4.1-4.3 Symbols, equations and Formulae
Chemical formulae
The structural formula describes how the atoms of a given molecule are connected.
This can be done with either a diagram (shown formula) or a written formula (simplified structural formula), The empirical formula determines the simplest whole-number ratio of each element's atoms in a compound.
The molecular formula indicates how many atoms of each element are present in one molecule of the compound or element.
E.g. H2 has two hydrogen atoms, while HCl contains one hydrogen atom and one chlorine atom.
Structural formula (simplified)
CH3CH2CH2CH3
Molecular formula
C4H10
Empirical formula
C2H5
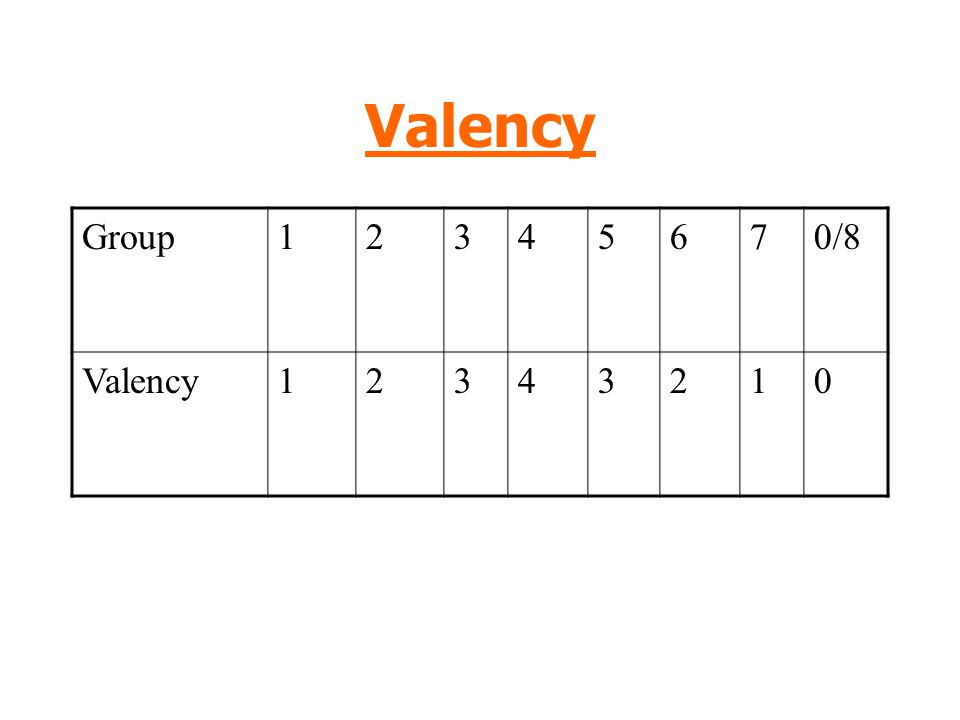
Using valency to deduce formulas
The concept of valency is utilized to deduce compound formulas.
The valency, or combining power, of an atom indicates how many bonds it can form with another atom. Carbon, for example, belongs to Group IV, which means that a single carbon atom can form four single bonds or two double bonds.
Each group's constituents have the following valencies:
What is the formula of aluminium sulfide?

