
Science 2023
Space science
Structure of the Universe
Emu in the sky
Emu is running - April and May
Time to collect eggs
Emu sitting - June and July
Still time to collect eggs
Eggs start to hatch - August
No collecting of eggs
Emu drinking - November
Water holes are full
Uses of emu in the sky
ceremonies
time to hunt
The brothers of Kingfish clan where forbidden to eat any kingfish. The brothers caught kingfish after kingfish but had to throw them all back. Eventually one of the brothers became so hungry, he ate the kingfish. The sun women, Walu, saw him and in anger, created a water sprout that lifted the brothers up into the sky.
Cosmic background noise - CMB is the ‘noise’ (radiation) left over from the big bang and represents the temperature of space in the whole universe. The temperature is not the same everywhere, therefore density is not the same everywhere. Discovered May 20, 1964.
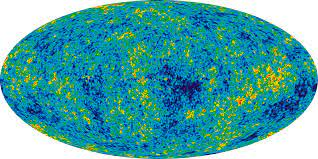
Cosmic microwave background - cosmic background microwave background is a form of electromagnetic radiation left over from the Big Bang. As the universe expands, the wavelength of the CMB radiation is stretched, causing it to shift towards longer wavelengths, or lower frequencies, this is known as redshift.
Cosmic background noise provides evidence for the big bang - the fact that we can see the microwaves expand means the waves started at a single point.
The Milky Way - the Milky Way is a galaxy that includes the Solar System. It is a huge collection of stars, dust and gas in a spiral system. Our sun is 25,000 light years away from the centre of the galaxy. We can see the stars and gas clouds of the Milky Way across the skies on Earth. We can also see other galaxies from Earth.
Universe mind map -
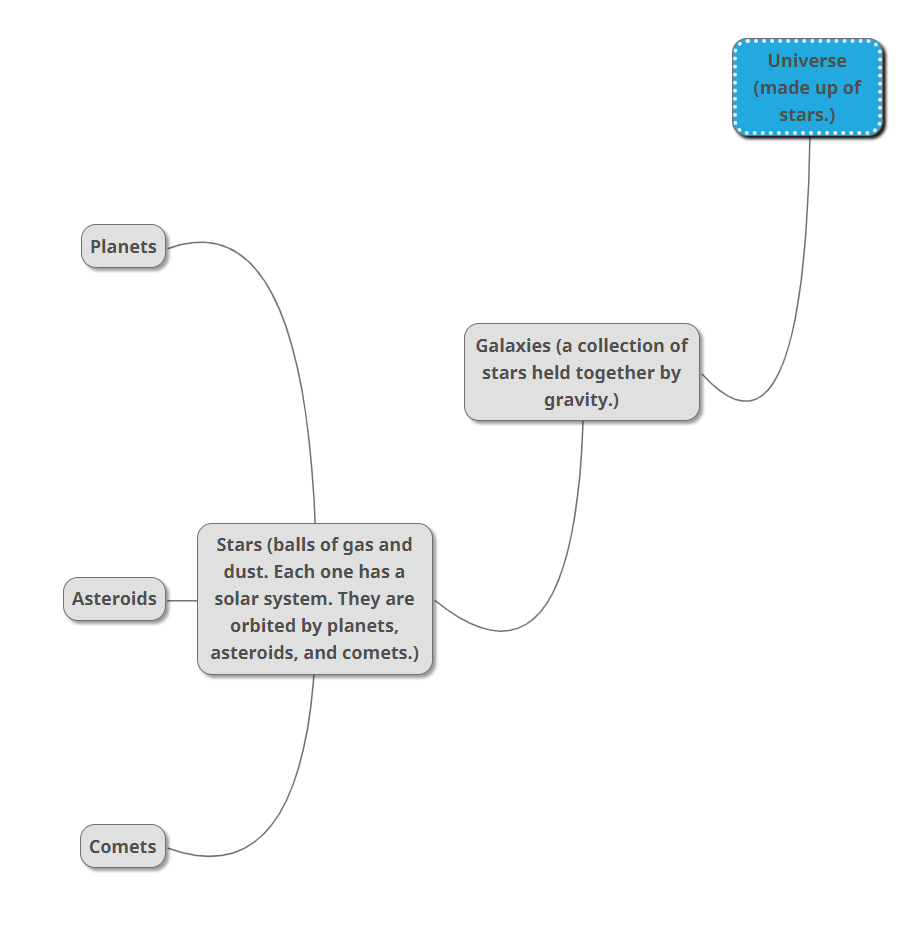
Galaxies - galaxies are a collection of gas, dust and billions of stars and their solar system.
