Bioenergetics
Metabolism- body's chemical processes that convert food and drink into energy, and all the processes that occur within cells to sustain life and function, including energy production and waste removal
Anabolic reactions - synthesize or build new molecules, build up
Catabolic reactions - breakdown of molecules, break down
Reactions take place in the cytosol (outside of myofibrils)
Where does it take place?
inside a muscle cell (actin and myosin)
In the cytosol there is organelles - most of this process of metabolism happens in the fluid
Has a lot of mitochondria because it requires a lot of ATP - other half happens here
SR - matters more with calcium storage
Cell membrane (sarcolemma) - outside of the muscle cell
Endergonic Reactions - requires energy to be added in order for reaction to take place
Exergonic Reaction - Use energy for our food to synthesize ATP
Coupled Reaction - Liberation o f energy in an exergonic reaction drives an endergonic reaction
Break down of glucose into CO2 and H2O -
glucose molecules - ring of carbons (has 4), carbon can bind to themselves or other molecules - creates the backbone of other molecules
Endergonic and Exergonic reaction of C6H12O6 + 6O2 - strips glucose of hydrogen and has a waste product of heat, CO2, and H20 = 6 O2 + 6 H2O
Elements -
number above element is the atomic number - number of protons, neutron, and electrons
Atomic mass - mass of the protons, electrons, neutrons and the average (protons + neutrons)
Rows - period, same number of electron shells
Columns - a lot of same chemical properties, how many valence electrons they have (outer shells)
Full outer shell means they are not very reactive
With bonding we care about the number of valence electrons
Carbon has 6 neutrons & protons
Electrons just exists around the energy levels of our neutrons
What happens when CO2 gets dumped into water - pH decreases and H+ goes up
Types of chemical reactions/bonds
Ionic - gives away and electrons and one take the electron (weak)
Ex: NaCl (table salt)
Atomic mass of 11 - has one valence electrons, first shell is 2 second shell is 8
Chlorine 17 - 2 in first shell, 8 in the next shell, and 7 are left over - needs one more and gets it from sodium, now both are happy because sodium gave chloride its one valence electron
Hydrogen -
Covalent - shares valence electrons with each other (strong), needs a lot of energy to break it
Can be polar - has a charge, when no balanced
Can be non polar - when balanced
Non-balanced covalent bond
Water - one side is negative and one is positive
Its important because it can dissolve things in water
redox-reaction
Oxydation
Removing an electron
Reduction
Addition of an electron
Oxidation & Reduction are always Coupled reactions
A molecules that loses electron is oxidized
A molecule that gains an electron is reduced
Roles of NAD and FAD in electron transport
Nicotinamide Adenine Dinucleotide (NAD)
Oxidized form = NAD+
Reduced form = NADH
Flavin adenine dinucleotide (FAD)
Oxidized form = FAD
Reduced form = FAD H2
Both NAD and FAD play an important role in transfer of electrons
Enzymes - enemy ex are proteins that help speed (catalyze) up reactions
reduce the amount of energy needed for the chemical reactions to happen
Big protein molecules that are folded up so only certain things can bind to them in certain places (binding sites)
The shape on the enzyme place a role of what the enzyme can do
PH of blood or skeletal muscle, or heat can change of shape of what enzyme can do
Types of Enzymes
ends in ase - means its an ezyme
Kinases
Adds a phosphates to a molecule
Activates that molecule
Quickly added phosphate to proteins to make it do its job right away
Creatine Kinase - breaks down creating phosphate and increase energy
Dehydrogenases
Removes hydrogen atoms
Lactate Dehydrogenase - help break down pyruvate and turn it into the lactate molecule
Oxidases - catalyzes oxidation-reduction reactions involving oxygen
Cytochrome Oxidase - a malfunction in this it is death, cannot recreate more energy
Isomerases - Rearrangement of the stricture of molecules
Diagnostic value of measuring enzyme levels in the blood - healthy people, cellular enzymes are not found in the blood.
However, in diseases that damage cells, these cells release enzymes into the blood.
Enzyme levels in blood serve as “biomarkers” of disease and/or tissue damage.
Example of diagnostic application.
Elevated lactate dehydrogenase or creatine kinase in the blood may indicate a myocardial infarction.
