Natural Killer Cells
3: NK cells were originally described as large granular lymphocytes which can develop natural cytotoxicity against tumour cells without prior contact. No priming by APCs required
4: NK cells develop from haematopoietic stem cells and through a common lymphoid progenitor. They change in expression levels of surface receptors throughout the 5 stages of development. Proliferation requires cytokine IL-15.
5: CD56(^bright)CD16(^low/-) NK cells are immunomodulatory and cytokine producing. They’re found in secondary lymphoid tissues. CD56(^dim)CD16+ NK cells make up the majority of NK cells and are 90% blood NK cells and are cytotoxic.
NK cells are found throughout body but localised in the spleen, lymph nodes, bone marrow and peripheral blood. Found in most barrier/lining tissues. Carry out immunosurveillance throughout vasculature and are found in mucosal tissues inc lungs, small and large intestines
6: Diversity of peripheral blood human NK cells can be identified by single-cell RNA sequencing. Very heterogenous group
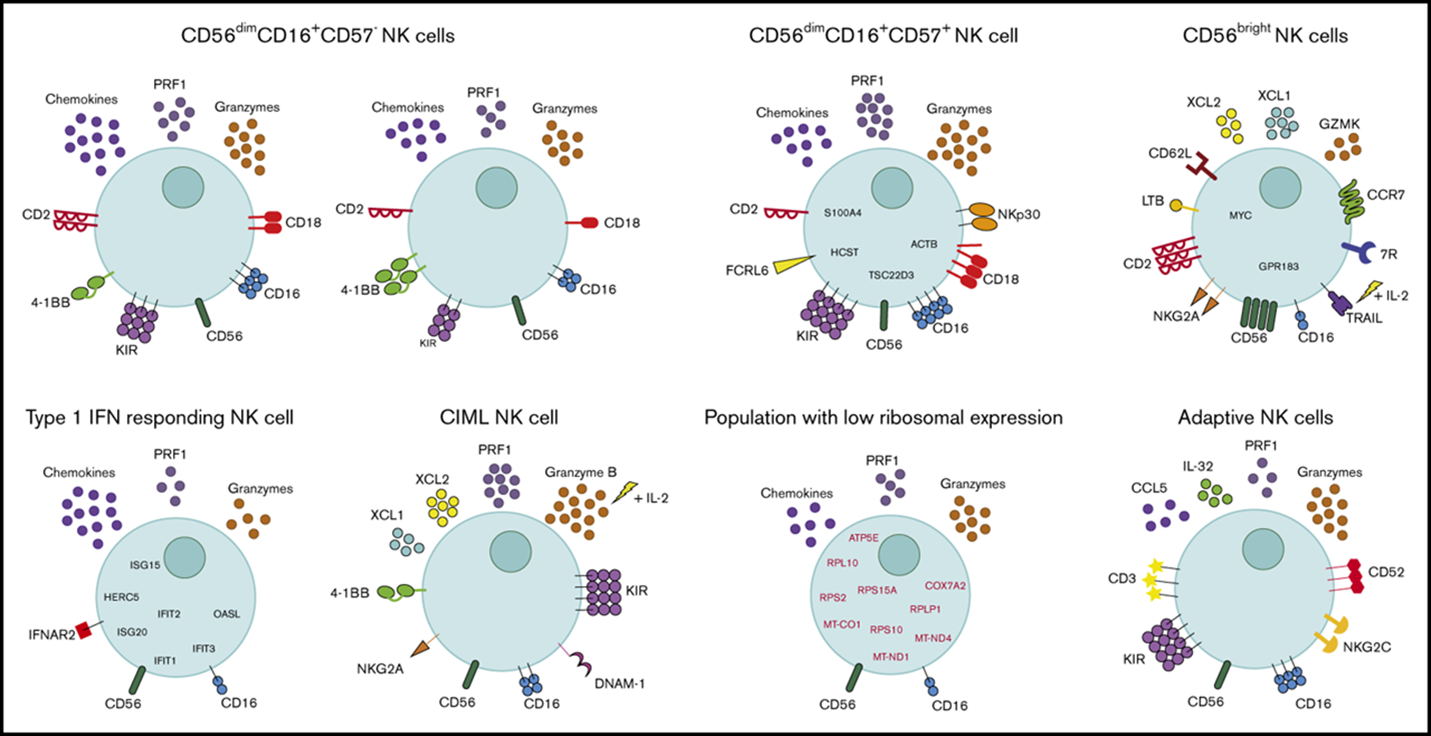
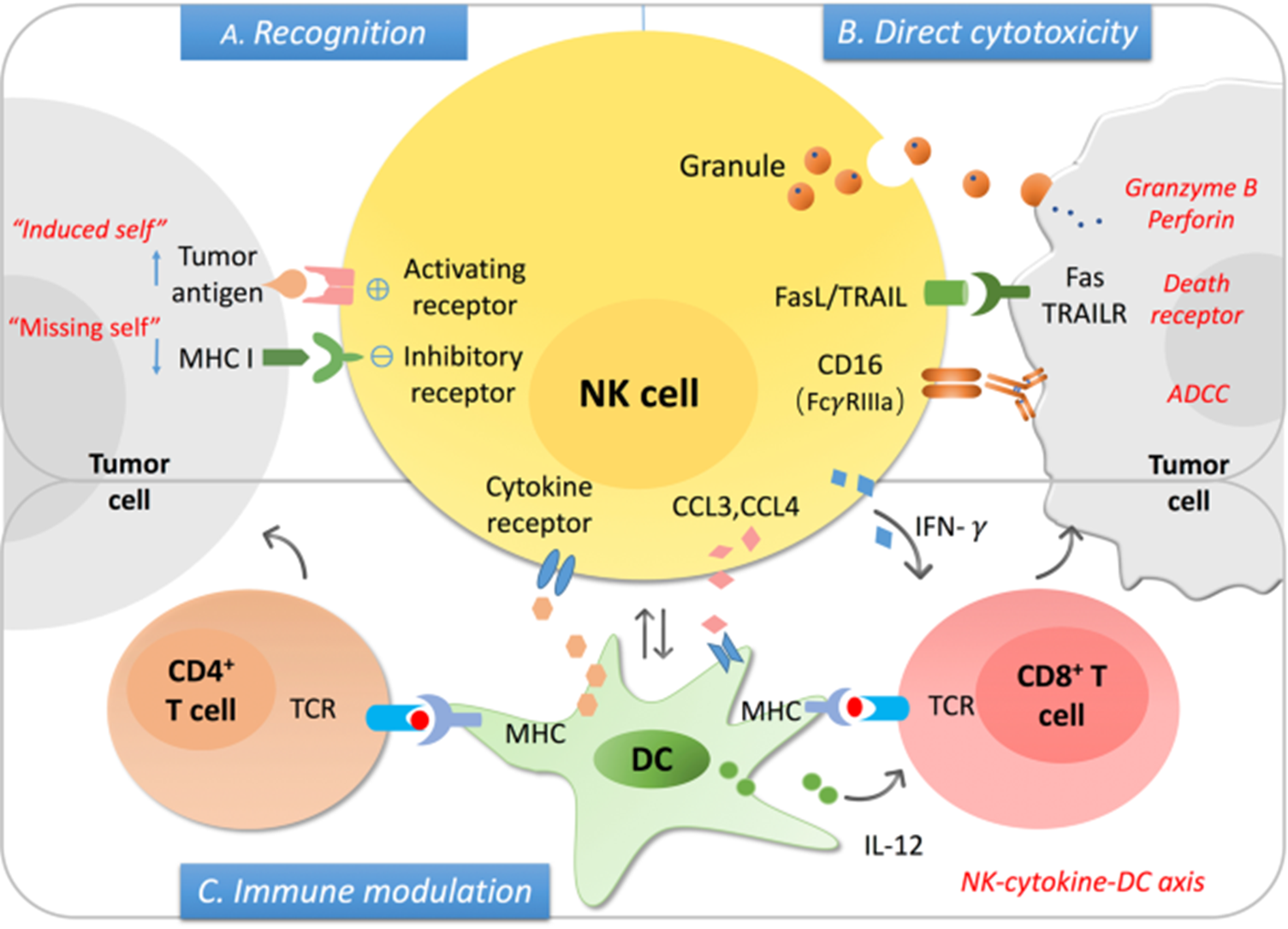
7: NK cells secrete cytokines and chemokines and influence the immune response. They kill infected and transformed cells and are very similar in activity to cytotoxic T cells. However, unlike cytotoxic T cells their cytoplasm contains constitutively expressed granules whereas T cells need to be stimulated to produce granules. They also interact with infected or malignant cells with reduced expression of MHC I (missing self model). NK cells can only kill self cells by direct contact, not a pathogen.