Stars - a star is a luminous ball of gas held together by its own gravity. Stars are mostly made of hydrogen and helium.
Difference between a star, a planet, a moon and an asteroid - Stars have nuclear fusion, and they produce light. A moon has a body that orbits a planet, an asteroid orbits a star. A planet is a body that orbits the sun.
Role of gravity in the universe - gravity is the force which holes the universe together. It creates a path of objects around another body.
Unit of measurement in the universe - the unit of measurement used in space is light years. Light years is the distance light travels in one year. One light year equals 9.46 trillion kilometres. For example, if a star that was 20 light years away exploded right now, we will see the explosion 20 years from that time it exploded.
Life cycle of a star
Stars can appear in different colours such as orange, yellow, blue and white and they can vary in sizes. Our sun is small compared to many stars. It is still huge compared to the Earth and other planets.
Hertzprung Russell diagram -
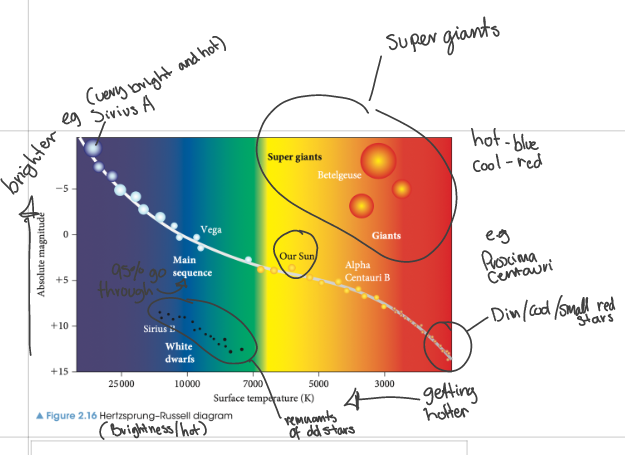
The Hertzsprung-Russell diagram is a graph that plots the luminosity of stars against their surface temperature. It is used to classify stars and understand their evolution (cycle).
Hot, bright stars are located in the upper left corner, while cool, dim stars are located in the lower right corner.
The main sequence, where most stars spend the majority of their lives, runs diagonally from the upper left to the lower right.
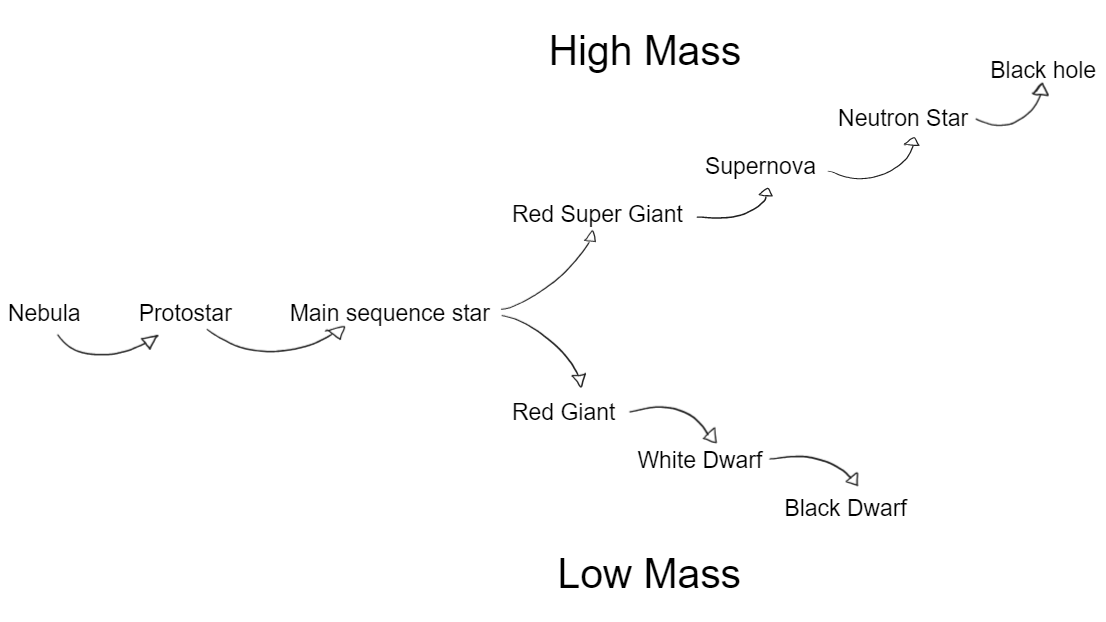
The cycle:
Nebula - stars come from clouds of dust and gas called nebulae which are mainly of hydrogen. All star begin their life as part of a nebula.