Factors that influence enzyme activity
Temperature
A small rise in body temperature increases enzyme activity.
Exercise results in increased body temperature.
Large increase in body temperature (greater than 41°C) can denature enzymes and decrease activity.
pH
Changes in pH (increase or decrease) can decrease enzyme activity.
High intensity exercise decreases muscle pH.
Fuels for Exercise-Carbohydrates
Glucose
Blood sugar
Why can we store a lot of glucose molecules
They are solute (dissolve in water), water will leave the side with less solute and go to the side with more solute
All the water would leave our blood and go into our cells
Glycogen
Storage form of glucose in liver and muscle
Gets stored in big chains and acts as a single solute
Synthesized by enzyme of glycogen synthase
1-2% opt the muscle volume is glycogen
Each gram of glycogen gets stores with about 3 grams of water in the muscle (1.5L)
About 2 cups of sugar is stored as glycogen
60-70% of fuels for high intensity exercise comes from glycogen
Glycogenlysis
Breakdown of glycogen to glucose
Fuels for exercise -fats
Fatty Acids
Chains of carbon atoms linked to carboxyl group at one end (C, H, and O)
Primary type of fat used by muscle cells for energy
Triglycerides
Fatty acids are stored in the body as triglycerides
Composed of 3 molecules of fatty acids and one molecule of glycerol
Glycerol - Not a fat but a type of alcohol
They are stored in many cells like skeletal muscle
Lipolysis - regulated by a family of enzymes called lipases
Glycerol that is released by lipolysis is not a direct sources of energy for muscles, but is used by the liver for glucose
Phospholipids - lipids combined with a phosphate group and synthesizes in every cell
Not a fuel sources for skeletal muscles
Roles can vary
Provides structural integrity
Proved insulation sheath around nerve fibers
Steroids - not used as an energy source during exercise
Common steroid is cholesterol
Cholesterol - component of all cell memebranes
Can be synthesized in every cell and consumed in all foods
Needed for synthesis of sex hormones estrogen, progesterone, and testosterone
High blood cholesterol levels have been implicated in the development of coronary artery diseases
Proteins
Proteins - are made up of small units called amino acids, with the human body requiring 20 different types to create the proteins found in cells and blood.
Out of these, nine are essential amino acids that the body cannot produce and must be obtained through diet.
Proteins are linked together by peptide bonds and provide about 4 kcal/g of energy.
To be utilized as energy sources, proteins must first be broken down into their amino acids.
They can support energy needs during exercise in two key ways.
One method involves the conversion of the amino acid alanine into glucose in the liver, which can be stored as glycogen and later converted back to glucose for muscle use.
Second medthod, several amino acids, such as isoleucine, alanine, leucine, and valine, can be transformed into metabolic intermediates in muscle cells, contributing directly to energy production in bioenergetic pathways.
High Energy Phosphates - Immediate source of energu for musclular contraction (ATP)
ATP - Adenosine Triphosphate - Universal energy donor (1-2sec)
Sturcture consists of thre emain parts
An adenine portion
A ribose portion
Thre elinked phosphates
Formation of ATP occure when combining
Adenosine Diphosphate & Inorganic phsphate & energy
High energy bond is when joining ADP & Pi
The ATPase breaks this bond energy is released and can be used to do work
Exergonic reaction - breadown of foodstuffs to form ATP from endergonic reaction
Bioenergetics
Muscle cells have a limited supply of ATP, which is essential for muscle contraction during exercise. To meet the continuous demand for ATP, muscle cells utilize three main metabolic pathways at the onset of contraction. The first is the breakdown of phosphocreatine (PC) to quickly generate ATP. The second pathway involves glycolysis, where glucose or glycogen is degraded to produce ATP without the need for oxygen, classifying it as anaerobic. The third pathway is aerobic metabolism, which generates ATP using oxygen. These metabolic processes work together to ensure a rapid supply of energy during muscular activity.
