Y11 Biology Unit Notes End of Year Exam
Unit Notes
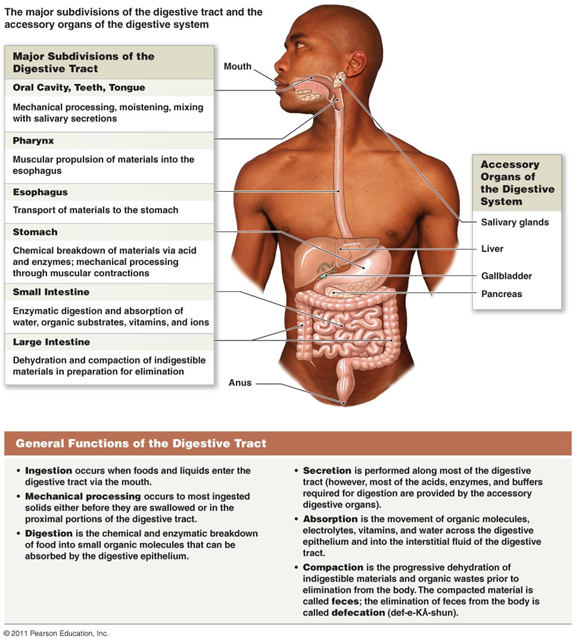
Digestion
Digestion is the process of mechanical and chemical breakdown of nutrients into smaller components in order to be absorbed into the body (blood stream)
Nutrients
Carbohydrates include monosaccharides (fructose and glucose) that combine to form disaccharides (sucrose and maltose) and polysaccharides (starch and glycogen). They consist of the elements carbon, hydrogen and oxygen.

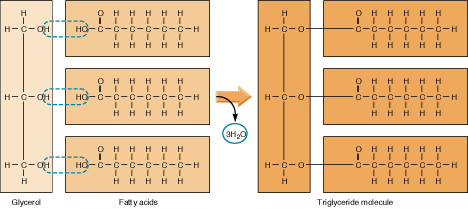
Lipids include glycerides (made from fatty acids and glycerol) and steroids. They consist of the elements carbon, hydrogen and oxygen.
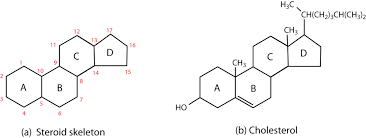
NOTE: although carbohydrates and lipids consist of the same elements, the ratios and molecular structures differ predictably.
Proteins are one or more peptides which consist of a sequence of amino acids. They consist of the elements carbon, hydrogen oxygen and nitrogen (and sulfur in many cases).
Metabolism
Metabolism is the web of all enzyme-catalyzed reactions in a cell or organism.
Metabolism is separated into two categories.
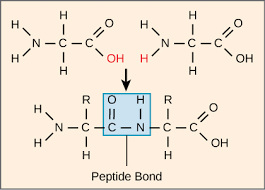
Anabolism is the synthesis of complex molecules from simpler molecules. This includes formation of macromolecules from monomers by condensation reactions.
Condensation reactions are chemical reactions in which reactants are joined together to form a product with water as a by product.
Consider:
Carbohydrates: Monosaccharide + Monosaccharide --> Disaccharide + Water
Example: Glucose + Glucose --> Maltose + Water
Lipids: 3 Fatty Acids + Glycerol --> Triglyceride + Water
Proteins: Amino Acid + Amino Acid --> Dipeptide + Water
Catabolism is the breakdown of complex molecules into simpler molecules. This includes breakdown of macromolecules into monomers by hydrolysis reactions.
Hydrolysis reactions are chemical reactions in which reactants are separated using water to form products.