8: NK cell activation method via balance of opposing signals. NK cells express wide variety of activating and inhibitory receptors. The balance of receptor stimulation determines NK activation and cell killing. NKs don’t express antigen specific receptors.
9: NK cells kill when they sense normal self-MHC proteins - ‘missing self’ model. Virally infected cells and tumour cells tend to downregulate MHC I to evade CD8+ T cell recognition and killing. Self recognition by inhibitory receptors inhibits cell killing. Activating receptors recognise ligands upregulated on infected, stressed and tumour cells.
10: the balance of signals determines NK cell activity - tolerance (inhibition), missing self (loss of MHC), or stress induced self (upregulation of stress induced ligands).
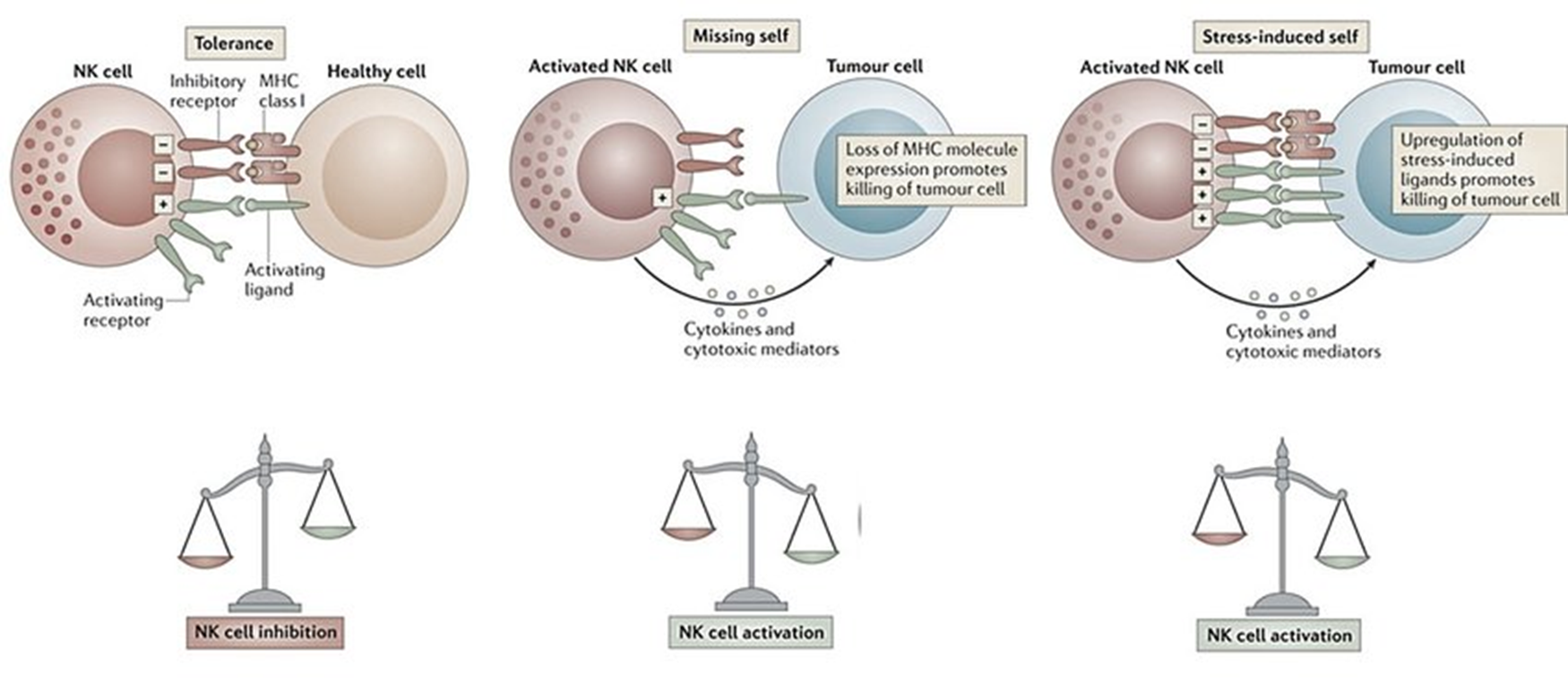
11: Major inhibitory NK receptors include killer immunoglobulin-like receptor (KIR) eg CD94-NKG2A.