Anaerobic ATP Production - most rapid method of producing ATP involves the donation of a phosphate group and its bond energy from PC to ADP to form ATP

Reaction catalyzed by creatine kinase is crucial for energy production in muscle cells during exercise
onset of physical activity, ATP is rapidly broken down into ADP and inorganic phosphate (Pi), but it is simultaneously resynthesized through the phosphocreatine (PC) reaction.
because muscle cells can only store limited amounts of phosphocreatine, the total ATP generated through this process is restricted
This combined energy system, known as the ATP-PC system or phosphagen system (5-10sec)
vital for providing energy during the initial moments of exercise - high-intensity activities lasting up to 5 to 15 seconds.
EX exercises: sprinting 100 meters, high jumping, quick weight-lifting
However, the limited stores of phosphocreatine can hinder performance during short, intense exercises.
led to discussions about the potential benefits of creatine supplementation for enhancing athletic performance.
Second Pathway of ATP Production (still Anaerobic)
Glycolysis - is a vital metabolic pathway that facilitates the conversion of glucose into pyruvate or lactate, ultimately resulting in the production of energy in the form of ATP (30 sec-2 min)
involves the breakdown of glucose, a six-carbon sugar → two three-carbon molecules known as pyruvate or lactate, depending on oxygen
takes place in an anaerobic environment - it does not rely on oxygen to proceed
circumstances where oxygen is present, the pyruvate produced can be further utilized in the mitochondria for aerobic respiration
leading to a more efficient generation of ATP
Glycolysis serves as the crucial initial step in the aerobic metabolism of carbohydrates
cells can rapidly mobilize energy reserves, underscoring its significance in energy metabolism
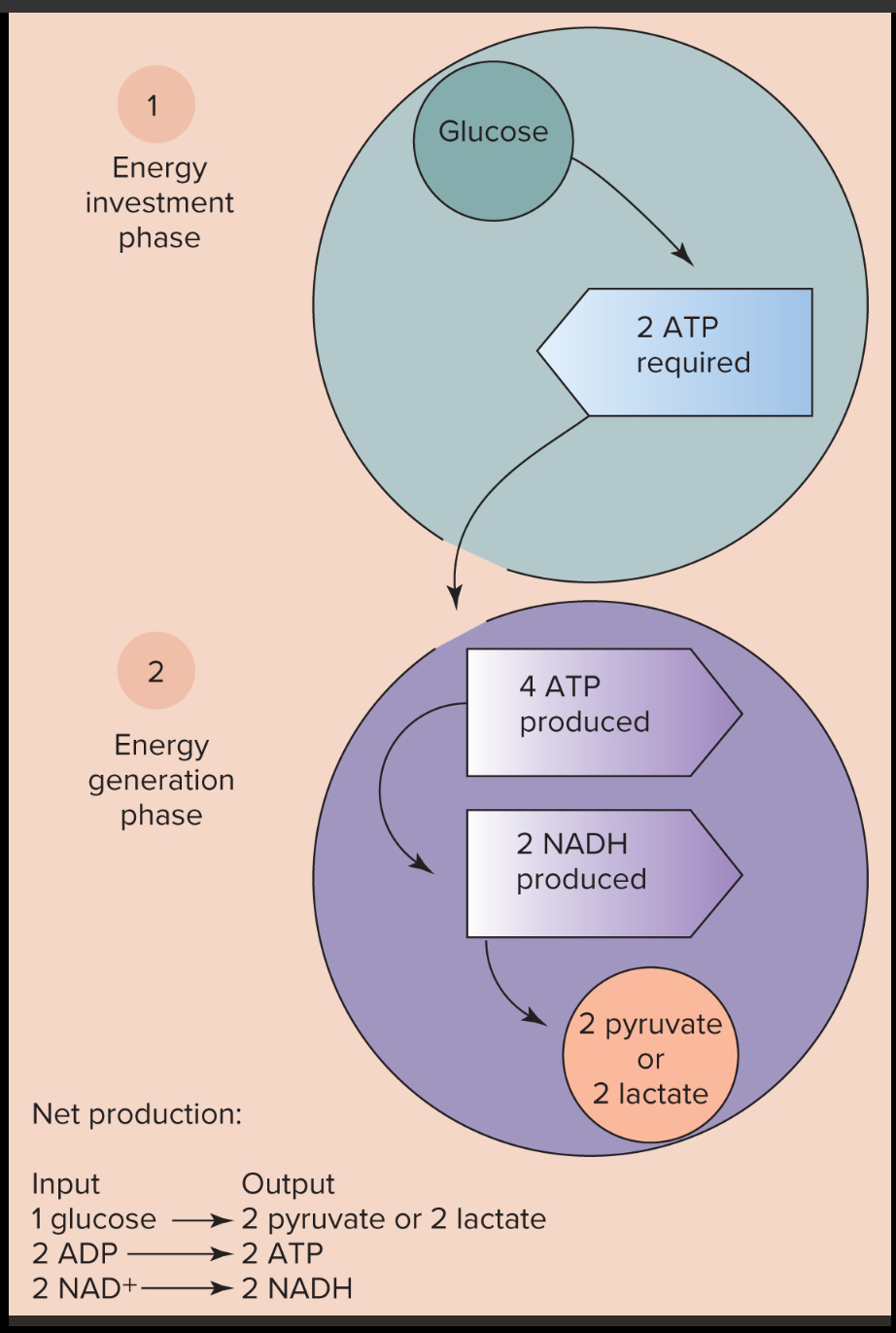
Aerobic ATP Production
Occurs inside the mitochondria and involves interaction of Citric Acid Cycle and Electron Transport Chain
Citric Acid Cycle
The citric acid cycle, commonly known as the Krebs cycle or tricarboxylic acid cycle, plays a crucial role in cellular respiration. Its main function is to facilitate the complete oxidation of acetyl CoA, which can be derived from carbohydrates, fats, or proteins. During this process, the cycle utilizes NAD+ and FAD as important electron carriers, helping to transfer energy efficiently within the cell.
Electron Transport Chain
