Chapter 9: Molecular Geometry and Bonding Theories
Molecular Shapes
Lewis structures show bonding and lone pairs but do not denote shape.
However, we use Lewis structures to help us determine shapes.
Common shapes for molecules with two or three atoms connected to a central atom.
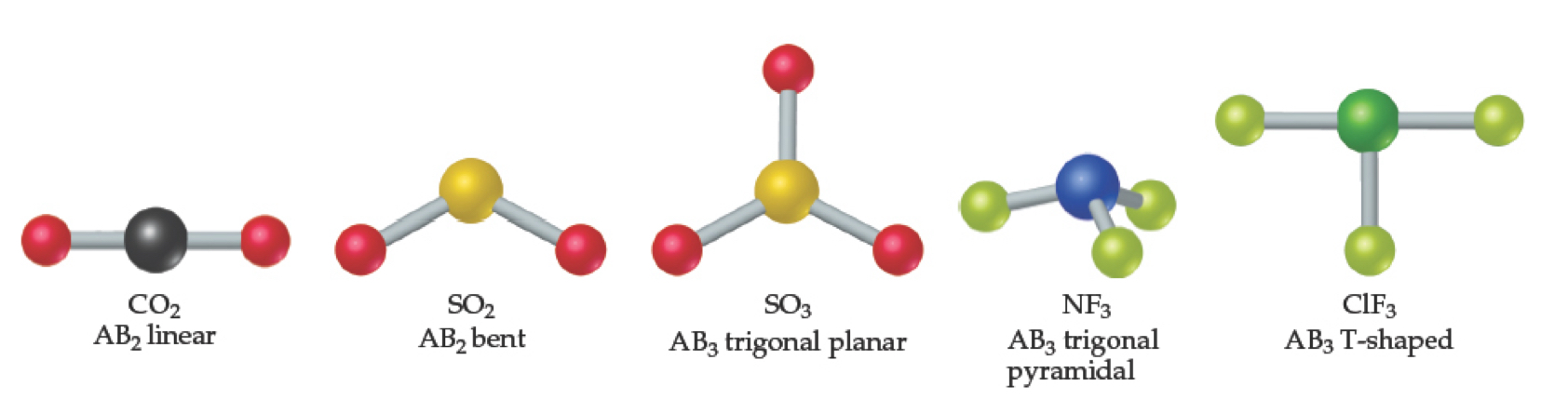
What Determines the Shape of a Molecule?
Bond angles and bond lengths determine the shape and size of molecules.
Electron pairs will repel each other.
Electron pairs are as far apart as possible, which helps determine the shape of the molecule.
Valence Shell Electron Pair Repulsion (VSEPR)
This tells us electrons don’t want to be near each other.
Electron Domains
Electron domains - a region of space around a central atom where electrons are most likely to be found.
Lone pairs = 1 electron domain
Single bonds = 1 electron domain
Double bonds = 1 electron domain
Triple bonds = 1 electron domain
In the following example, the central atom, A, has 4 electron domains:
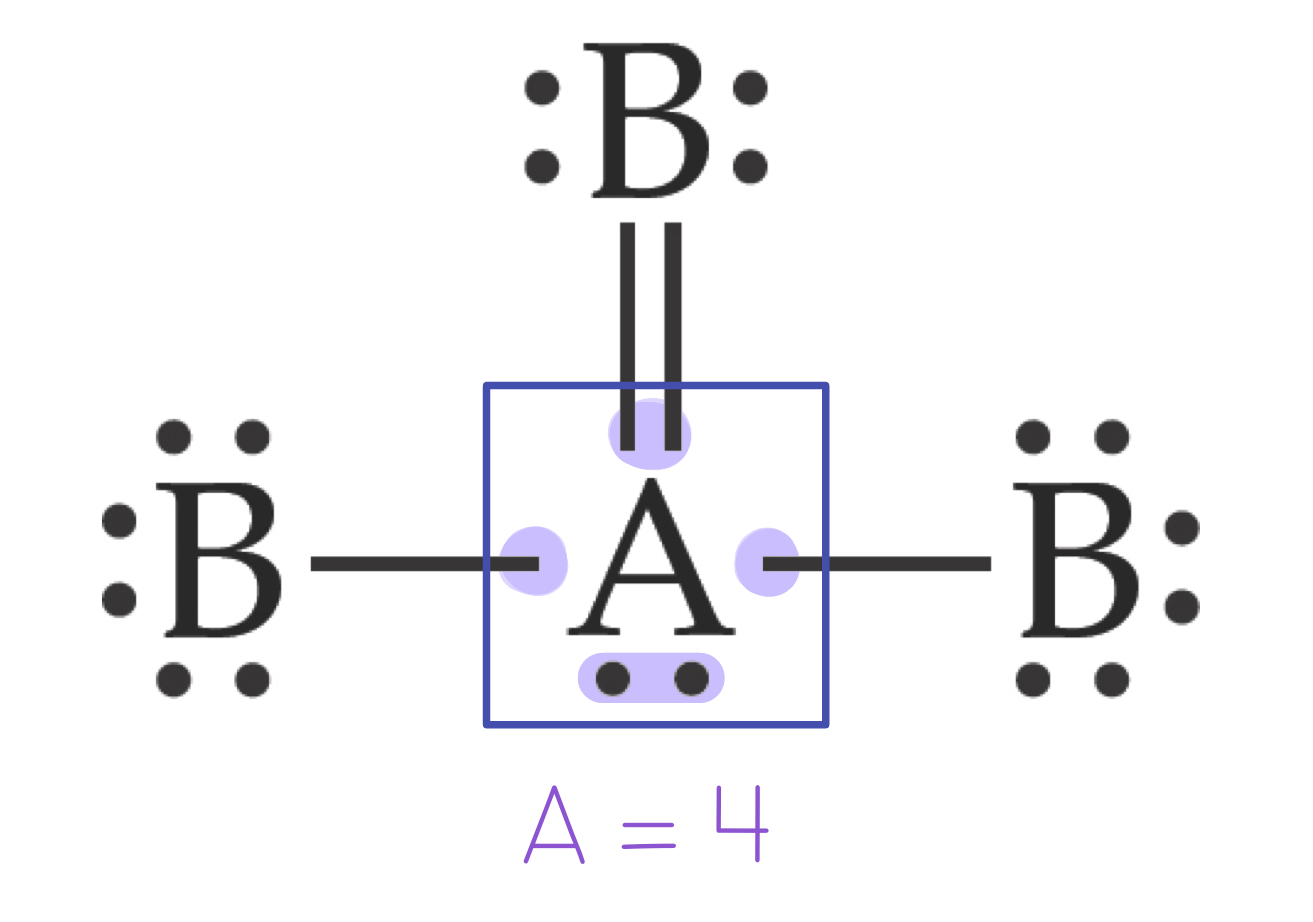
Valence Shell Electron Pair Repulsion (VSEPR) Model
VSEPR Theory - a set of rules that are used to look at a 2D Lewis structure of a molecule and determine what the molecule's 3D shape will be based on the repulsion between electron pairs, which helps predict the arrangement of atoms around the central atom.
Electrons or pairs of electrons want to be as far apart as possible.
They have negative charges, which leads to the repulsive forces.
Atoms also need space.
Arrangement of electrons results in different angles.
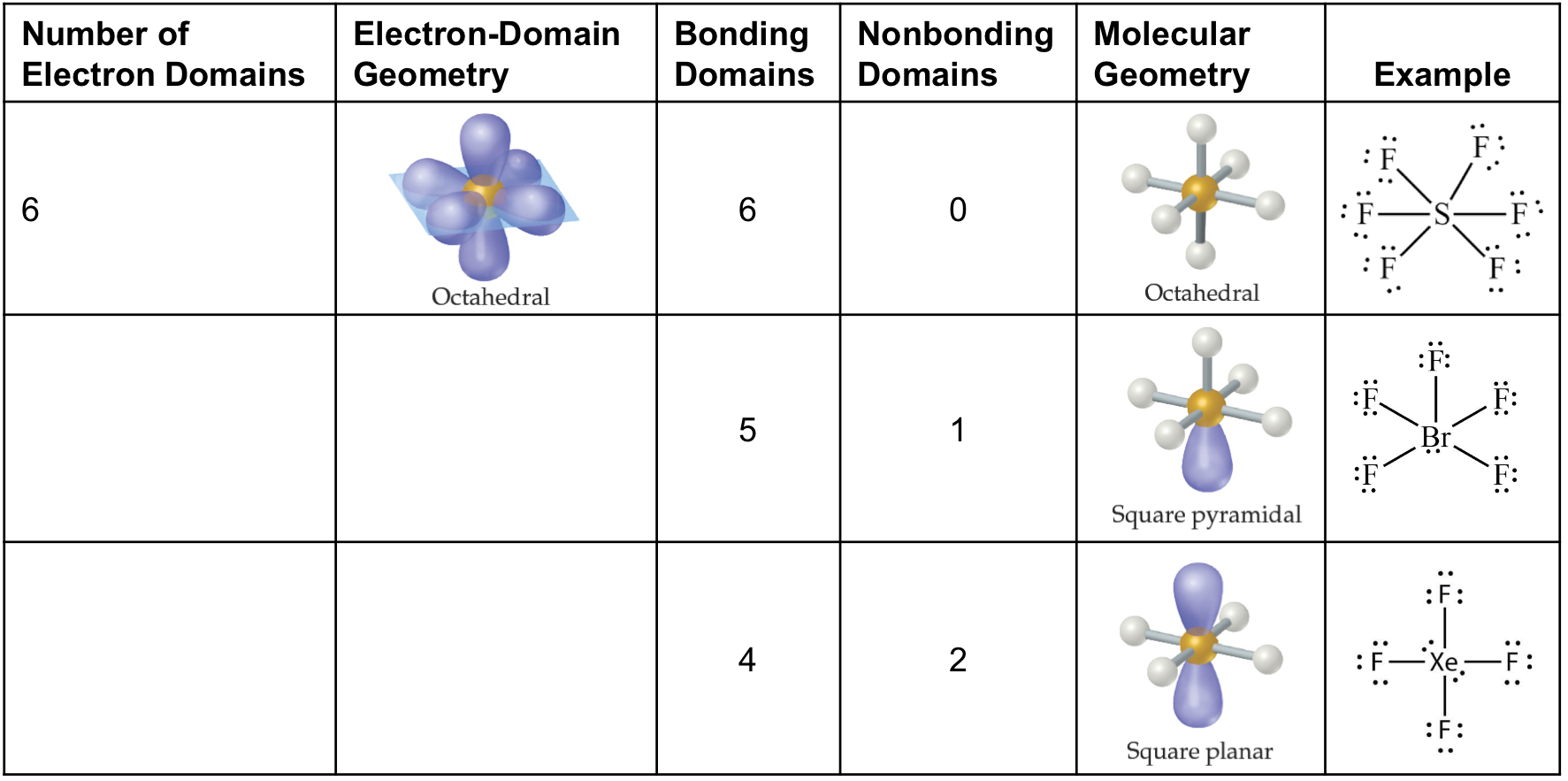
Electron Domain Geometries
To determine the electron domain geometry, count the total number of lone pairs, single, double, and triple bonds of the central atom.