KIRs are highly diverse and polymorphic, and recognise HLA-A, B and C in humans. 1 NK cell can express many different KIRs.
Key KIR CD94-NKG2A binds target cell HLA-E. Surface cell HLA-E indicates overall level of MHC-I biosynthesis by presenting components of MHC to NK cells, and if cell is expressing adequate MHC-I levels cell killing is prevented.
12: Major activating NK receptors include NKGD2D and CD16 (Fcgamma RIII).
NKG2D ligands inc MHC-I-like molecules which don’t associate with beta 2 microglobulin. These receptors are expressed by stressed and tumour cells.
CD16 receptors bind IgG associated with cell surface antigen- antibody acting as adaptor between NK cell and infected cell. Cross linking of CD16 triggers antibody dependent cell mediated cytotoxicity.
13: Nectins and nectin-like molecules belong to Ig superfamily. Ligands for NK cells. Expressed on epithelial cells and help form junctions between cells. Highly expressed on tumour cells and become accessible when epithelial structure disrupted. They stabilise binding to NK cells during IS formation and if signal is strong it can result in cytotoxicity.
NK cells express receptors which recognise nectins and nectin-like molecules on target cells eg CD226 recognises nectin 2 and nectin-like 5, CD96 recognises nectin-like 5 and CRTAM recognises nectin-like 2
14: Close contact between the target cell and NK cell required hence the synapse. The NK cell and target conjugate and are anchored by LFA-1 and beta 1 integrins