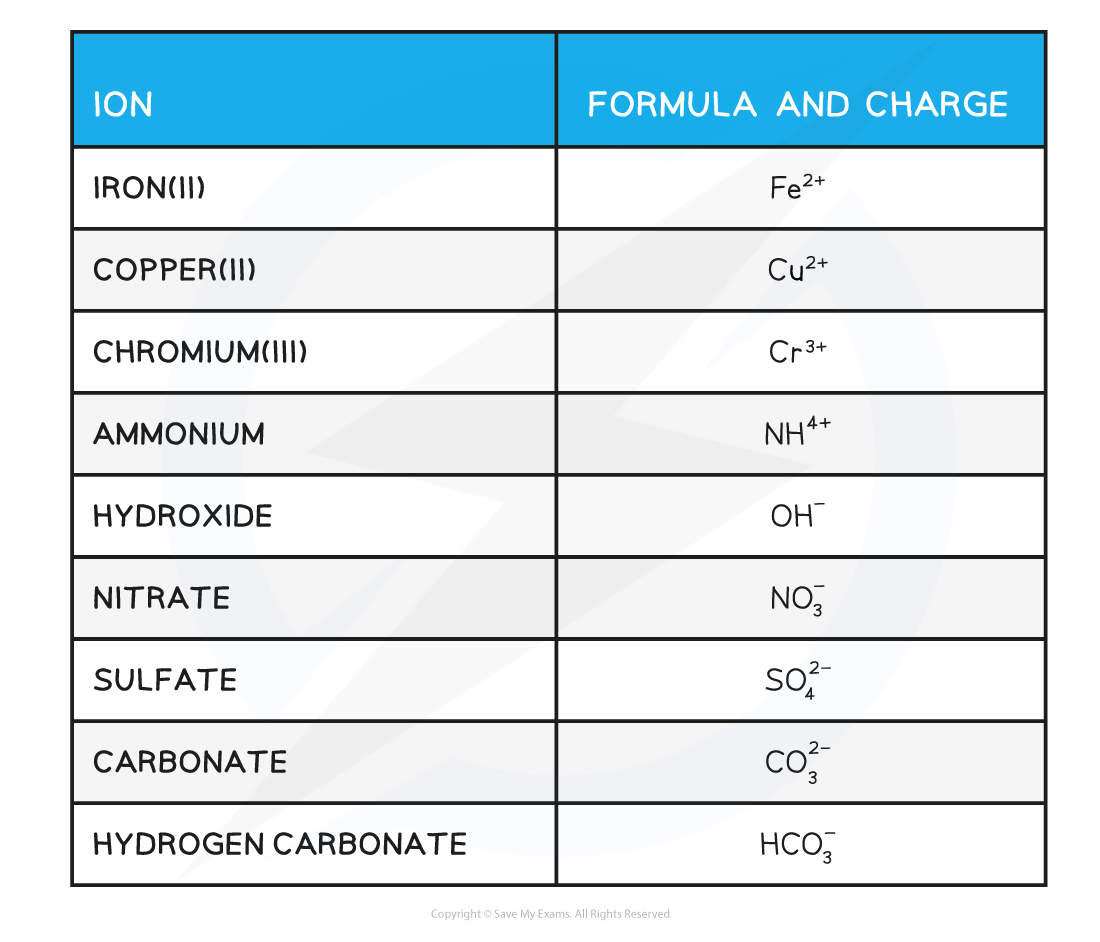
Ionic Compound Formulae Deduction
If you know the charge on the ions, you can compute the formulas for these compounds.
The charges of several common ions are listed below. The table includes a number of common compound ions.
These ions are referred to as polyatomic ions by certain chemists.