Electron Domain Geometries - Bond Angles
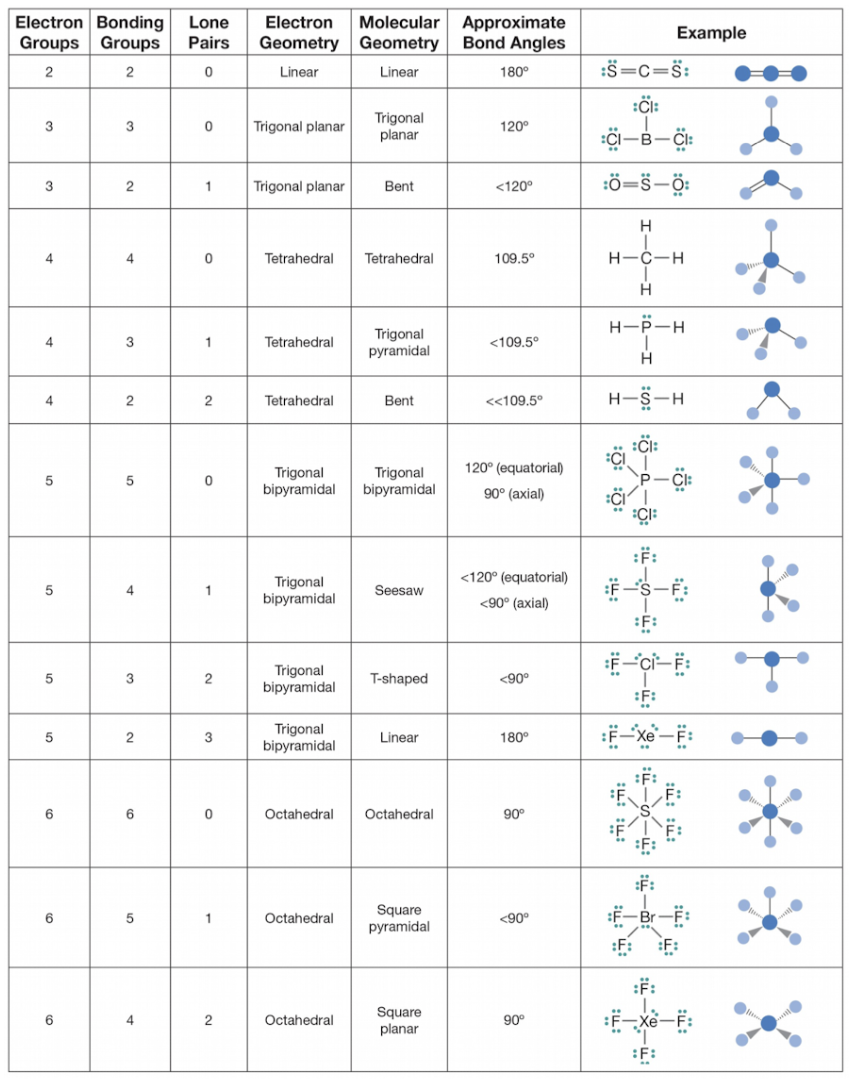
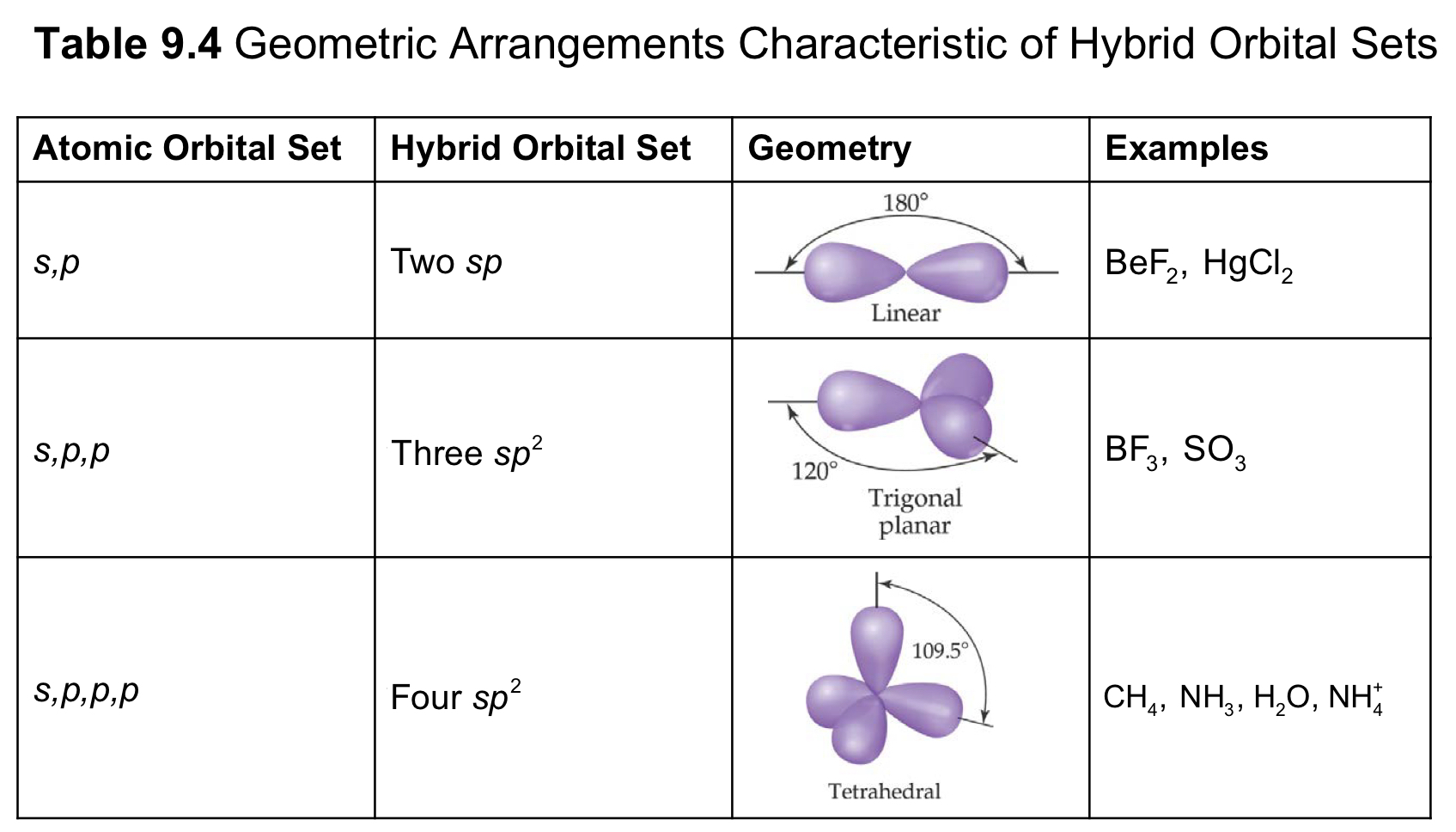
Number of Electron Domains | Electron Domain Geometry | Predicted Bond Angle |
2 | Linear | 180° |
3 | Trigonal planar | 120° |
4 | Tetrahedral | 109.5° |
5 | Trigonal bipyramidal | 120° and 90° |
6 | Octahedral | 90° |
Molecular Geometries
Draw the best Lewis structure.