The removal of electrons plays a critical role in cellular respiration, as these electrons carry the potential energy obtained from food molecules. This energy is utilized in the electron transport chain to facilitate the conversion of ADP and inorganic phosphate (Pi) back into adenosine triphosphate (ATP). While oxygen does not take part in the reactions of the citric acid cycle, it serves as the final acceptor of electrons and hydrogen at the conclusion of the electron transport chain, leading to the formation of water (H2O) through the reaction of hydrogen (H2) and oxygen (O).
Oxidative Phosphorylation - Process of aerobic production of ATP (indefinetly)
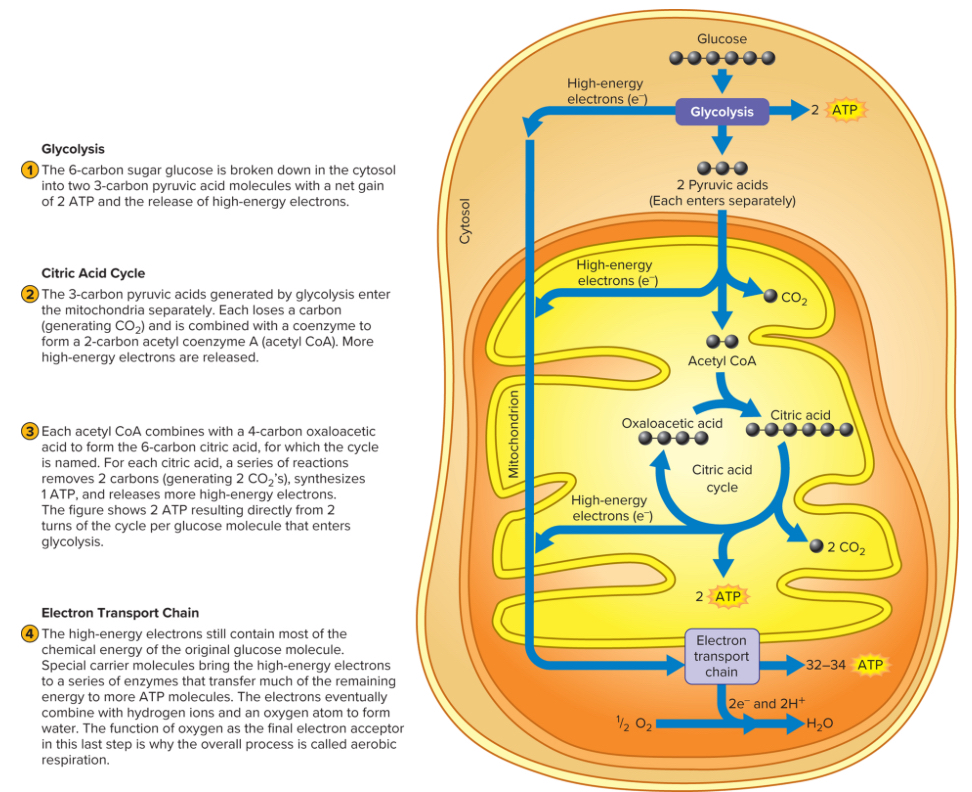
Citric Acid Cycle
Begins with the formation of the two-carbon molecule acetyl-CoA, which is derived from the breakdown of carbohydrates, fats, or proteins. A crucial step in this process is the conversion of pyruvate, a three-carbon molecule produced during glycolysis, into acetyl-CoA, with the release of carbon dioxide (CO2)
Once formed, acetyl-CoA combines with a four-carbon molecule called oxaloacetate to produce citrate, a six-carbon compound. The cycle then progresses through a series of reactions that generate two molecules of CO2 and regenerate oxaloacetate, allowing the cycle to continue. Each glucose molecule entering glycolysis yields two pyruvate molecules, which, in the presence of oxygen, are transformed into two acetyl-CoA molecules. Consequently, each glucose results in two complete turns of the citric acid cycle
The main function of the citric acid cycle is to extract electrons from various substrates, which are transported by NADH and FADH to the electron transport chain. During each cycle, three NADH and one FADH are produced. NADH is more energy-rich, providing sufficient energy to generate 2.5 ATP molecules per pair of electrons passed through the electron transport chain, while FADH yields 1.5 ATP per molecule.
The citric acid cycle also leads to the direct production of guanosine triphosphate (GTP), a high-energy compound that can convert ADP into ATP through substrate-level phosphorylation. Majority of the energy produced in the cycle is linked to the formation of NADH and FADH, which are primarily responsible for powering the electron transport chain and ATP synthesis. Mainly centered on the role of carbohydrates in generating acetyl-CoA for the citric acid cycle.
Beta Oxidation - Process of Converting Fatty Acids to Acetyl-CoA