The Charges of Common Ions Table
The overall sum of the charges of an ionic compound should be 0
You therefore need to work out the ratio of the ions to ensure this is the case
When you write the formula of a compound ion it is necessary to use brackets around the compound ion where more than one of that ion is needed in the formula
For example copper(II)hydroxide is Cu(OH)2
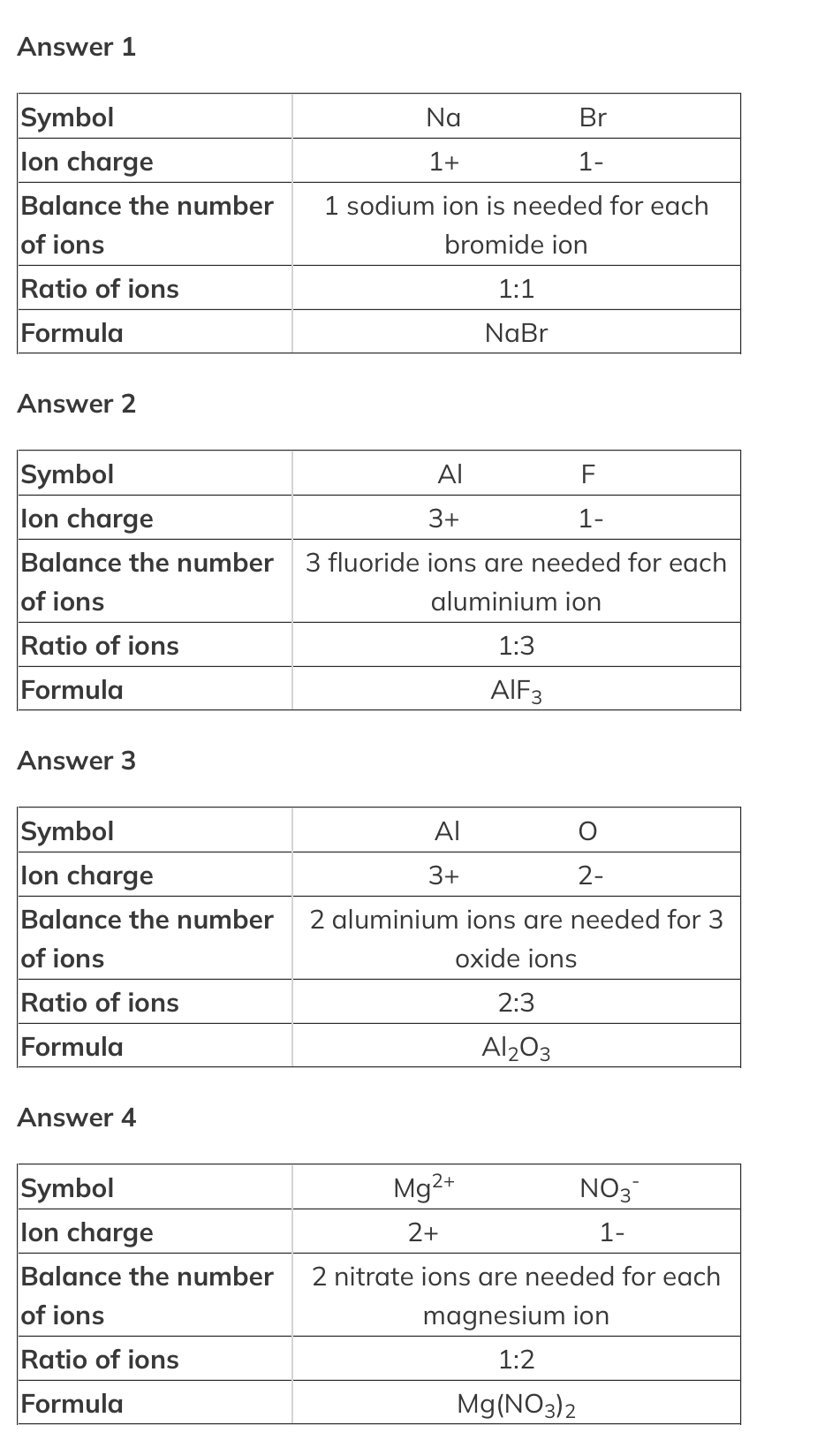
What is the formula of?