Determine the electron domain geometry.
Use the arrangement of the bonded atoms to determine the molecular geometry.
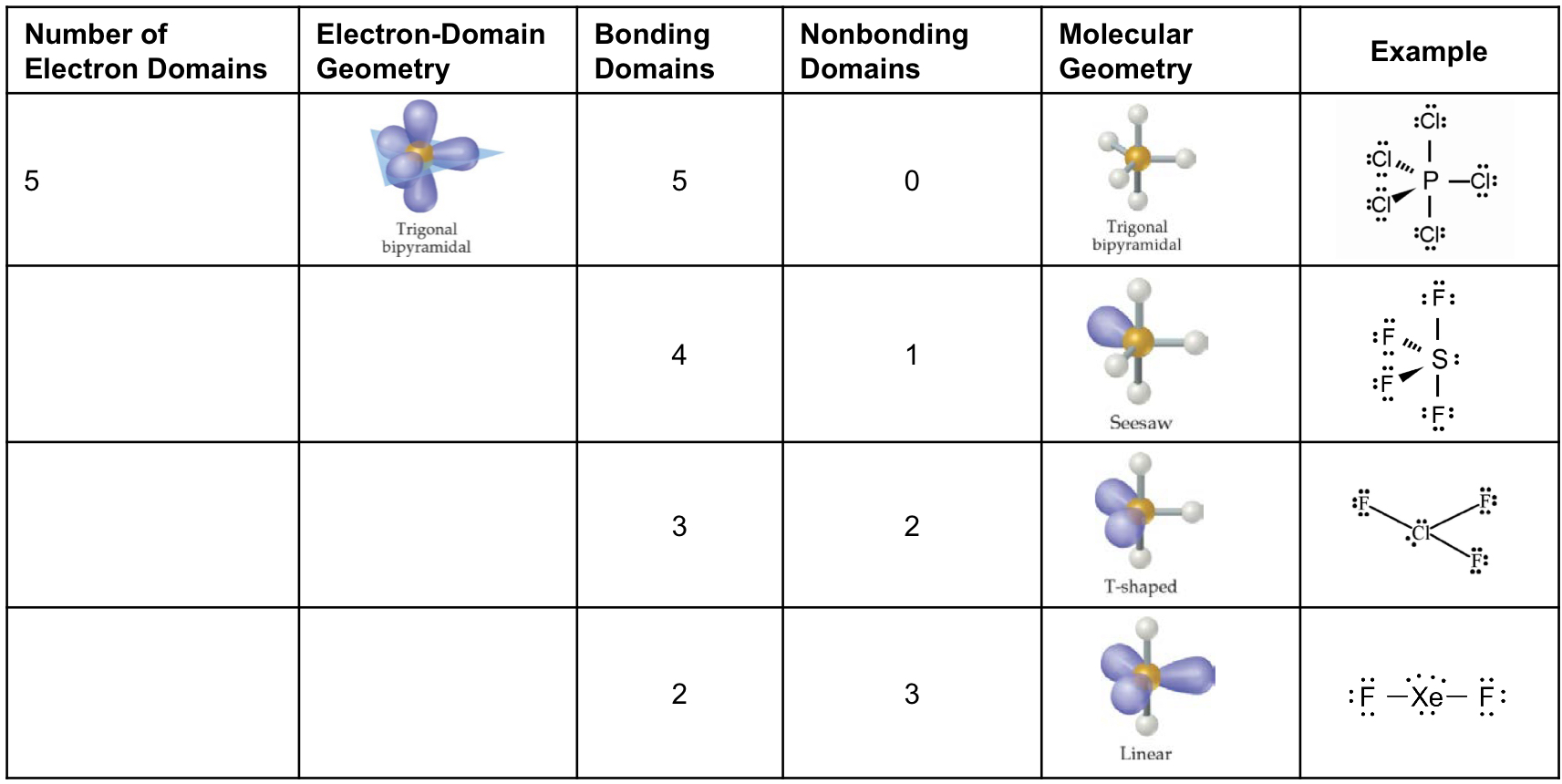
Molecular Geometry Table
Determining Molecular Shapes Practice
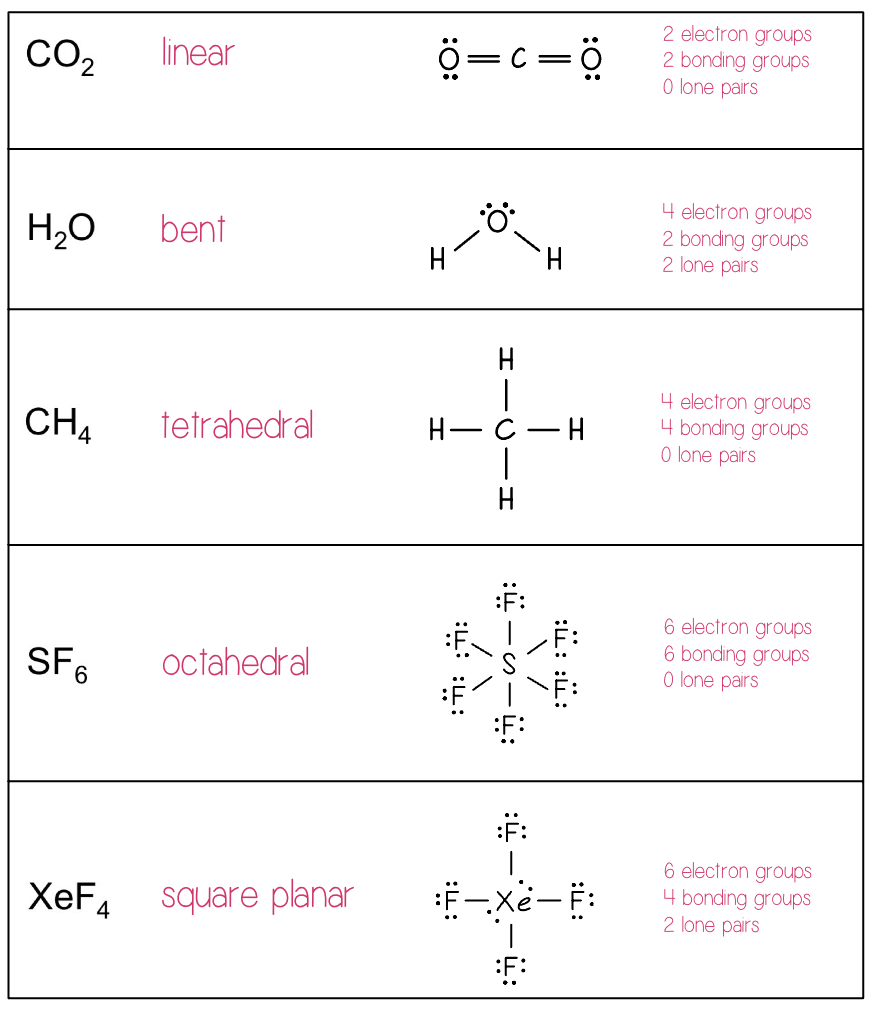
Linear Electron Domain
Only one molecular domain: Linear (180°)
If there are only two atoms in the molecule, the molecule will be linear no matter what the electron domain is.
