups around the central atom, they will occupy positions in the shape of two square-base pyramids that are base-to-base with the central atom in the center of the shared bases.
This results in the electron groups taking an octahedral geometry.
This is called octahedral because the geometric figure has eight sides.
All corner positions are equivalent.
The bond angle is 90°.
Molecular Geometry
The actual geometry of the molecule may be different from the electron geometry.
When the electron groups are attached to atoms of different size, or when the bonding to one atom is different than the bonding to another, this will affect the molecular geometry around the central atom.
Lone pairs affect the molecular geometry tremendously.
They occupy space around the central atom, but are not “seen” as points on the molecular geometry.
The Effect of Lone Pairs
Lone pair groups “occupy more space” on the central atom.
since their electron density is exclusively on the central atom rather than shared like bonding electron groups
Relative sizes of repulsive force interactions:
Lone Pair – Lone Pair > Lone Pair – Bonding Pair > Bonding Pair – Bonding Pair
This affects the bond angles, making the bonding pair – bonding pair angles smaller than expected if there are lone pair(s) around the central atom.
Bent Molecular Geometry/Shape: Derivative of Trigonal Planar Electron Geometry
When there are three electron groups around the central atom, and one of them is a lone pair, the resulting shape of the molecule is called a trigonal planar — bent shape or trigonal bent.
The bond angle is less than 120°.
Derivatives of the Tetrahedral Electron Geometry
When there are four electron groups around the central atom, and some are lone pairs, they will occupy one or two of the characteristic corners in the tetrahedron.
When there are four electron groups around the central atom, and one is a lone pair, the result is called the trigonal pyramidal.
When there are four electron groups around the central atom, and two are lone pairs, the result is called the tetrahedral bent.
The bond angles for both trigonal pyramidal and tetrahedral bent are less than 109.5° due to the larger repulsion for the lone pair(s).
Derivatives of the Trigonal Bipyramidal Electron Geometry
When there are five electron groups around the central atom, and some are lone pairs, they will occupy the equatorial positions because there is more room.
When there are five electron groups around the central atom, and one is a lone pair, the result is called the seesaw shape.
When there are five electron groups around the central atom, and two are lone pairs, the result is called the T-shaped.
When there are five electron groups around the central atom, and three are lone pairs, the result is a linear shape.
The bond angles between equatorial positions are less than 120°
The bond angles between axial and equatorial positions are less than 90°
linear = 180° axial–to–axial