Consider:
Carbohydrates: Disaccharide + Water --> Monosaccharide + Monosaccharide
Example: Maltose + Water --> Glucose + Glucose
Lipids: Triglyceride + Water --> 3 Fatty Acids + Glycerol
Proteins: Dipeptide + Water --> Amino Acid + Amino Acid
Collision Theory
Collision Theory states that a chemical reaction will occur if 3 criteria are met:
Reactants (including enzymes and substrates) collide
with the correct orientation (i.e., in the "right" place)
with enough energy (E ≥ Ea)
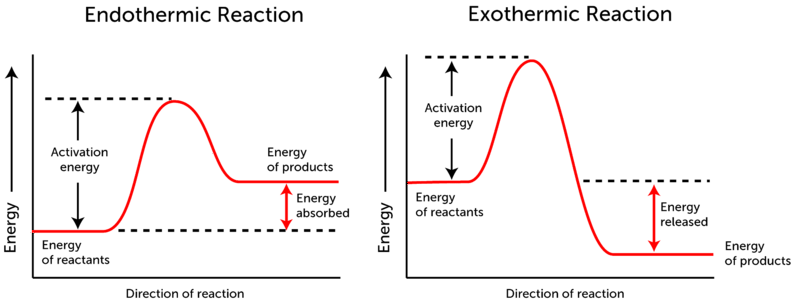
Ea = activation energy = the minimum amount of energy required for a chemical reaction to occur)
It is helpful to recall Kinetic Theory (it is usually applied to gasses but generally applies to all matter) whose main principles are:
All matter is made of particles
All particles are in constant motion
The motion is random but linear
Collisions are elastic
Matter – anything that has mass and volume
Reaction rate
Reaction rate is the change in a quantity per unit time.
In terms of reactants, it is the rate at which they disappear. This is a negative change.
In terms of products, it is the rate at which they appear. This is a positive change.
Equation
The equation is change in amount ÷ change in time.
Amount may be volume (of a gas), mass, temperature (change is a consequence of the reaction), height, length and so forth.
Graph
Amount - time graph
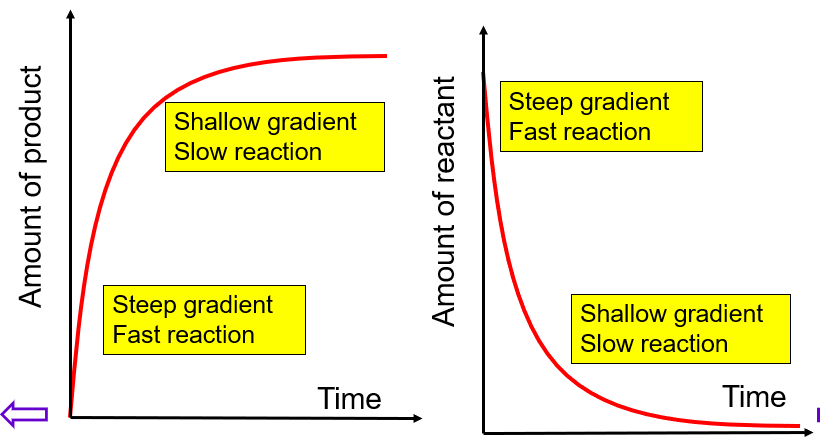
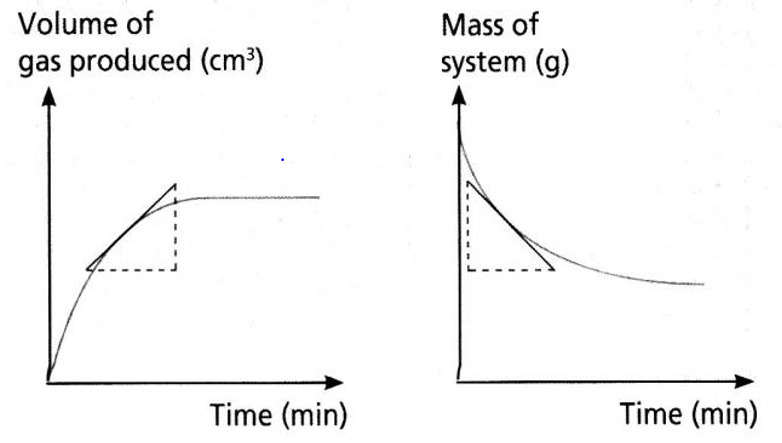
Average rate is the change in amount / time (the slope)
Instantaneous rate is the slope of a tangent at a given point
As the reaction progresses, the concentration of reactants decreases. This leads to lower frequency of collision with the correct orientation and sufficient energy. Thus, rate decreases as reactions progress - highest at the beginning and lowest at the end.