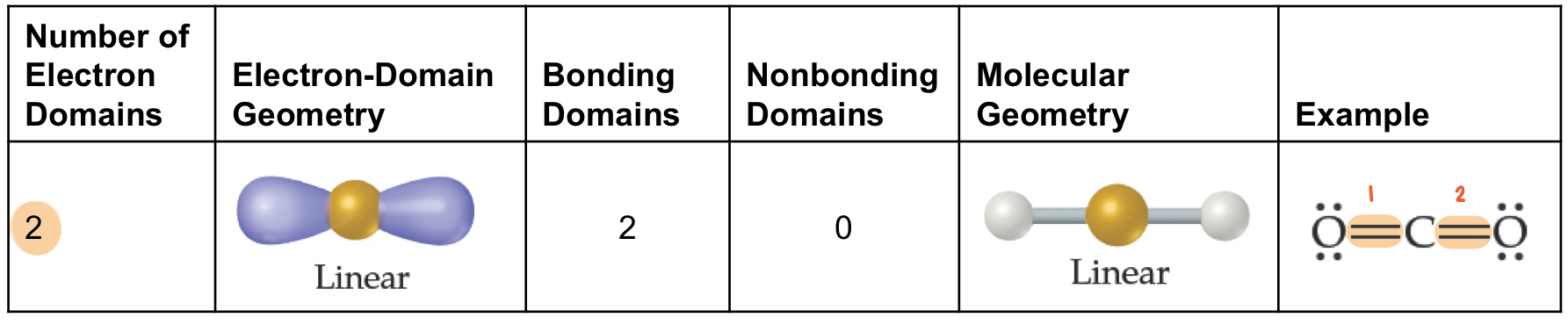
Trigonal Planar Electron Domain
There are two molecular geometries
Trigonal planar, if 3 bonds and 0 lone pairs (120°)
Bent, if 2 bonds and 1 lone pair (<120°)
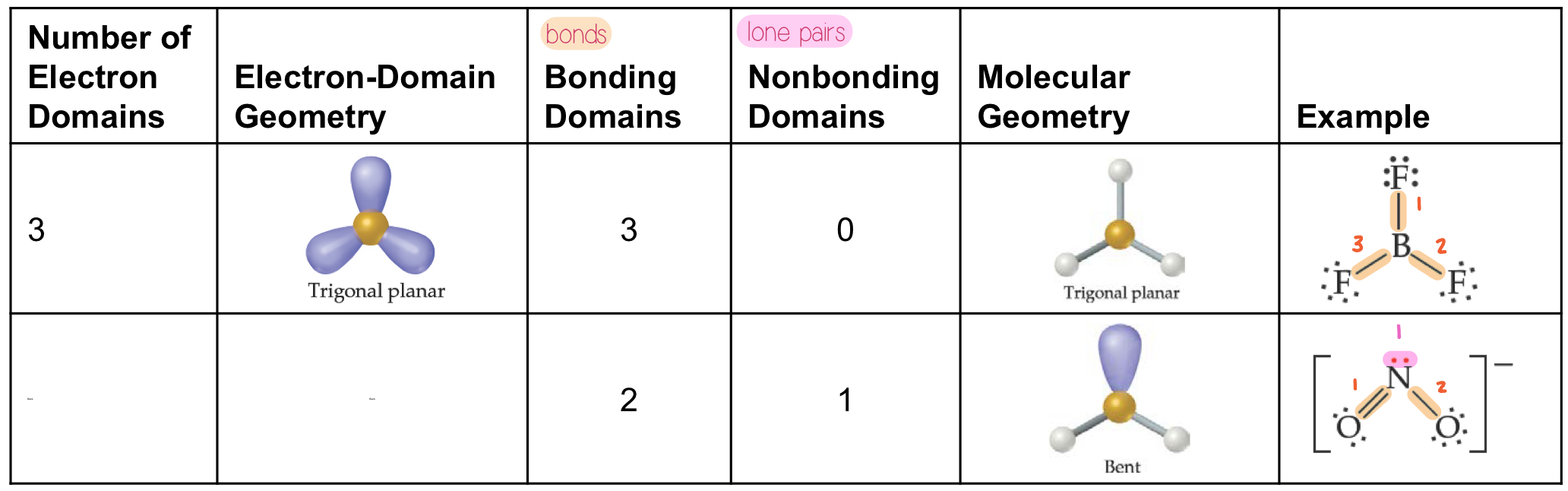
Tetrahedral Electron Domain
There are three molecular geometries
Tetrahedral, if all are bonding pairs (109.5°)
Trigonal pyramidal, if one is a lone pair (<109.5°)
Bent, if there are two lone pairs (<<109.5°)
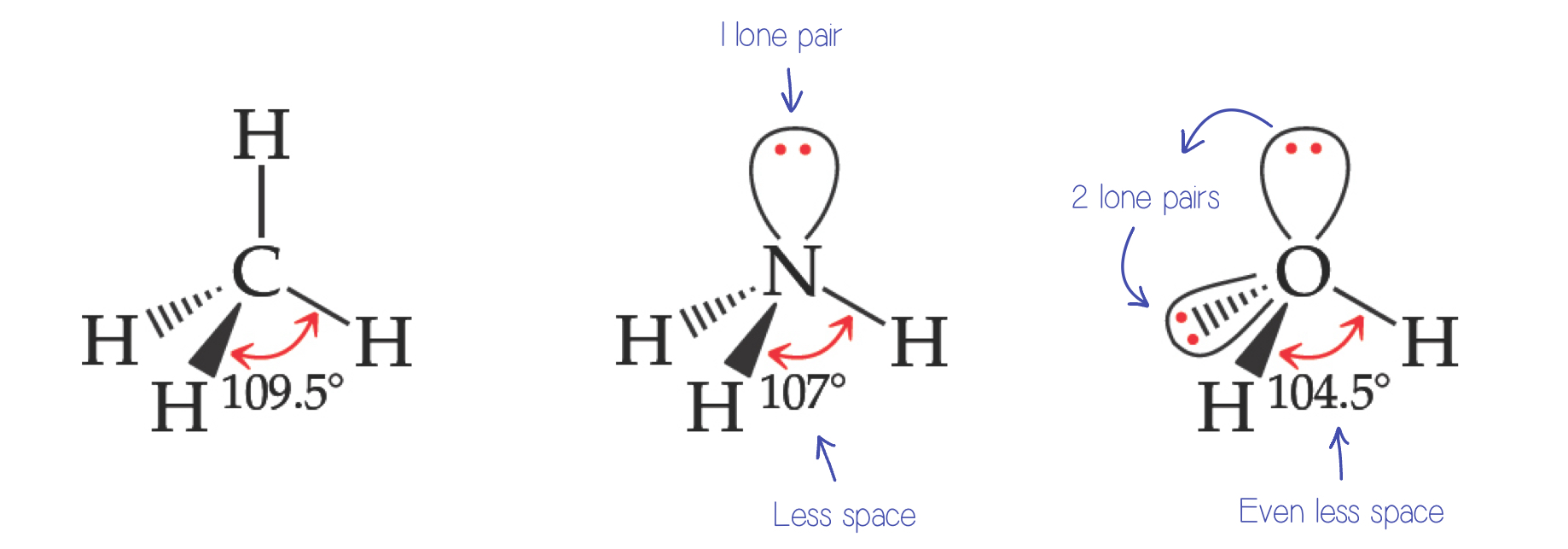
Nonbonding Pairs and Bond Angle
Nonbonding pairs are physically larger than bonding pairs.
When 2 electrons are in a bond, they take up less space.
This results in a decrease in the bond angle between the bonding pairs, leading to a distorted shape in the molecule.
Their repulsions are greater; this tends to compress bond angles.
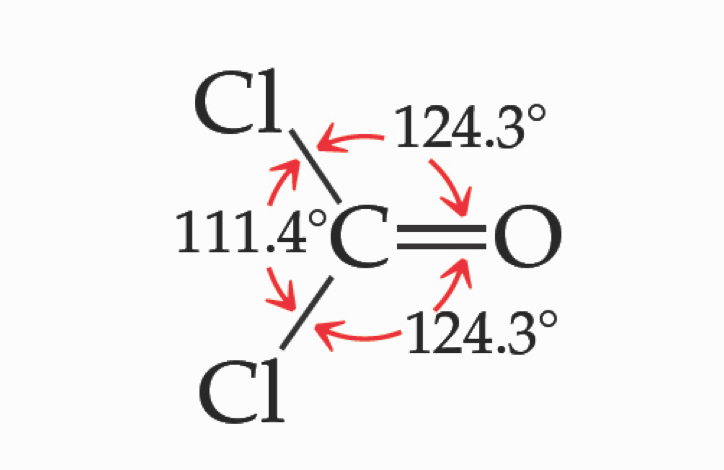
Multiple Bonds and Bond Angles
Double and triple bonds have larger electron domains than single bonds.
They exert a greater repulsive force than single bonds, making their bond angles greater.
Consequently, the presence of multiple bonds can also influence the molecular geometry by leading to increased bond angles between surrounding atoms, as they require more space due to their electron density.
Expanding Beyond the Octet Rule
Remember that some elements can break the octet rule and make more than four bonds (or have more than four electron domains).
The result is two more possible electron domains
Five - trigonal bipyramidal
Six - octahedral
Trigonal Bipyramidal Electron Domain
There are two distinct positions in this geometry:
Axial (vertical, 90°)
Equatorial (horizontal, 120°)
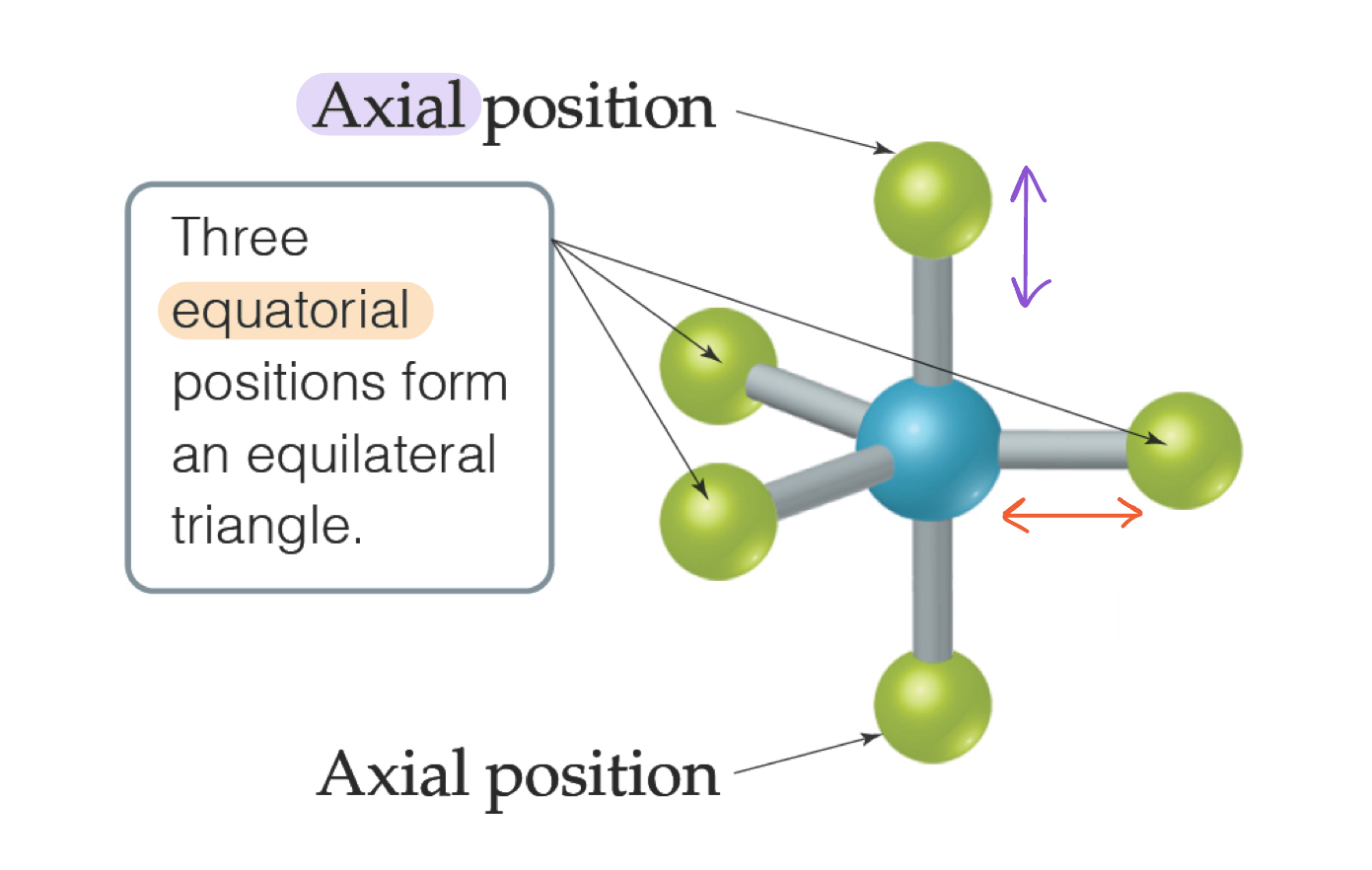
Lone pairs occupy equatorial positions.