sodium bromide
aluminium fluoride
aluminium oxide
magnesium nitrate
Writing Equations and Balancing
Word Equations
These show the reactants and products of a chemical reaction using their full chemical names
The arrow (which is spoken as “goes to” or “produces”) implies the conversion of reactants into products
Reaction conditions or the name of a catalyst can be written above the arrow
An example of an word equation for neutralisation is:
sodium hydroxide + hydrochloric acid → sodium chloride + water
The reactants are sodium hydroxide and hydrochloric acid
The products are sodium chloride and water 💦
Compounds' names
For compounds consisting of 2 atoms:
If one is a metal and the other a nonmetal, then the name of the metal atom comes first and the ending of the second atom is replaced by adding -ide
E.g NaCl which contains sodium and chlorine thus becomes sodium chloride
If both atoms are nonmetals and one of those ishydrogen, then hydrogen comes first
E.g. Hydrogen and chlorine combined is called hydrogen chloride
For other combinations of nonmetals as a general rule, the element that has a lower group number comes first in the name
E.g. carbon and oxygen combine to form CO2 which is carbon dioxide since carbon is in Group 4 and oxygen in Group 6
For compounds that contain certain groups of atoms:
There are common groups of atoms which occur regularly in chemistry
Examples include the carbonate ion(CO32-), sulfate ion (SO42-), hydroxide ion (OH-) and the nitrate ion (NO3-)
When these ions form a compound with a metal atom, the name of the metal comes first
E.g. KOH is potassium hydroxide, CaCO3 is calcium carbonate