Fats in the body are primarily stored as triglycerides within fat cells and muscle fibers. When the body needs to release this stored fat, triglycerides undergo breakdown, resulting in the release of fatty acids. For these fatty acids to be utilized as fuel during aerobic metabolism, they must first be converted into acetyl-CoA through a process known as beta oxidation. This process takes place in the mitochondria and involves a series of enzymatic steps, starting with an activated fatty acid and culminating in the formation of acetyl-CoA
Beta oxidation consists of four sequential reactions that effectively "chop" fatty acids into two-carbon fragments, producing acetyl-CoA. Once generated, acetyl-CoA serves as a crucial fuel source for the citric acid cycle, also known as the Krebs cycle, ultimately leading to the production of ATP through the electron transport chain.
Electron Transport Chain - Aerobic Poroduction of ATP occurs in Mitrochondria
The electron transport chain, also known as the respiratory chain or cytochrome chain, plays a crucial role in the aerobic production of ATP
Process utilizes the potential energy stored in reduced hydrogen carriers, primarily NADH and FADH, to convert ADP into ATP
NADH and FADH do not directly interact with oxygen
Instead, the electrons from the hydrogen atoms they carry are passed through a sequence of electron carriers known as cytochromes
As these electrons move through the chain, they release energy, which is harnessed to rephosphorylate ADP into ATP
small amounts of free radicals—highly reactive molecules—are generated during this electron transfer.
during periods of muscular exercise, the rate of electron flow through the chain increases without corresponding increases in radical production within the mitochondria.
Free Radicals - formed in mitochondria
Process of oxidative phosphorylation, essential for aerobic ATP production, involves the movement of electrons down the electron transport chain
This pathway also generates free radicals, as some electrons can "leak" from the chain, creating reactive molecules with unpaired electrons
Free radicals can interact with other cellular molecules, leading to potential damage.
It was traditionally believed that aerobic metabolism during exercise increased free radical production in the mitochondria of active muscles. However, recent studies indicate that while muscular exercise does elevate the levels of free radicals, the majority of their production in skeletal muscle mitochondria actually occurs while the muscles are at rest, not during exercise.