There are four distinct molecular geometries in this domain:
Trigonal bipyramidal, if all electrons are in bonds (120° equatorial, 90° axial)
Seesaw, if 4 bonds and 1 lone pairs (<120° equatorial, <90° axial)
T-shaped, if 3 bonds and 2 lone pairs (<90°)
Linear, if 2 bonds and 3 lone pairs. (180°)
Octahedral Electron Domain
All positions are equivalent in the octahedral domain.
There are three molecular geometries:
Octahedral, if 6 bonds and no lone pairs (90°)
Square pyramidal, if 5 bonds and 1 lone pair (<90°)
Square planar, if 4 bonds and 2 lone pairs (90°)
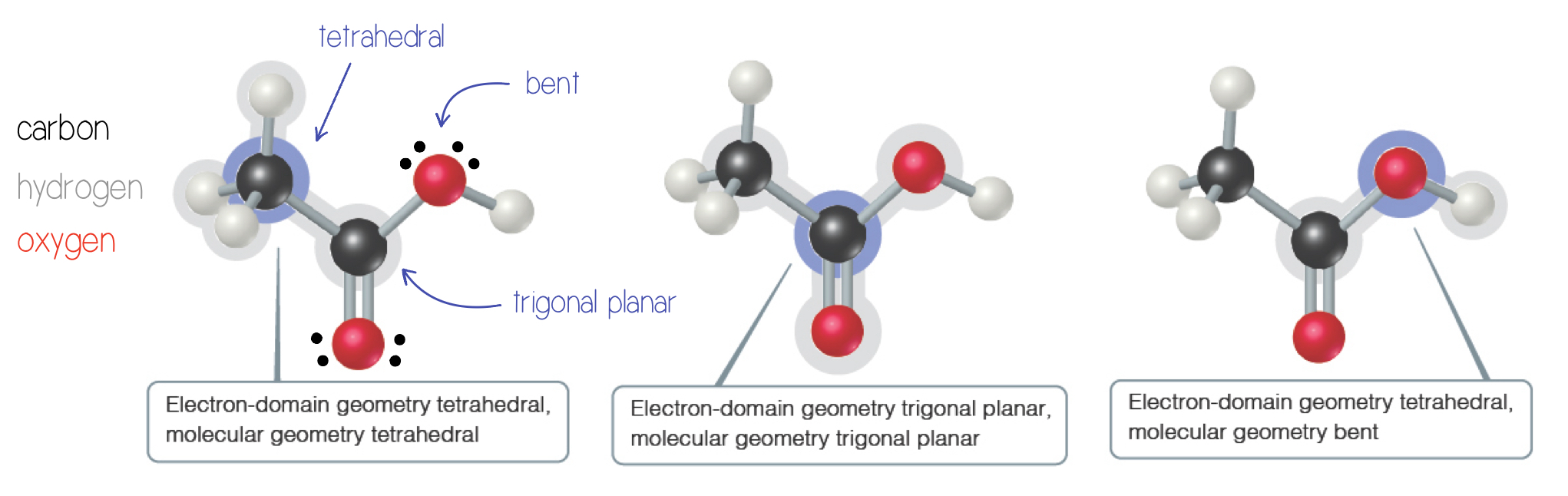
Shapes of Larger Molecules
For larger molecules, look at the geometry about each atom rather than the molecule as a whole.
Polarity of Molecules
Ionic bonds (m+nm) are polar, instead of sharing electrons, they transfer electrons.
Non-polar covalent bonds share electrons fairly equally.
Polar covalent bonds have an unequal sharing of electrons.
Ask yourself:
Covalent or ionic?
If covalent:
Are the bonds polar? (Do they have a bond dipole?)
1. No: The molecule is non-polar.
2. Yes: Continue, do the average position of \delta+ and \delta- coincide? (Is the overall dipole moment equal to zero?)
1. Yes: The molecule is non-polar.
2. No: The molecule is polar.
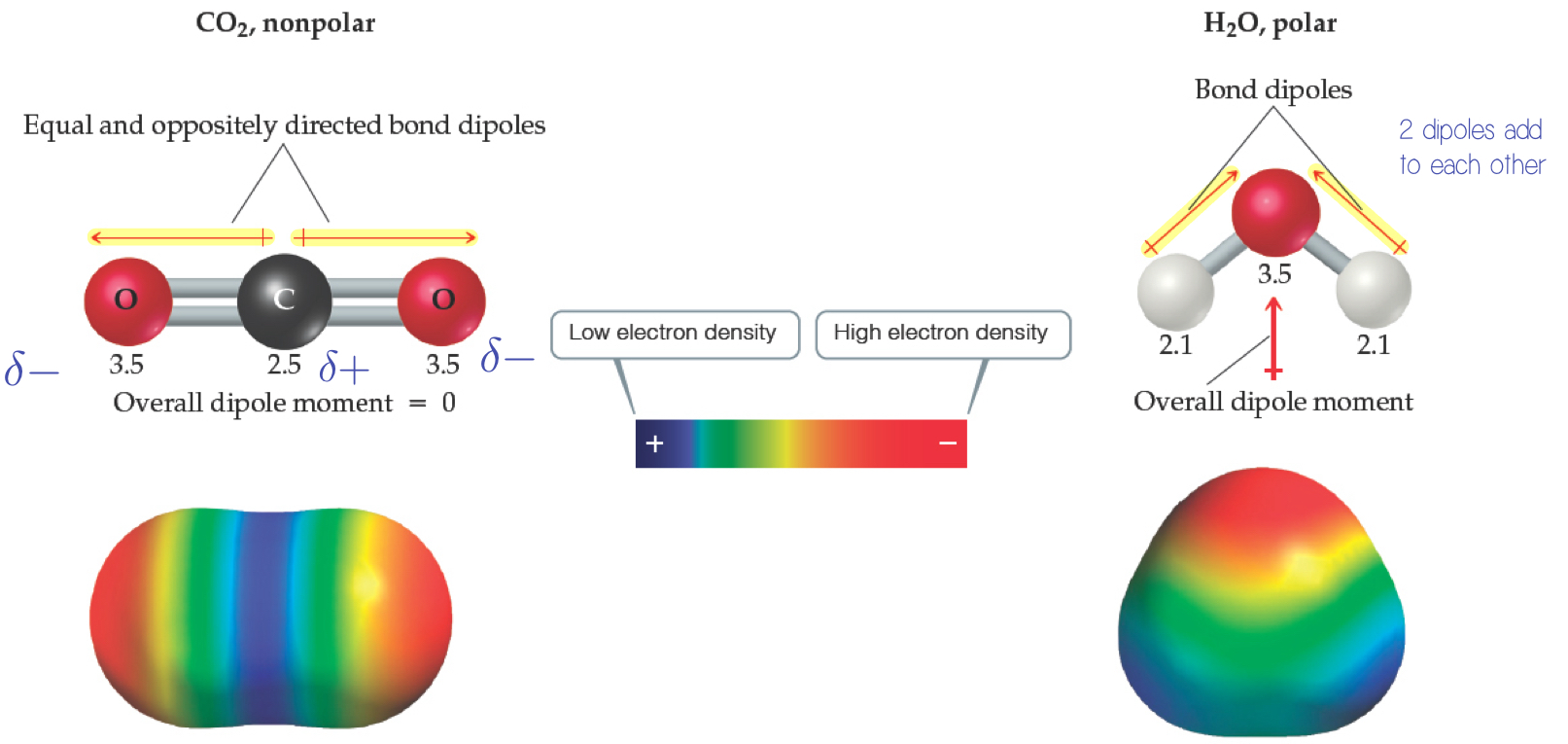
Comparison of the Polarity of Two Molecules
A non-polar molecule is equal, so dipoles will cancel.
