Photo-physics of molecules
Bioluminescence
Light emission by chemical reactions in living organisms .
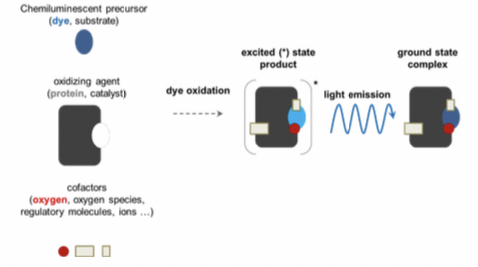
Recipe for bioluminescence
Chemiluminescent precursor
• substrate- dye molecule, chromophore(light emission).
Oxidizing agent
• Catalyst - Protein molecule
Cofactors
• oxygen, regulatory materials, ions
Steps
Interaction between the chemiluminescent precursor(dye molecule) and the oxidizing agent.
Dye oxidized to form excited state product.
The molecule releases energy; equal to the difference between excited and ground state, in form of light.
E2 - E1 = hf = hc/ wavelength
* wavelength in the range of UV-VIS light
Ground state complex formed.
Examples:
a. Firefly; precursor: luciferin oxidizing agent: luciferase. Wavelength = 562nm.
b. Blue Jellyfish; precursor: coelenterazine oxidizing agent: apoquorin. Wavelength= 480nm.
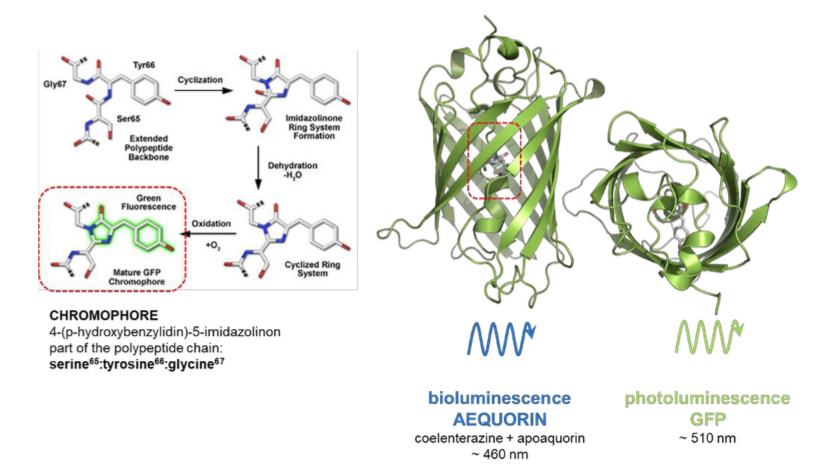
Green Fluorescent Protein (GFP)
Besides bioluminescence the blue jellyfish can also emit by photoluminescence. In this case ==the protein acts as the fluorophore; Green fluorescent protein==. The chromophore is part of the polypeptide chain and energy to excite it is provided by bioluminescent light(coelenterazine-apoquorin). Light emitted is greenish due to 510nm wavelength.
The gene encoding GFP can be fused to a specific target in other organisms by genetic manipulation.
The targets are visible through the fluorescence emission of GFP.
GFP is constitutively active (emits fluorescence) in other organisms, not only in jelly fish.
Types of fluorophores
• Intrinsic fluorophores: Aromatic amino acids; most proteins possess intrinsic fluorescent due to fluorescence amino acids(Tryptophan, tyrosine and phenylalanine). The intrinsic fluorescence of proteins is not visible to humans eye. GFP; in jelly fish.
Application - Restricted flexibility, e.g; fluorescence spectroscopy.
• Extrinsic fluorophores: It is sensitive to environmental parameters(biosensors) and regulates fluorescence emission properties. Synthetic dye molecules, GFP in other organisms, quantum dot, fluorescent antibody.
Application - Spectral flexibility, e.g; Immunofluorescence, flow cytometry.
Fluorescent labels
The binding of antibody to the target(antigen), the antibody is flourescent due to gluorphores present.
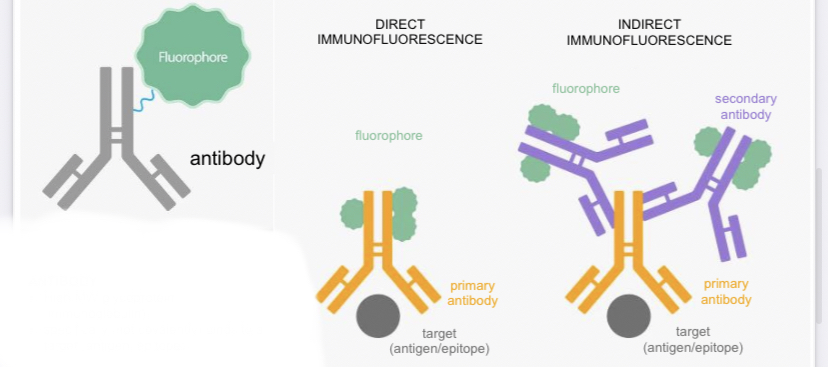
Types of immunofluorescence
Direct immunofluorescence:
target + primary antibody & fluorophore
Indirect immunofluorescence:
target + primary antibody + secondary antibody & flurophore.
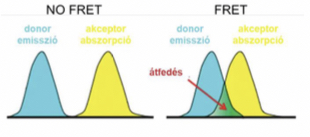
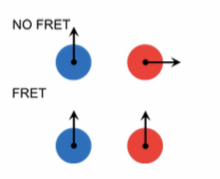
Förster Resonance Energy transfer(FRET)
It is energy transfer on dipole-dipole interactions of excited state donor and acceptor with appropriate spectral characteristics.
Requirements
Overlap between emission spectrum of donor and absorption spectrum of acceptor.
Distance between donor and acceptor (approximately 2-10nm, critical distance = R0)
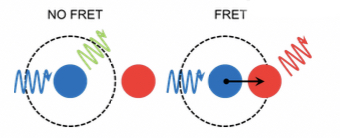
Relative orientation of donor and acceptor
Efficiency of FRET; transfer efficiency (E) depends on the distance between the donor and the acceptor (r), as well as their characteristic critical distance (Ro):

The transter efficiency can be related to the fluorescence emission of the donor measured in the absence(Fda) and presence of acceptor(Fd):
E = 1 - Fda/Fd
FRET as a molecular ruler
Relationship between transfer efficiency and donor-acceptor distance allows FRET to determine distance between molecules in order of wavelength in nm.
Biosensors
Acts as substrate for sensory domain. Fluorescence is sensitive to environmental influence.
Photoswitchable fluorophores
Photobleaching is an irreversible photochemical destruction of the fluorophore due to the excitation.
Disadvantages.
a) Anti-photobleaching medium (pl. glucose oxidase - catalase - mercaptoethanol)
b) Lower exposure time
c) Lower excitation intensity
d) Resistant fluorophore
Advantages
FRAP(fluorescence recovery after photobleaching)
Technique of FRAP
Molecule is labelled by fluorescent dye, a fluorescent microscope with low intensity is used to detect fluorescent emission. Then illuminate the sample with high intensity light, you can recover the fluorescence by viewing with low intensity light. The time required for half maximal recovery (t1/2) can be derived; ==the shorter the time the more mobile the molecule.==