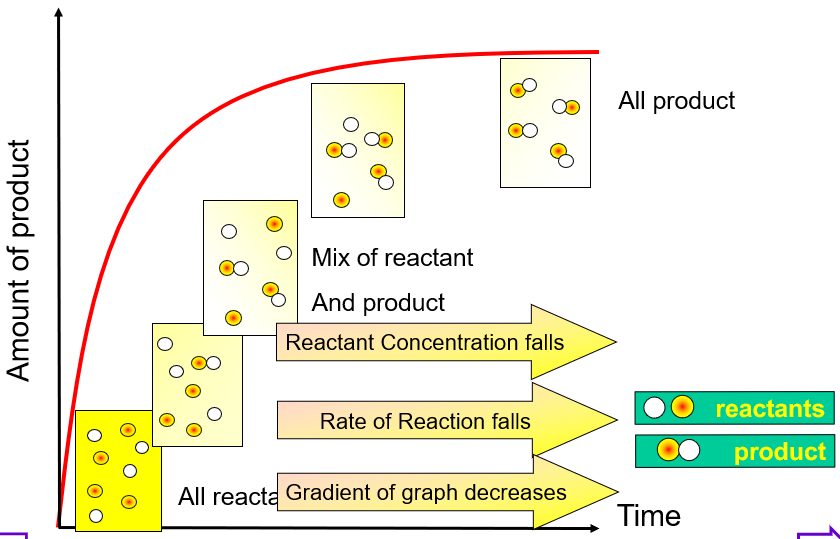
Rate - factor graph
Rate is read from the y-axis.
Enzyme
Definition
An enzyme is a biological (protein) catalyst.
A catalyst increases reaction rate but is not used up (it is recycled).
An enzyme is biological which means that it is found in living things. It is a protein.
Digestive enzymes

Enzyme specificity
an enzyme binds to and reacts with specific substrates according to the lock-n-key model. This explains substrate-enzyme specificity.
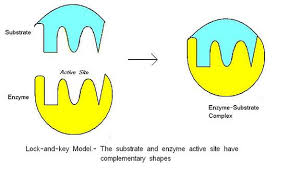
The enzyme is the lock.
The active site is the keyhole where the reaction takes place in the enzyme.
The substrate must fit the active site – the shape of the substrate is complementary to the shape of the active site.
The substrate is the key that fits into the active site which is the keyhole.
The substrate is the reactant.