A polar molecule is unequal, so the dipole add to each other.
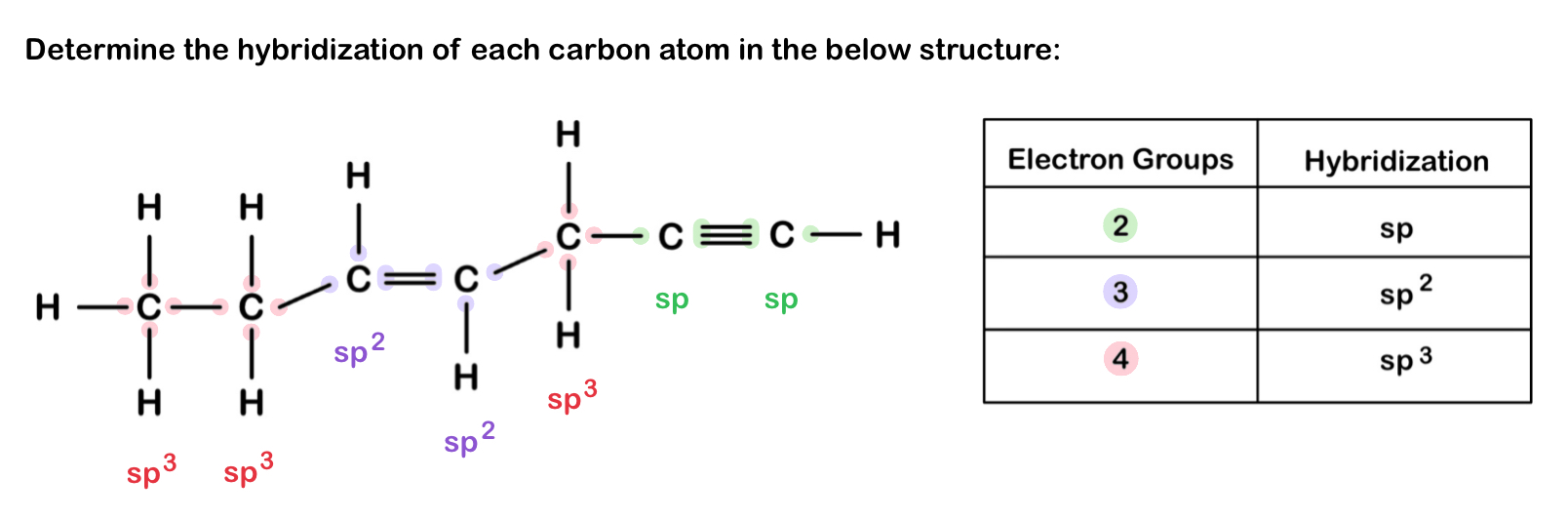
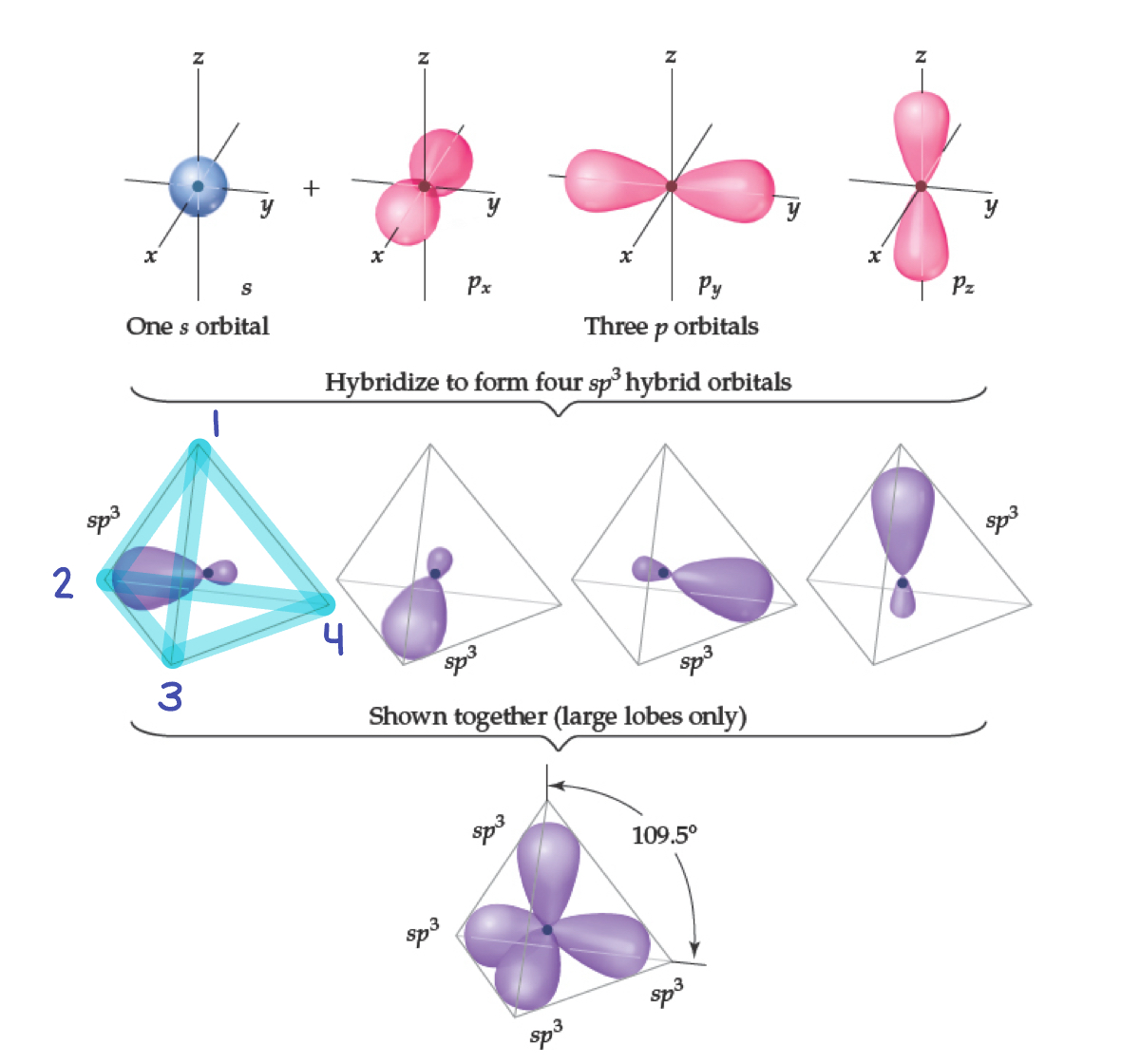
Hybrid Orbitals
Hybrid orbitals form by “mixing” of atomic orbitals to create new orbitals of equal energy, called degenerate orbitals.
Hybridization is the process of combining different atomic orbitals (s, p, d) to create unique hybrid orbitals that meet the bonding requirements of atoms in a molecule.
When two orbitals “mix”, they create two orbitals; when three orbitals “mix, they create three orbitals; and so on.
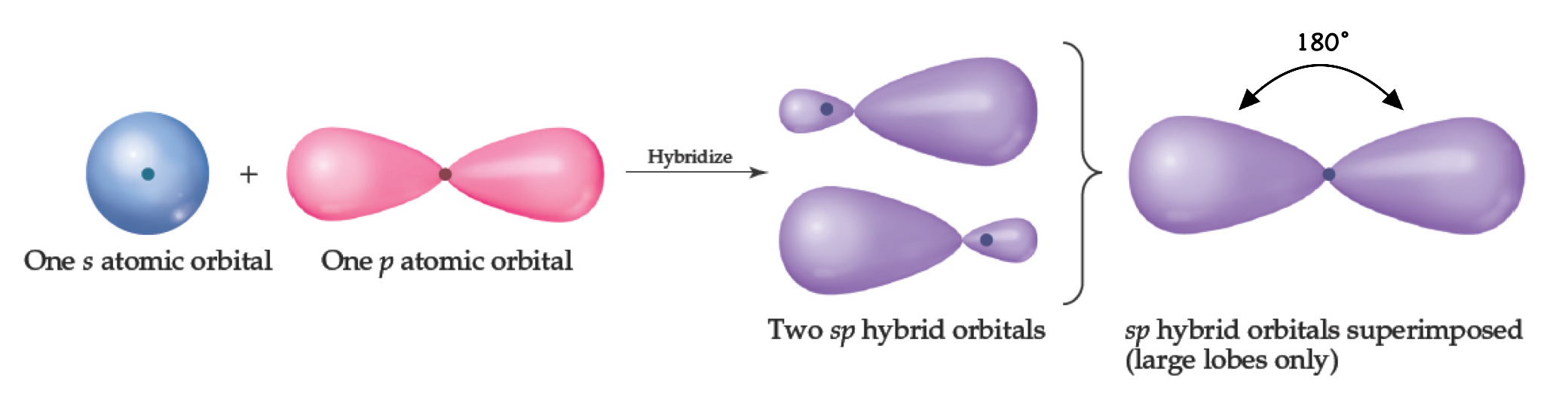
sp Orbitals
Mixing s and p orbitals yields 2 degenerate orbitals that are hybrids of the two orbital.