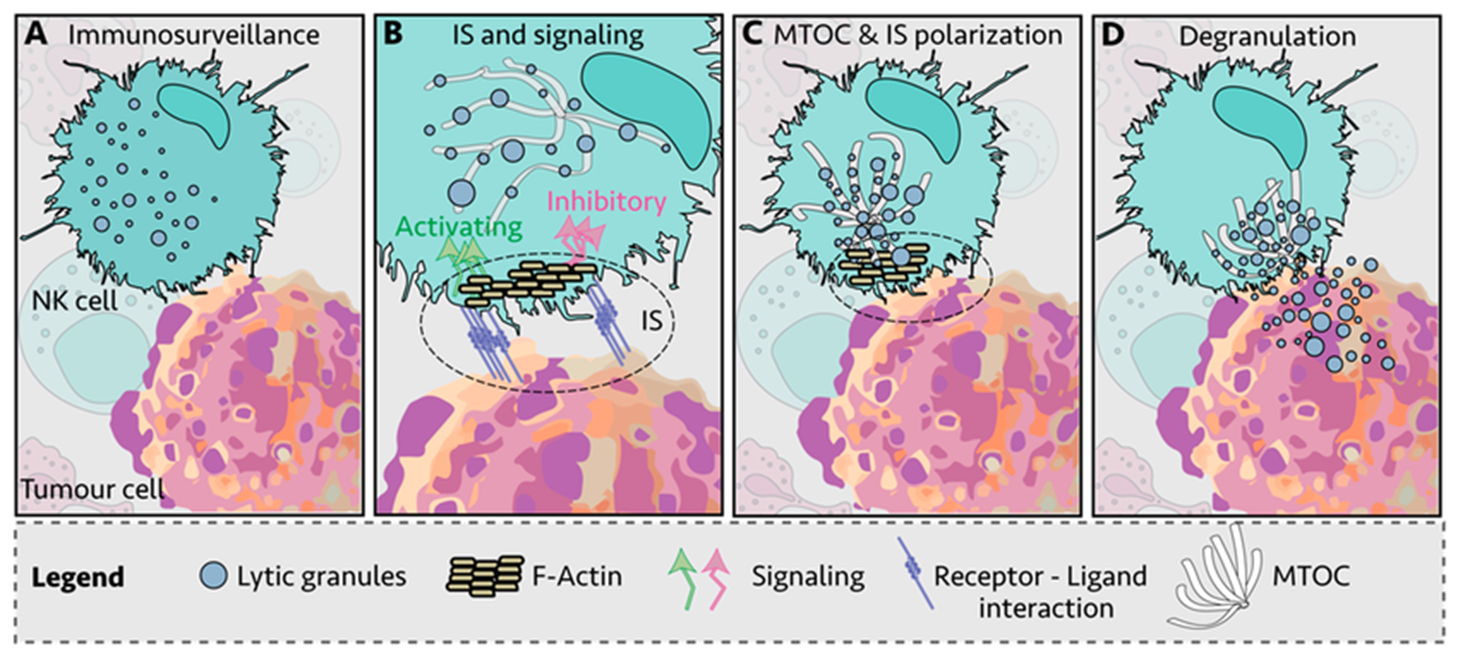
15: Granule release may rely on 2 signals but often more are needed. LFA1 interacting with ICAM1 → granule polarisation within NK cells.
CD16 interacts with IgG Fc receptor → ADCC
Co-engagement of both receptors before granules are released. Interactions of other receptors with ligands may → cell killing. Their activation is similar to Tc cells as it involves perforin and caspase 3 activation inducing apoptosis.
17: There are multiple cell death pathways with different triggers, signalling cascades and immunological consequences.
Necrosis is the abrupt disruption of membrane integrity, upregulated discharge of intracellular contents into the environment and is highly inflammatory - very destructive, in response to severe and unexpected (sudden) stress.
Apoptosis is programmed cell death. Extrinsic apoptosis is the binding of surface ligands by external signals and has TNF family ligands. Internal apoptosis is the internal sensing of death signals and intracellular stress.
Ferroptosis due to iron overload
18: Fas mediated cell killing: the Fas ligand (FasL) is present in membranes of activated Tc cells and NK cells. Fas is an apoptosis-signalling receptor molecule belonging to the TNF-R family found on many cells. It contains a conserved intracytoplasmic death domain that indirectly activates the caspase enzymatic cascade when FasL binds.