NOTE: the lock-n-key model is actually outdated and a revised model, the induced fit model, is used
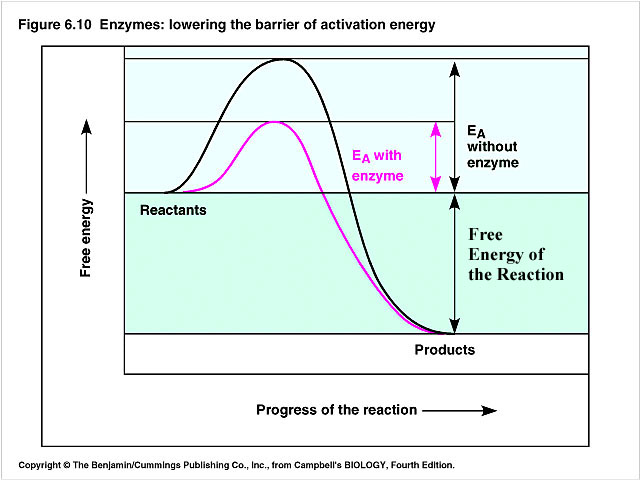
Enzyme catalysis
an enzyme increases reaction rate but is not permanently changed in the reaction
An enzyme reacts with a substrate through an alternate reaction pathway
The activation energy of alternate reaction pathway is less than that of the reaction pathway without the enzyme
Factors
Factors that affect reaction rate in enzymes are:
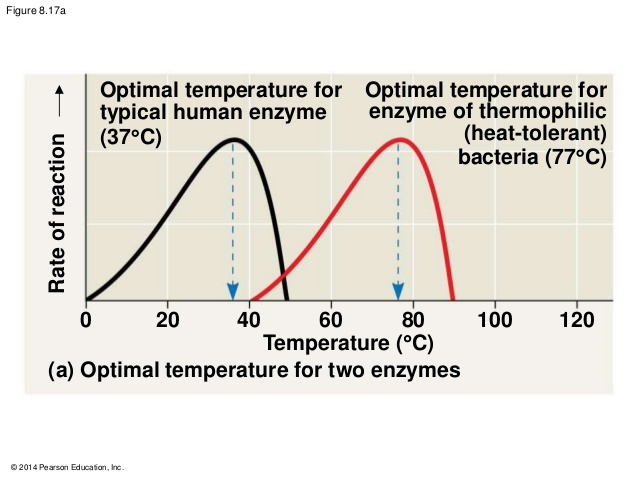
Temperature
changing temperature → changes the average kinetic energy of the particles
→ changes the speed of the particles → changes the frequency of collisions → changes the probability of successful collisions → changes reaction rate
AND
→ changes the number of particles with E ≥ Ea → changes the frequency of successful collisions → changes reaction rate
UP UNTIL THE ENZYME DENATURES – the active site changes shape so that the substrate no longer binds to it and rate declines. There is an OPTIMUM temperature.
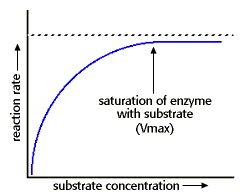
Concentration
changing concentration → changes the number of particles within a specified space → changes the frequency of collisions → changes the probability of successful collisions → changes reaction rate
UP UNTIL ALL ACTIVE SITES ARE USED ON THE ENZYME SUCH THAT ANY ADDITIONAL CHANGE HAS NOT EFFECT – rate reaches a maximum.
Surface area
changing surface area → changes the number of particles exposed → changes the frequency of collisions → changes the probability of successful collisions → changes reaction rate
UP UNTIL ALL ACTIVE SITES ARE USED ON THE ENZYME SUCH THAT ANY ADDITIONAL CHANGE HAS NOT EFFECT – rate reaches a maximum.
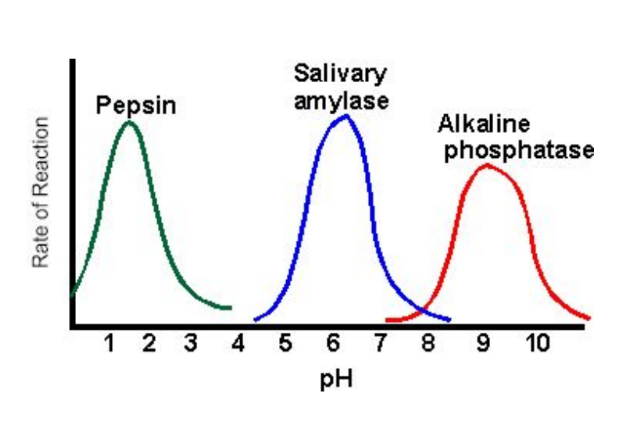
pH
changing pH → modifies the interaction of the substrate with the active site
UP UNTIL THE ENZYME DENATURES – the active site changes shape so that the substrate no longer binds to it and rate declines. There is an OPTIMUM pH.