Electron Transport Chain Oxidative Phosphorylation:
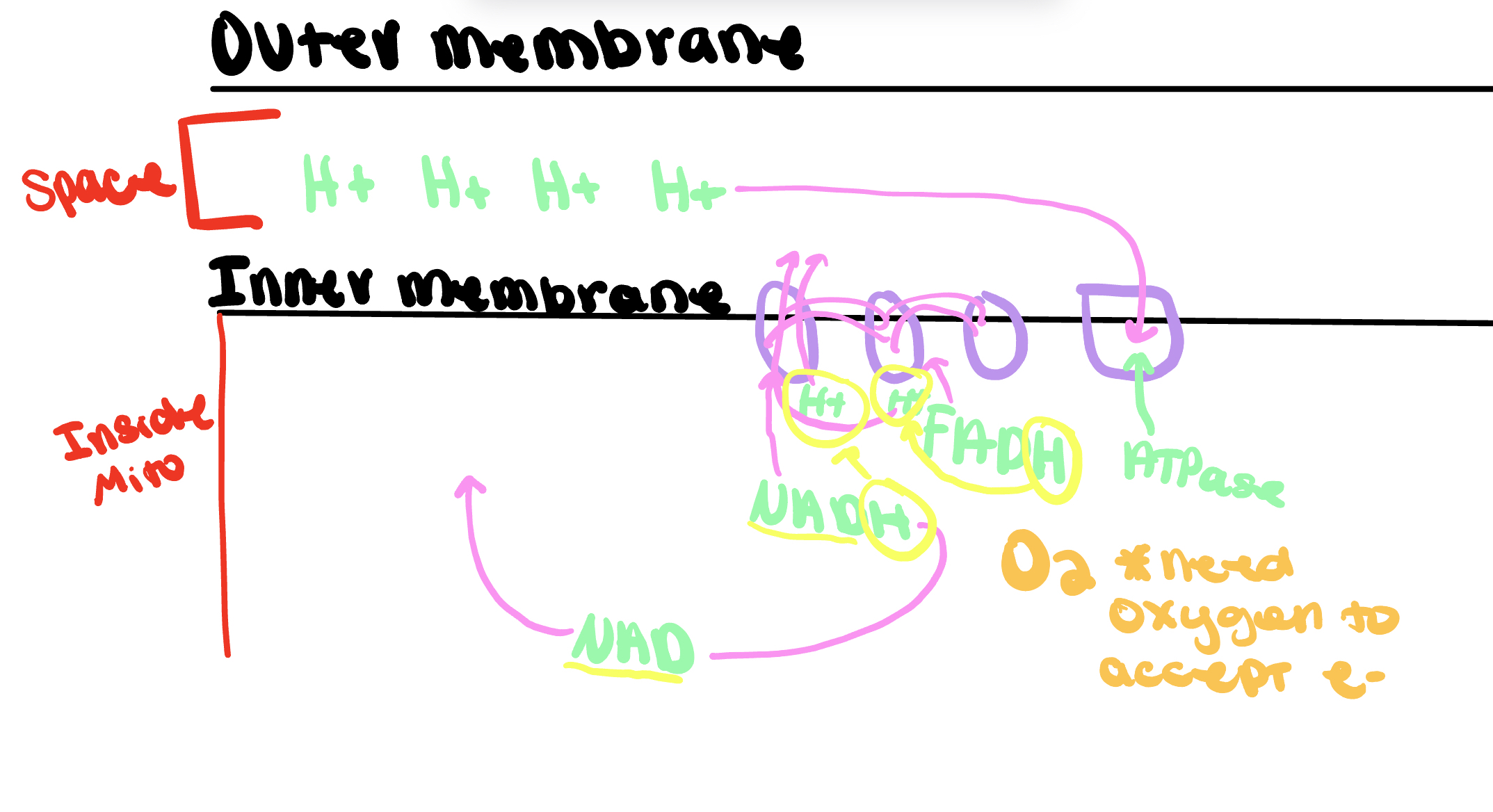 Same example below just shown better:
Same example below just shown better:
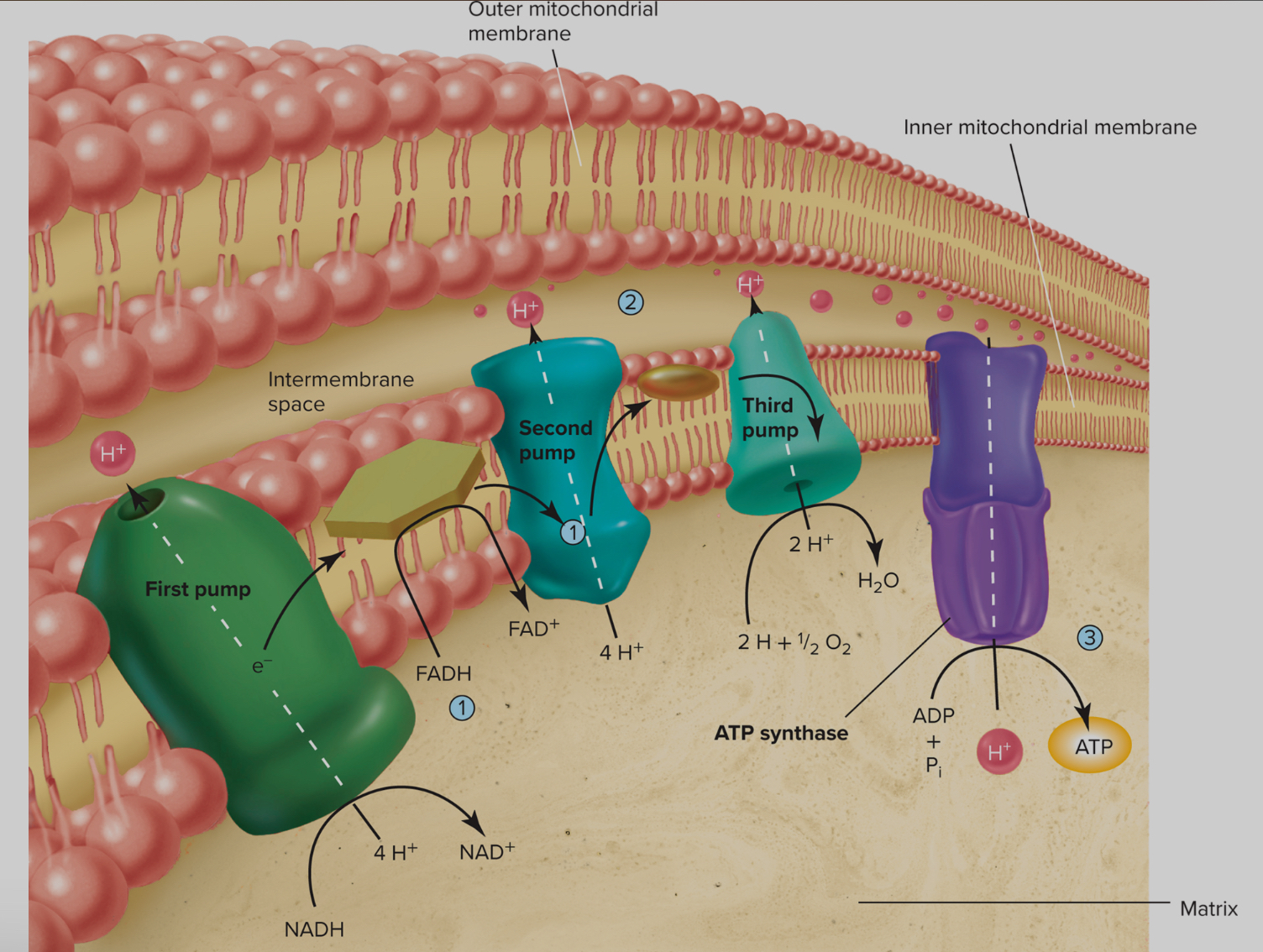
Regulation of Bioenergetics-
Rate Limiting Enzymes - An enzyme that regulates the rate of a metabolic pathway
Modulators of rate-limiting enzymes:
Levels of ATP & ADP+P
Why levels of ADP? - if you have a lot of ADP signals to all these systems to ramp up their production
High levels of ATP inhibit ATP production
Low levels of ATP and high levels of ADP+P, stimulate ATP production
Calcium stimulates aerobic ATP production
Calcium starts off muscle contraction, tell muscle cell it is doing work