The sp hybrid orbitals each has two lobes like a p orbital.
One of the lobes is larger and more rounded, as is the s orbital.
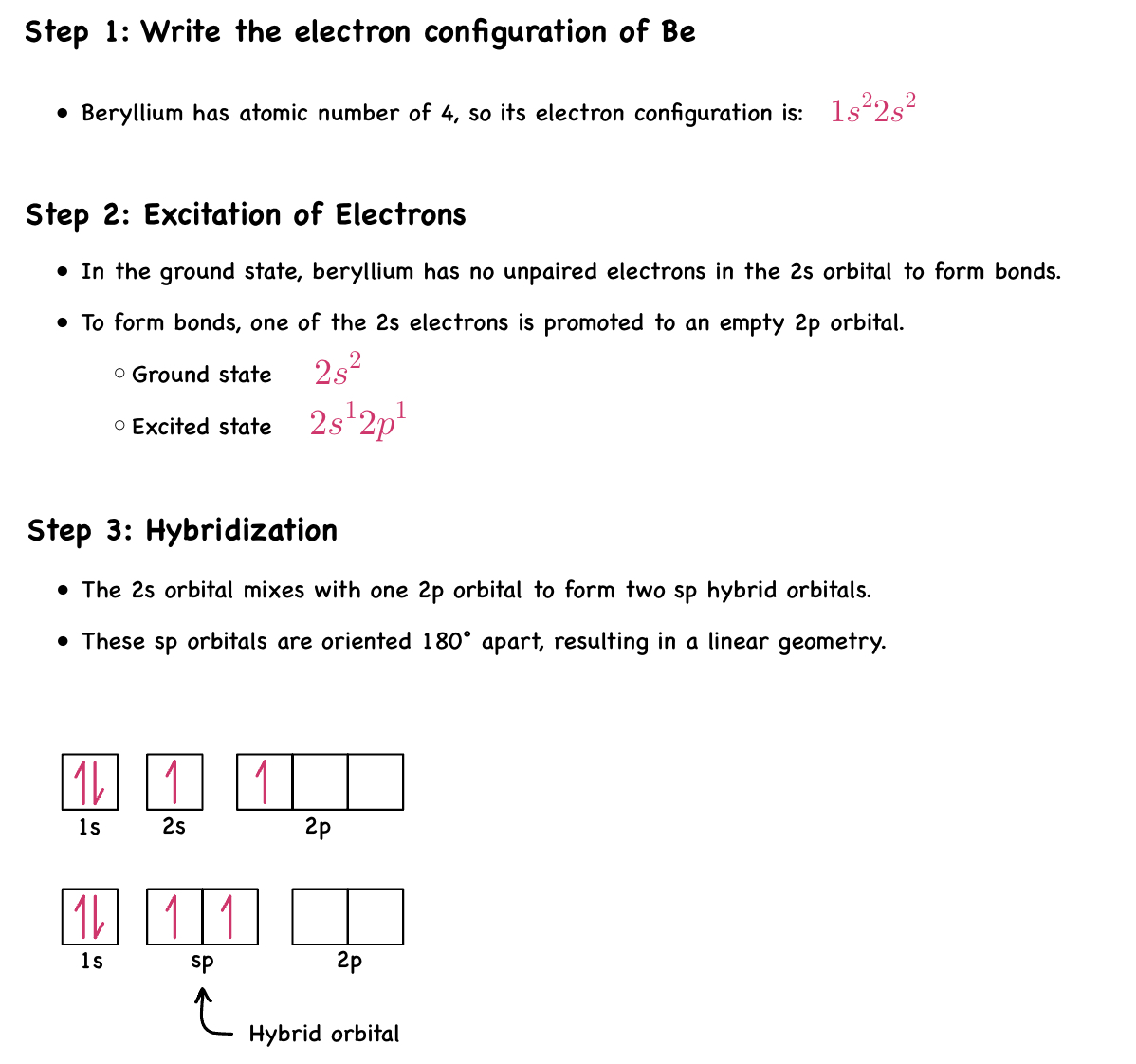
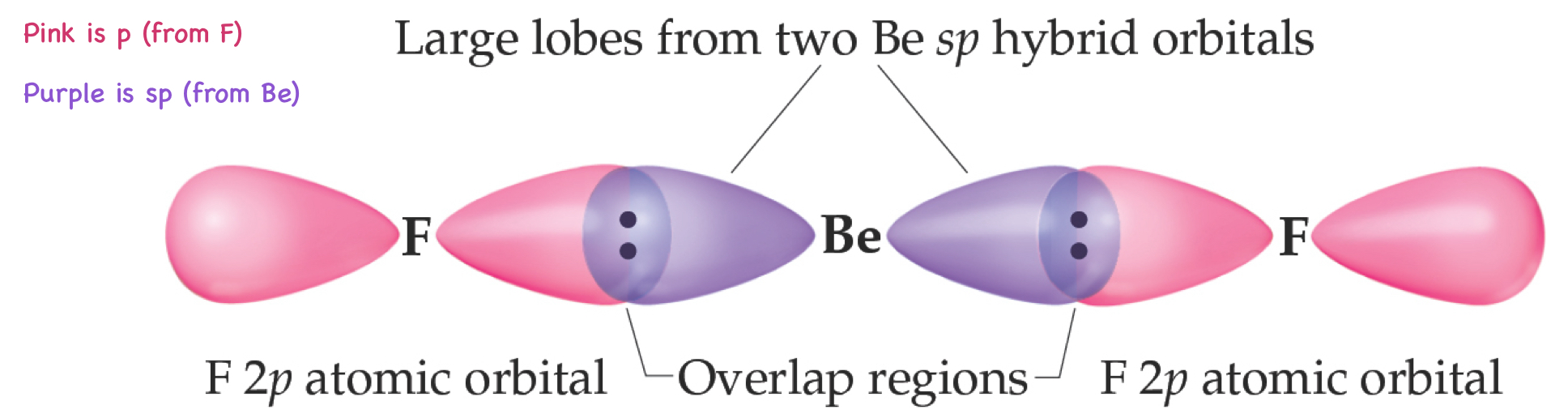
Be - sp Hybridization
Position of sp Orbitals
These two degenerate orbitals would align themselves 180° from each other.
This is consistent with the observed geometry of Be compounds (like BeF2) and VSEPR: linear.
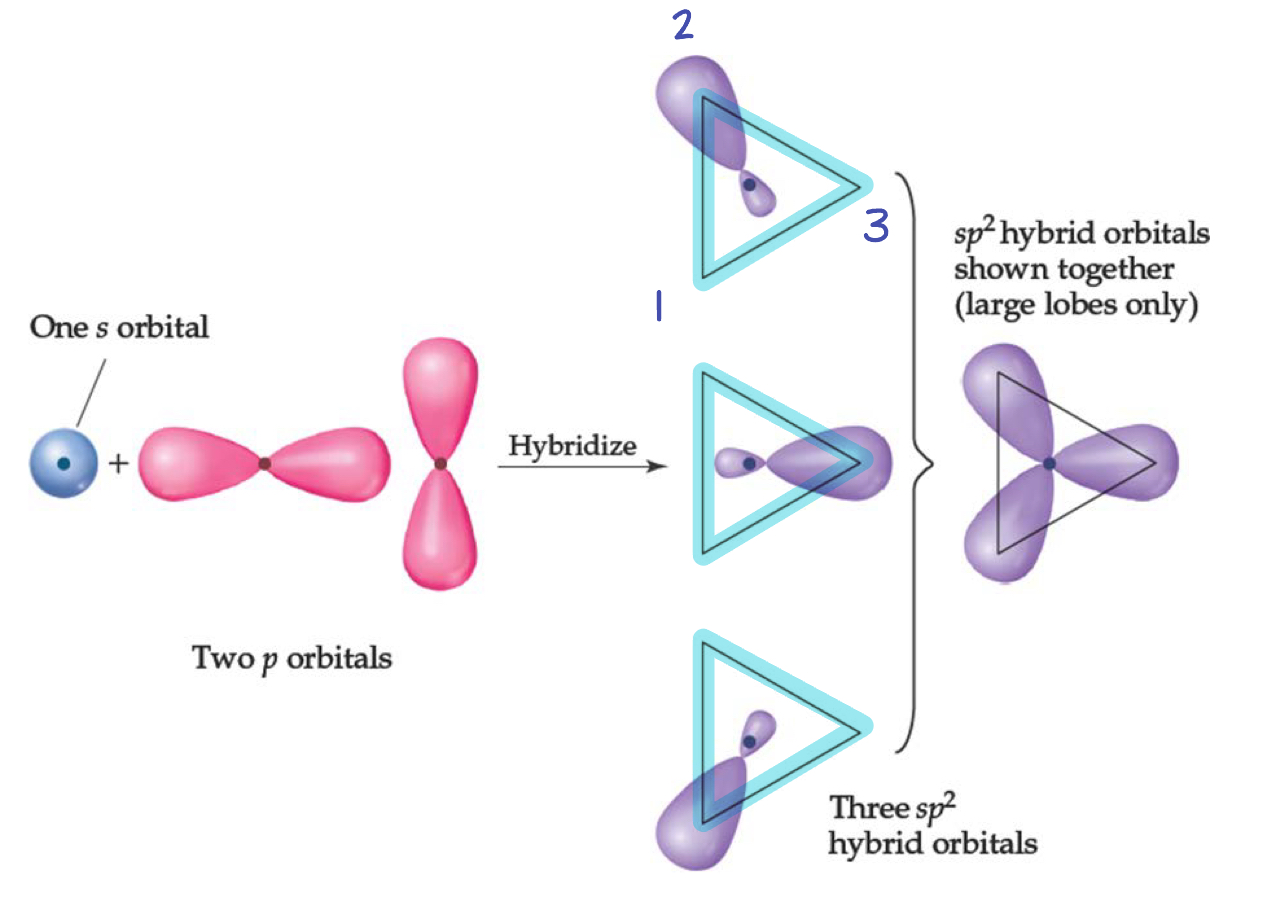
Boron - Three Electron Domains Gives sp2 Hybridization
Boron has three electrons groups when bonded to 3 atoms with no lone pairs.