Writing and balancing equations
Chemical equations use the chemical symbols of each reactant and product
When balancing equations, there needs to be the same number of atoms of each element on either side of the equation
The following nonmetals must be written as molecules: H2, N2, O2, F2, Cl2, Br2 and I2
Work across the equation from left to right, checking one element after another
If there is a group of atoms, for example a nitrate group (NO3-) that has not changed from one side to the other, then count the whole group as one entity rather than counting the individual atoms.
Examples of chemical equations:
Acid-base neutralisation reaction:
NaOH (aq) + HCl (aq) ⟶ NaCl (aq) + H2O (l)Redox reaction:
2Fe2O3 (aq) + 3C (s) ⟶ 4Fe (s) + 3CO2 (g)
n each equation there are equal numbers of each atom on either side of the reaction arrow so the equations are balanced
The best approach is to practice lot of examples of balancing equations
By trial and error change the coefficients (multipliers) in front of the formulae, one by one checking the result on the other side
Balance elements that appear on their own, last in the process
Example 1 😻
Balance the following equation:
aluminium + copper(II)oxide ⟶ aluminium oxide + copper
Unbalanced symbol equation:
Al + CuO ⟶ Al2O3 + Cu


Sometimes it can be hard to know what the correct state symbol is and we have to look for clues in the identity of substances in a reaction
Generally, unless they are in a solution:
Metal compounds will always be solid, although there are a few exceptions
Ionic compounds will usually be solids
Non-metal compounds could be solids, liquids or gases, so it depends on chemical structure
