Unit 10: Intermolecular Forces and Solutions
Intermolecular Forces
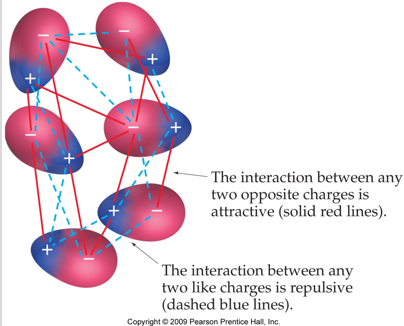
intermolecular forces (IMFs) - force that exists between atoms; forces vary in strength; all weaker than intramolecular forces
dispersion forces - forces that exist between all molecules, they are sometimes called London Forces.
- as electrons move around the outside of a molecule and at any particular moment just happen to end up together at one end, they form a temporary dipole… a positive or negative pole that exists for just a tiny part of a second
- these tiny dipoles react to the tiny dipoles in other molecules around them. if a molecule is larger it will have more dispersion forces. these forces don’t really do much, they just keep molecules moving around. they are only important in nonpolar molecules
dipole-dipole forces - dispersion forces are caused by temporary dipoles, polar molecules have permanent dipoles; generally stronger than dispersion forces
hydrogen bonding - the strongest intermolecular forces. they are not bonds, they are just really strong dipole-dipole forces. they occur when hydrogen is bonded to one of the highly electronegative atoms (F, O, or N). there are forces that give water most of its special qualities. 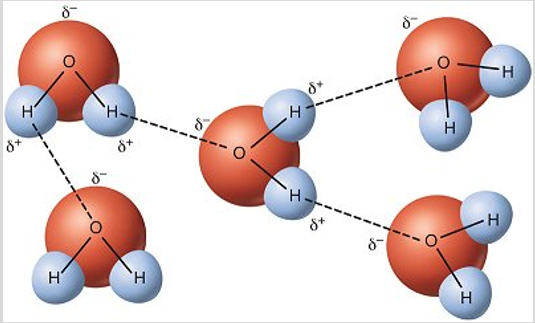
liquids
individual atoms can move around, but liquids are more dense (than gas)
particles are close together and the interactions between them hold their particles together
liquids cannot be compressed
viscosity - a measurement of the resistance of a liquid to flow. in general, viscosity decreases with heat, it increases with larger particles of more forces between the particles
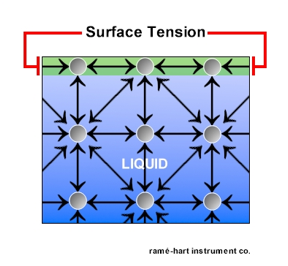
surface tension- a property of the surface of a liquid that allows it to resist an external force (ie. floating a paperclip on top of water, even though that are denser that water) this property is caused by cohesion of like molecules, and is responsible for many of the behaviors of liquid (also what pulls water into a ball while falling)
cohesion - the tendency of liquid particles to attract one another