Trigonal planar
1s + 2p = 3sp
Carbon: sp3 Hybridization
Carbon has four electron groups.
Tetrahedral
We get four degenerate sp3 orbitals.
Hybrid Orbital Summary
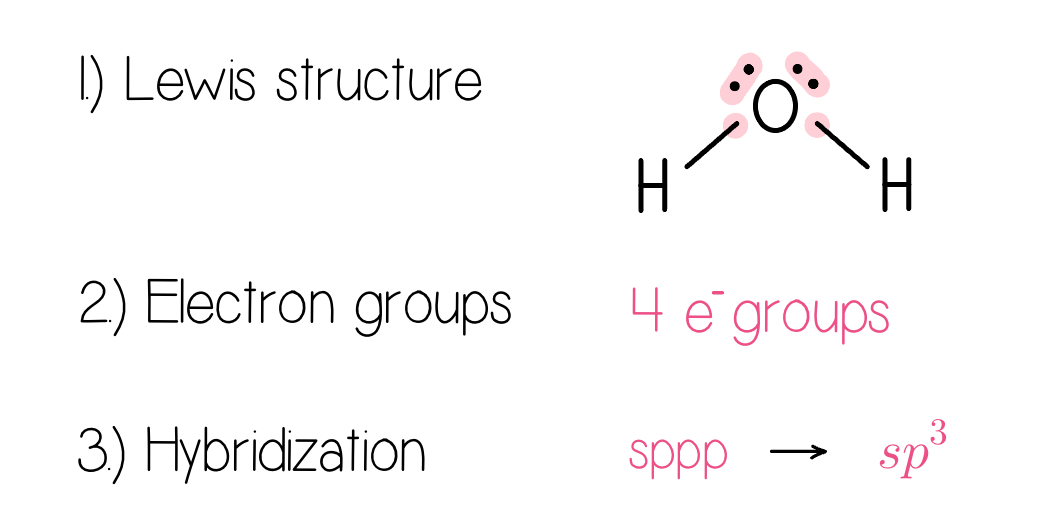
1.) Draw the Lewis structure.
2.) Use VSEPR to determine the electron domain geometry.
3.) Specify the hybrid orbitals needed to accommodate these electron pairs.
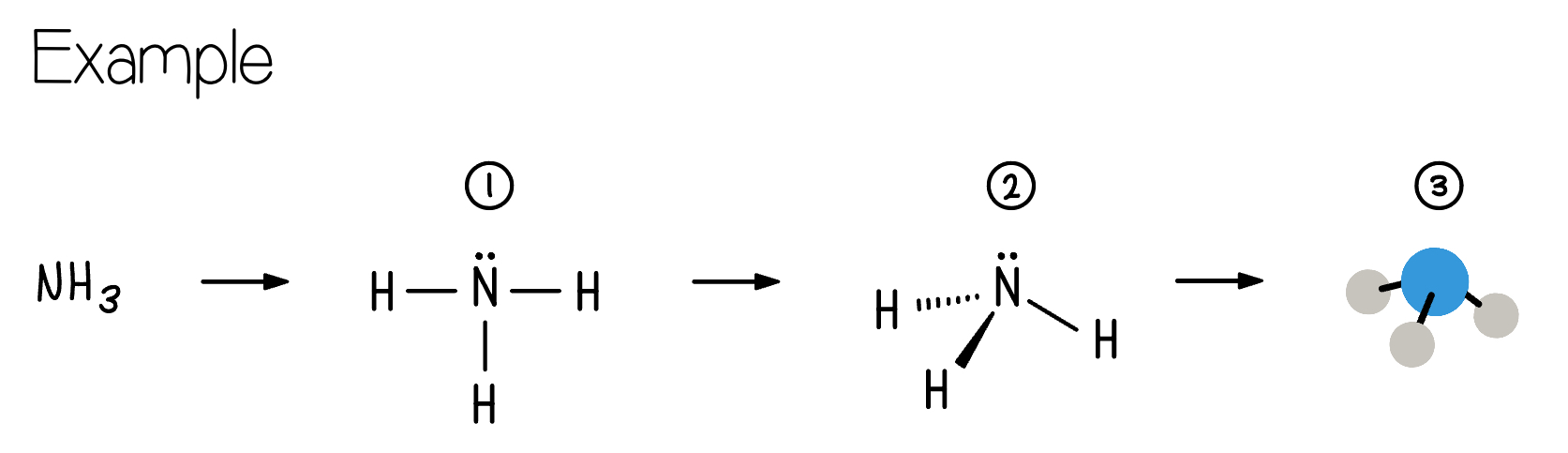
Example: For the molecule HO what is the hybridization on oxygen?
Types of Bonds
How does a double or triple bond form?
It can’t, if we only hybridized orbitals.
If we use orbitals that are NOT hybridized, we can have “sideways” overlap.
There are two types of bonds:
Sigma (\sigma) bond - a single bond
Pi bond (\pi) - a double bond = 1\sigma +1\pi
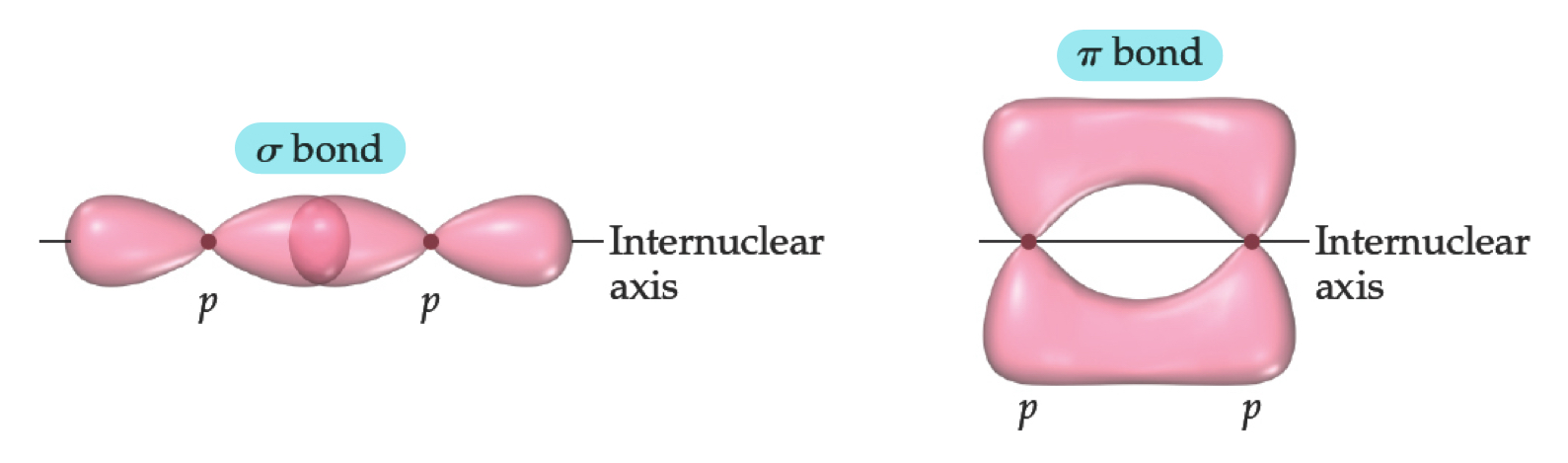
Sigma (\sigma) and Pi (\pi) Bonds
Sigma bonds are characterized by:
Head-to head overlap
Cylindrical symmetry of electron density about the internuclear axis.
Pi bonds are characterized by:
Sideways overlap
Electron density above and below the internuclear axis.
Single bonds are always \sigma bonds
Multiple bonds have one \sigma bond; all other bonds are \pi bonds
Single bonds - 1\sigma
Double bonds - 1\sigma + 1\pi
Triple bonds - 1\sigma + 2\pi
Localized and Delocalized Electrons
Localized electrons: Electrons that are confined to a specific bond between two atoms.
Delocalized electrons: Electrons that are spread out over several atoms, contributing to resonance structures.
Reference: Chemistry The Central Science (14th Edition)