Precipitates formed in solution count as solids
In the worked examples above the final equations with the state symbols would be
2Al (s) + 3CuO (s) ⟶ Al2O3 (s) + 3Cu (s)
MgO (s) + 2HNO3 (aq) ⟶ Mg(NO3)2 (aq) + H2O (l)
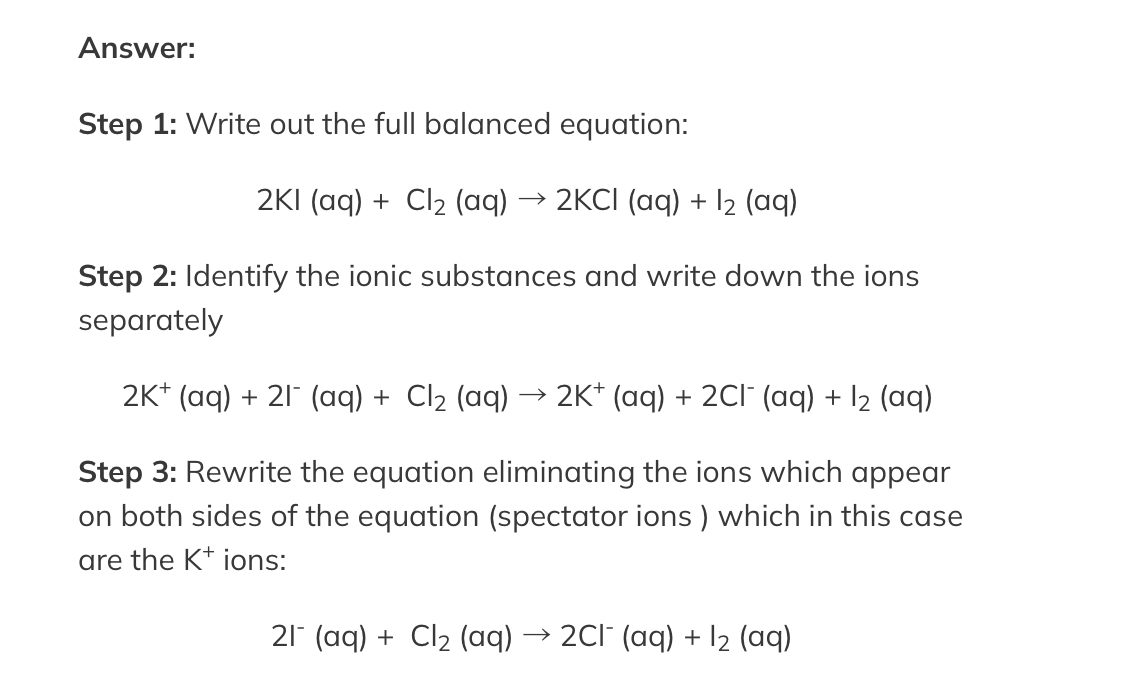
Balancing Ionic Equation
AR and MR
Relative atomic mass
The symbol for the relative atomic mass is Ar
The relative atomic mass for each element can be found in the periodic table along with the atomic number

Atoms are too small to accurately weigh but scientists needed a way to compare the masses of atoms
The carbon-12 is used as the standard atom and has a fixed mass of 12 units
It is against this atom which the masses of all other atoms are compared
Relative atomic mass (Ar*)* can therefore be defined as:
the average mass of naturally occurring atoms of an element on a scale where the 12C atom has a mass of exactly 12 units
The relative atomic mass of carbon is 12
The relative atomic mass of magnesium is 24 which means that magnesium is twice as heavy as carbon
The relative atomic mass of hydrogen is 1 which means it has one twelfth the mass of one carbon-12 atom
The relative atomic mass of an element can be calculated from the mass number and relative abundances of all the isotopes of a particular element using the following equation:
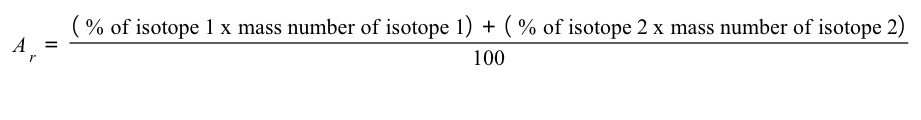
The table shows information about the Isotopes in a sample of rubidium

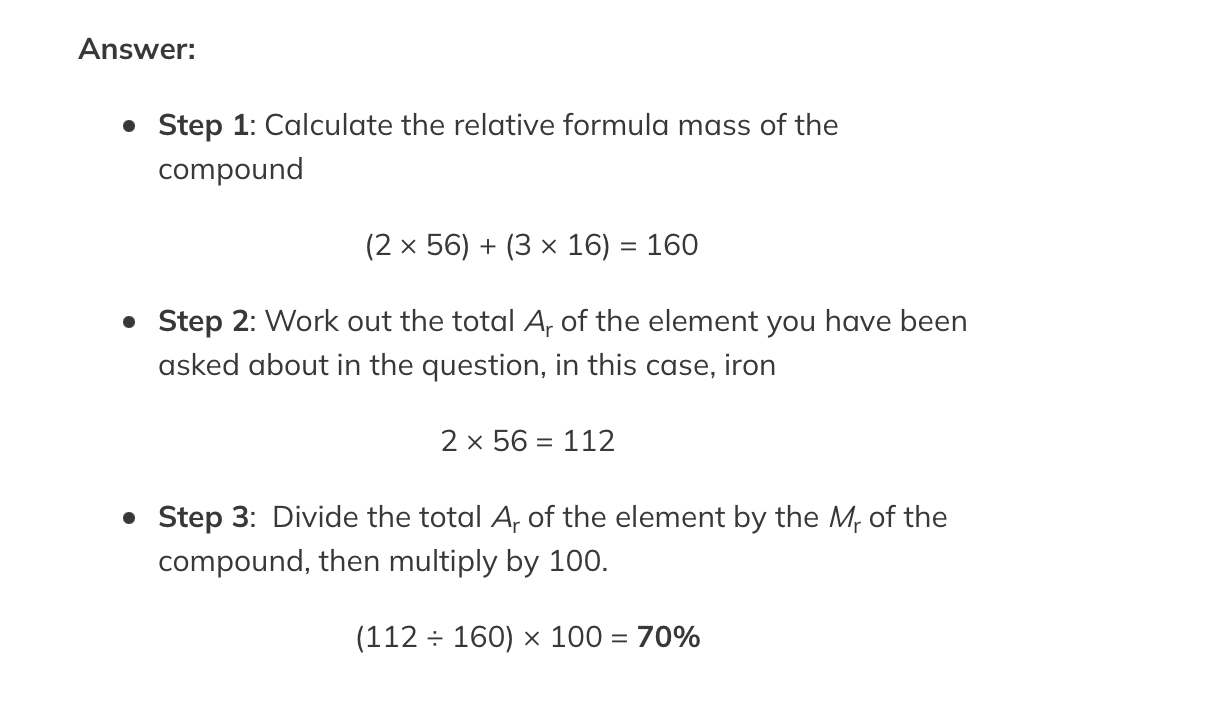
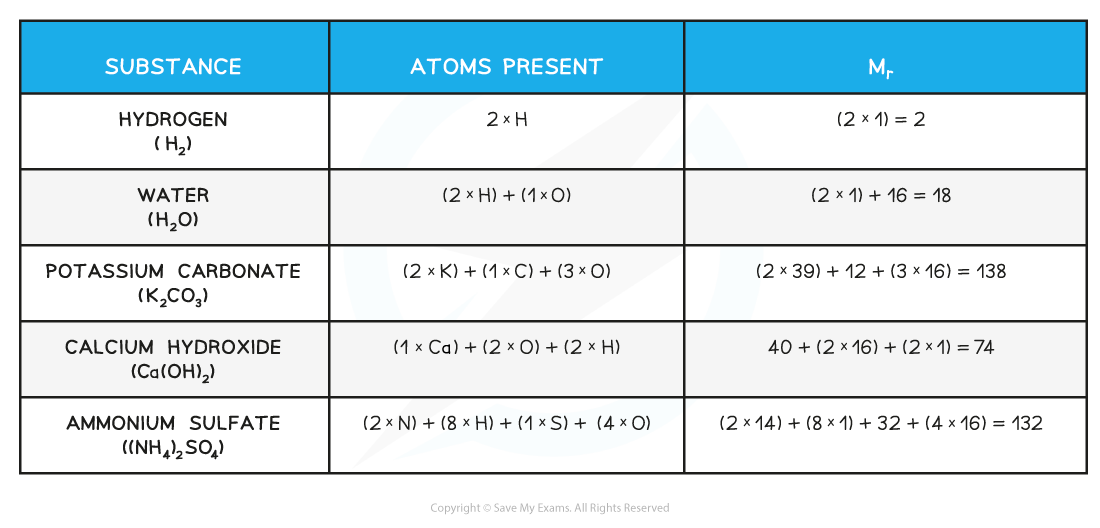
Relative formula mass Calculation
Calculating Percentage mass
Calculate the percentage of iron in iron(III) oxide, Fe2O3.
RAM (Ar): Fe = 63.5 O = 16