19: Caspases are proteases, meaning they degrade proteins and DNA. Caspase cascade is highly regulated and has many activators and inhibitors.
Initiator caspases = 2, 8, 9, 10, 11, 12
Effector caspases = 3,6 and 7
Caspase cascade has many sequential pathways and has many breaks and control points
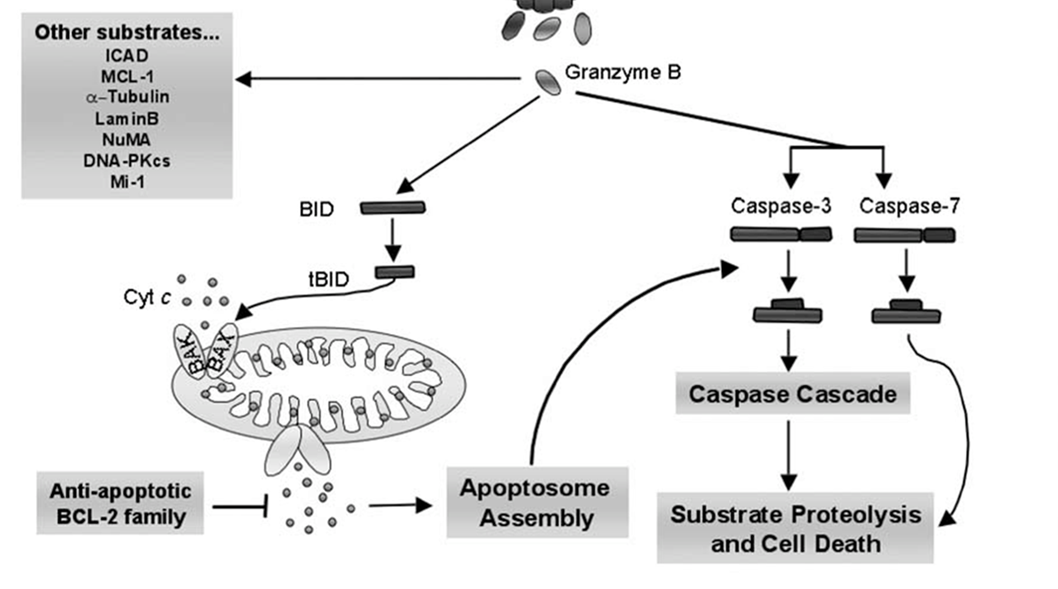
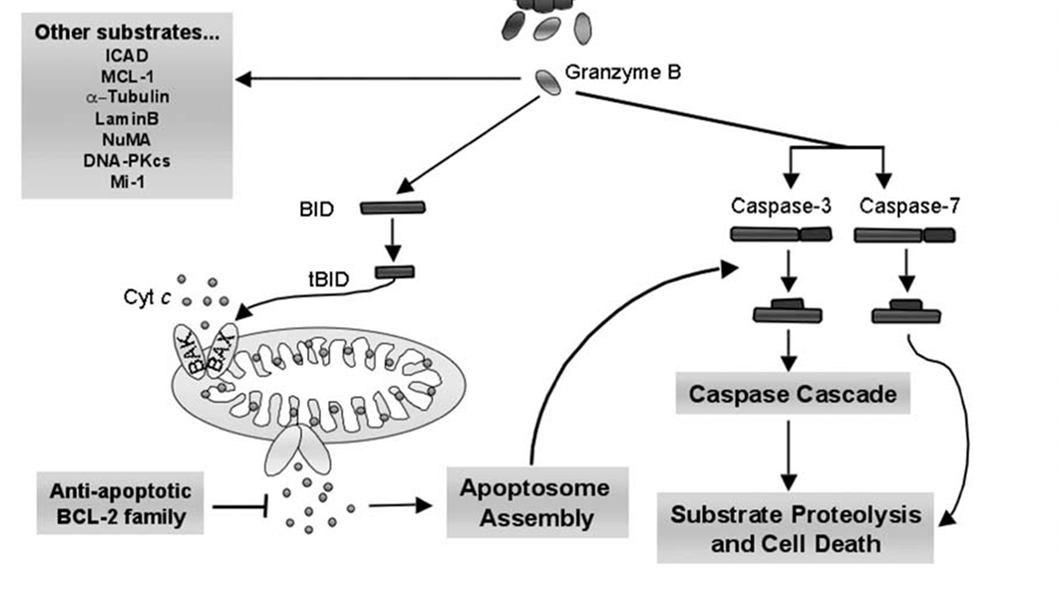
20:
Bax/Bak homo and heterodimers are inserted into the outer membrane as result of granzyme B activation
cytochrome C released from mitochondria as result
cyt C binds apoptotic protease activating factor 1 (Apaf1) and ATP
binds procaspase 9 → apoptosome
apoptosome cleaves procaspase 9 (initiator caspase) → active caspase 9
caspase 9 cleaves and activates procaspase → caspase 3
caspase 3 cleaves cytoskeletal and nuclear proteins
ICAD cleaved → CAD
DNA fragmentation
21: Apoptosis causes:
blebbing and condensing
nuclear fragmentation
apoptotic bodies form
eat me signals expressed on surface - rearrangement of membrane lipid PS from inner to outer leaflet for phagocyte detection
inflammation resolved
The uptake and clearance of apoptotic bodies is known as efferocytosis.
22: Apoptosis may directly act on cytosolic pathogens. Nucleases activated in apoptosis can degrade viral DNA, and apoptosis prevents virion assembly so infectious virus not released.
23:
need to know:
comparisons between activity of different immune cells - similarities and differences