Atoms/Bonding - Term 2
Subatomic particles:
There are three subatomic particles: protons (positively charged with a mass of 1), Neutrons (no charge with a mass of 1), and Electrons (negatively charged with a negligible mass of 1/1840). Most of these particles are found in the dense nucleus. Despite that, the atom is 99.99% empty space.
Subatomic Particle | Charge | Mass | Location |
Proton | Positive (+) | 1 | Nucleus |
Neutron | Neutral (0) | 1 | Nucleus |
Electron | Negative (-) | Negligible (1/1840) | Orbiting the nucleus |
The phrase "atoms" refers to particles that have no charge and are therefore neutral. The number of protons determines the element. Electrons are arranged in shells or energy levels.
Atomic Number
How many protons in the nucleus
Defines which element the atom is
Mass Number
Total mass of atom
Protons + neutrons
To find the number of neutrons: mass number - number of protons
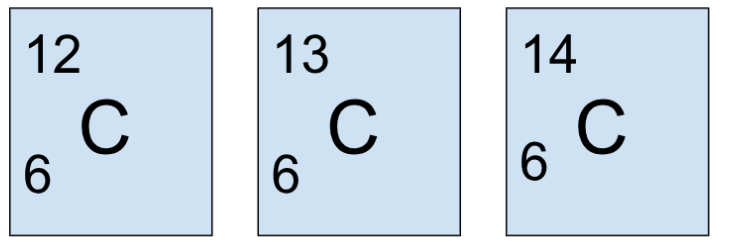
Isotopes
Isotopes are the same element with different numbers of neutrons and therefore different mass numbers.
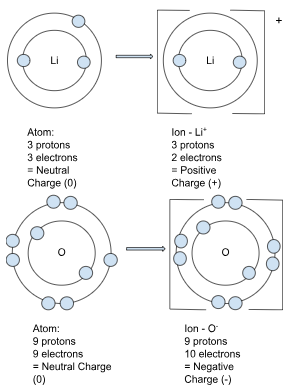
Ions:
Atoms will gain or lose electrons (e–) to achieve a full outer shell. Since electrons can be passed from one atom to another, they determine the overall charge of the ion. If there are more electrons than protons, it is negatively charged (anion - think onion=negative), and if there are fewer electrons than protons, it is positively charged (cation - think cat=postive).
Electron Shell | 1 | 2 | 3 |
Stable no of e- | 2 | 8 | 8 |
Electron Configuration:
The number of electrons in an atom or ion can be represented by its electron configuration. It is a list of numbers in order from innermost to outermost shell, which states the number of electrons in each shell.
For instance, a neutral carbon atom has 6 electrons (to neutralise its 6 protons), so the electron configuration for it would be 2,4. This indicates that 2 of the electrons are in the 1st shell (the maximum for the first shell) and 4 are in the second.
The Periodic Table:
The Periodic Table is organised in order of atomic number (number of protons). It is arranged in groups and periods. Groups are the columns, and periods are the rows.
Groups: They represent the number of electrons in the outermost (valence) shell
Periods: They represent the number of shells an element is likely to have
Using the position of an element on the periodic table, we can figure out what the charge of an element would be. Elements in groups 1 (alkali metals), 2 (alkaline earth metals), 13 and sometimes 14 form cations, while elements in groups 15, 16, 17 (halogens) and sometimes 14 form anions.
Group 1 - Alkali Metals:
(Hydrogen is not a part of Alkali metals but is part of Group 1)
Valence electron: 1
Chemical Properties: Reacts violently in water that will result in explosions with heavier elements
Physical properties: Soft, shiny and silvery.
Group 2 - Alkaline Earth Metals:
(Harder, less reactive than Group 1. Naturally found in the earth.)
Valence electron: 2
Chemical Properties: Form basic solutions when combined with water
Physical properties: Soft and silvery.
Group 3-12 - Transition Metals:
This valence electron pattern is not followed closely by the transition metals (groups 3-12), making their charges less predictable.
(Form coloured ions and compounds)
Physical properties: Hard and high density. High melting point and boiling point.
Group 17 - Halogens:
Reactive non-metals
Valence electrons: 7
Chemical Properties: React with metals to form ionic compounds called salts.
Physical properties: At room temperature fluorine and chlorine are gases, bromine is a liquid. and iodine is a solid
Group 18 - Noble Gases:
Unreactive non-metals due to stable valence shell.
Valence electrons: 8
Chemical Properties: Unreactive (inert). Don’t form ions.
Physical properties: Colourless, odourless and non-flammable.
Metals, Non-metals and Metalloids:
Metals are on the LEFT of the table. Nonmetals are on the RIGHT. Metalloids are elements that have properties of both metals and nonmetals. On the Periodic table, they are found in a diagonal line separating the metals and nonmetals. Examples include Boron, Silicon, Germanium and Tellurium. It is important to note that most elements on the periodic table are metals.
Metals:
Properties:
Good conductors of electricity
Malleable (bent/shaped easily)
Ductile (can be stretched into wires)
Lustrous (shiny)
Most of them have high melting and boiling points
Solid at room temperature (except mercury)
Dense and strong
Tend to lose electrons in reactions (forming positive ions)
Left side and middle of the periodic table
Explanation:
Free-moving electrons reflect light causing the shine and they also allow electrical conductivity because they can move freely
Delocalised electrons hold metal atoms together so they don’t break
High melting point because the strong electrostatic attraction between the ions is hard to break
Non-metals:
Properties:
Poor conductors of electricity
Brittle when solid (breaks or shatters easily)
Not malleable or ductile
Dull and not shiny
Lower melting and boiling points
Tend to gain electrons in reactions (forming negative ions)
Found on the right side of the periodic table
Explanation:
Don’t have free moving electrons to reflect light or conduct electricity
Held in rigous structures without flexible metallic bonds so it breaks easily
Low melting and bioling point because it has small molecules
Metallic bonding:
Is an attraction/bond between positively charged metal ions and delocalised electrons
Delocalised Electrons:
Can freely move through a metal without being attached to an atom
METALLIC LATTICES:
Composed of positive metal ions arranged in a regular pattern
Surrounded by delocalised electrons that move freely
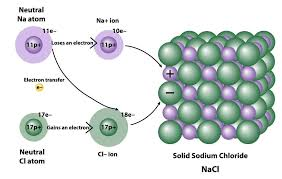
Ionic Bonding:
Bonding that occurs through electrostatic attraction between a cation and an anion.
Covalent Bonding:
Covalent bonding is bonding between two non-metals and is in molecule form. To satisfy the octet/duet rule (noble gas electron configuration), atoms share electron pairs instead of exchanging electrons. These shared electrons are called bonded pairs. Molecules that have two atoms are called diatomic molecules.
Single Bonds: eg. Hydrogen
When two atoms share one pair of electrons (one from each atom). The atoms require one electron to complete their valence shell.
Double Bonds: eg. Carbon Dioxide
When atoms share four electrons, two electrons from each atom. The atoms require two electrons to complete their valence shell.
Triple Bonds: Nitrogen
When atoms share 6 electrons, three from each atom. The atoms require three electrons to complete their valence shell.