adhesion - the tendency of liquid particles to be attracted to other materials
Types of Mixtures
mixture - a material system made up by two or more different substances which are mixed together but are not combined chemically
- mixtures can be either: homogeneous or heterogeneous
homogeneous mixture - a mixture in which the composition is uniform
heterogeneous mixture - a type of mixture in which the components can easily be identified as there are two or more phases present
suspensions - a mixture containing particles that settle out if left undisturbed
colloids - mixture of intermediate-sized particles (between the size of suspensions and solutions). they can’t be separated by settling out. these are very common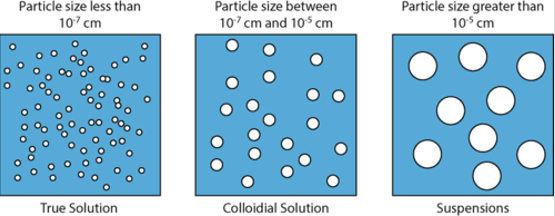
examples:
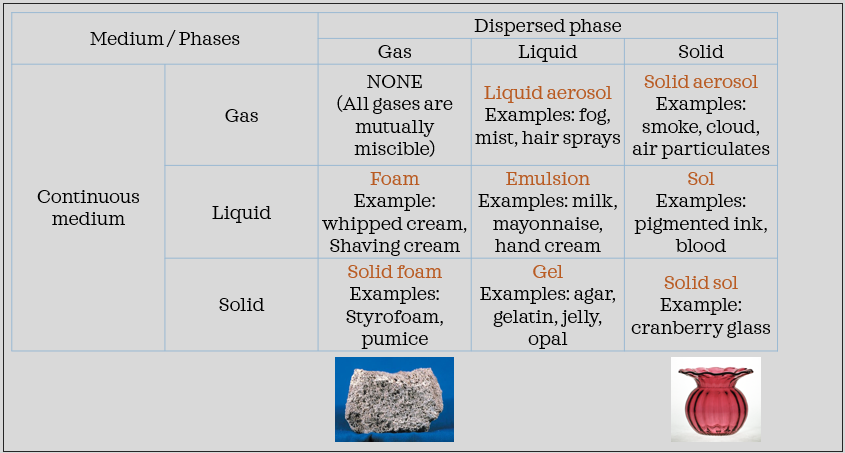
a solution is a homogeneous mixture composed of two substances. in such a mixture, a solute is dissolved in another substance known as a solvent. the solvent does the dissolving
Solution Concentration
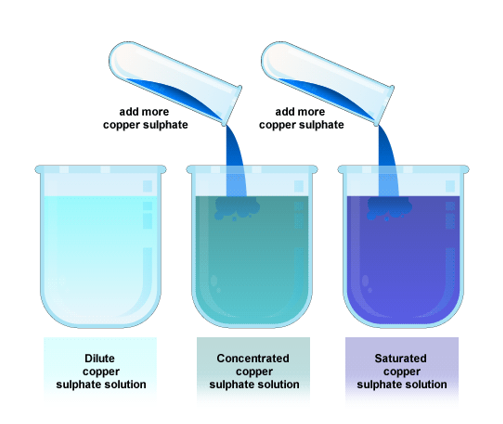
concentration - a measure of how much solute is dissolved in a solvent
sometimes we describe this quantitatively by saying that a solution is dilute (not much solute) or concentrated (a lot of solute) all the way to saturated (all the solute the solvent can hold)
molarity - the number of moles of solute dissolved per liter of solution; abbreviated with M, and when written it is underlined M (the most common and useful of the ways to measure concentration)
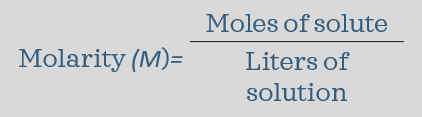
example problems:
A student dissolves 2.75g of sodium hydroxide, NaOH, in water to make 250 mL of solution. What is the molarity of the solution? (the molar mass of NaOH is 4.00g/mol.) 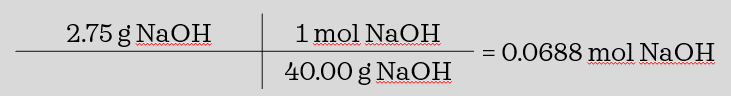

Potassium nitrate, KNO3, has a molar mass of 101.11g/mol. How much KNO3 is needed to make 100.0 mL of a 1.50 M solution of KNO3?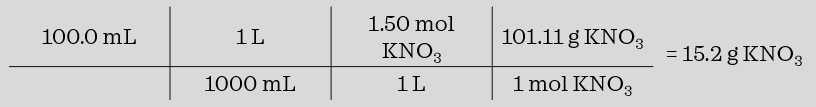
The Dilution Equation
a very helpful relationship exists in chemistry called the dilution equation. it is useful when you want to use a solution of one molarity to make a solution of another molarity.
M1V1 = M2V2
where M1 is the molarity of your known solution and M2 is the molarity of the solution you are trying to make. V1 is the volume of the M1 solution you need and V2 is the volume of the solution you are trying to make.
notice that molarity times volume gives a number of moles, so this equation can be used whenever the number of moles is going to stay the same.
more example problems:
If a student used 75 mL of a concentration HCl solution to make 1.5 L of a 0.50 M HCl solution, what was the original concentration of the stock solution?
According to our problem,
V1 = 75 mL = 0.075 L
V2 = 1.5 L
M2 = 0.50 M
We are looking for M1
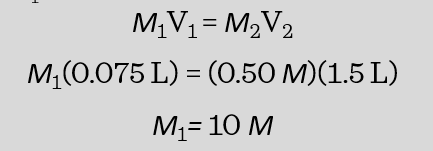

Solutions and Stoichiometry
remember that molarity is another ratio, in this case, moles of solute to volume.
what is the minimum volume of 0.750 M HCl that should be added to 1.50 grams of copper to completely react with it in the equation below.
Cu (s) + 2 HCl (aq) → CuCl2 (aq) + H2 (g)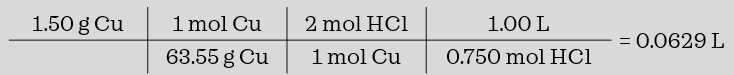 so we need at least 0.0629 L = 62.9 mL
so we need at least 0.0629 L = 62.9 mL
what volume of 0.415 M silver nitrate will be required to react with all the calcium bromide in 35.0 mL of 0.128 M?
2 AgNO3 (aq) + CaBr2 (aq) → Ca(NO3)2 (aq) + 2 AgBr (s) so, you would need 21.6 mL of 0.415 M AgNO3
so, you would need 21.6 mL of 0.415 M AgNO3
Factors Affecting Solvation
solvation - the process of attraction and association of molecules of a solvent with molecules or ions of a solute; as ions dissolve in a solvent they spread out and become surrounded by solvent molecules
ex. hydration is a solvation in which the solvent is water. as ions dissolve in a solvent, they spread out and become surrounded by solvent molecules
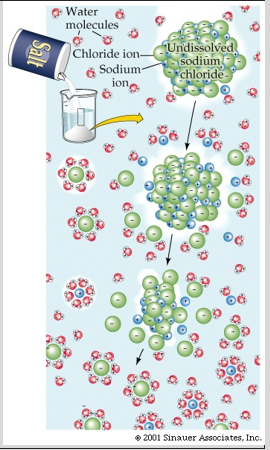
factors that affect solvation:
agitation
surface area
temperature
solubility - the property of a solute to dissolve in the solvent. the solubility of a substance depends on the nature of the solvent as well as on the temperature and pressure
unsaturated solution - one in which only a little of the possible amount of solute is dissolved
saturated solution - one in which the maximum amount of solute has been dissolved at the given conditions
supersaturated solutions - contain more of the solute than could be dissolved by the solvent under normal circumstances. these are created when a substance is more soluble at a high temperature, the the solution is called. if a crystal is added (or sometimes even the solution is shaken), crystals will form
Unit 10 Review
15 multiple choice questions (3 points each)
1 short answer application question (5 points)
3 problems (10 points each)
list and describe the intermolecular forces that exist between molecules
explain how intermolecular forces effect substances, especially liquids
describe how intermolecular forces and molar mass determine the boiling point of a liquid
discuss the similarities and differences of the following terms: viscosity, surface tension, cohesion, and adhesion
describe and give examples of homogeneous and heterogeneous mixtures
describe the properties of solutions, suspensions, and colloids
identify the different types of solutions and types of colloids
measure the concentration of solutions in terms of molarity
use solution molarity to solve stoichiometry problems
differentiate between dilute, concentrated, and saturated solutions
differentiate among saturated, unsaturated and super-saturated solutions
explain how solutions form and the process of solvation and hydration
define solubility and describe the factors that affect solubility
describe the factors that affect the rate at which a solute dissolves in a solvent