Protostar - gravity causes the dust and gas in nebulae to become denser and more concentrated. The temperature increases until hydrogen and other light elements undergo nuclear fusion. This produces energy and the core get brighter and hotter until a protostar is born.
Main sequence star - nuclear fusion of hydrogen atoms is a now in full flow and a protostar becomes a main sequence star. This star is much hotter and brighter then before, releasing energy and a flow of radiation from its core. A main sequence star is in equilibrium: it is stable because the forces within it are balanced. Our sun is currently in its main sequence stage… but we are safe. These processes take billions of years.
Cycle of smaller (low mass) stars after the main sequence:
Red giant - helium and other light elements in the star’s core fuse to form heavier elements. This process releases even more energy, causing the star to expand. The star gives off a red light, hence the name “red giant”.
White dwarf - a red giant will eventually run out of its “fuel” of light elements. Nothing else in it will fuse. It will lose its outer layers leaving behind the core of the star. This is so dense and hot that it glows white.
Black dwarf - a white dwarf will eventually cool down enough that it stops glowing white. Stars like the sun then fade out, go cold and enter the stage of a black dwarf.
Cycle of bigger (high mass) stars after the main sequence:
Red super giant - following the main sequence, a star begins to fuse together heavier elements. As it has more fuel, it swells out to a much larger size, giving off a lot more energy.
Supernova - a red supergiant will start to collapse in on itself. This compresses its core more until it reaches a critical point. This causes a massive shockwave or a cataclysmic explosion called a supernova. A supernova event can outshine an entire galaxy for several weeks. The explosion only lasts seconds, in those seconds it can release as much energy as the star has released up to that point. It can be as bright as the light from 10 billion stars
THEN
Neutron star - after a supernova, only the star’s compressed core is left behind. The core has turned into lots of neutrons. The neutron star is extremely dense. A small amount of the neutron star would way more than the earth.
OR
Black hole - the core left from the supernova will continue to collapse and keep getting smaller until the entire star collapses into an infinitely small point. The gravitational field is now so strong, nothing can escape from it, not even light of any electromagnetic radiation.
Visual map of cycle:
More information:
Stellar nebula - stellar nebula are clouds that form new stars. Gravity causes the hydrogen to fuse into helium.
Stars turn into giants/supergiants as they get older - stars turn into giants/supergiants as they get older because helium and other elements in the star’s core fuse to form heavier elements. This process releases even more energy, causing the star to expand.
Larger stars are hotter but live shorter. - larger stars are shorter lived because
they burn more fuel, so they run out of hydrogen sooner than smaller stars do.
Likely progress of our sun through the cycle - it will expand and run out of energy, becoming a white dwarf, then a black dwarf.
Supernova - the size of the red giant depends if the star will go into a supernova or not.
Difference between a neutron star and a white dwarf - a white dwarf is hotter and it is remains of a red giant. A neutron star is full of neutrons and comes from a super red giant.
Black hole - a black hole is remnants of super red giants. When gravity is so string it collapses and becomes a black hole.
The big bang
The big bang - how astronomers explain the way the universe began at a hot, dense, single point, then expanded and stretched into the universe we know today. It is still expanding.
The Doppler effect - 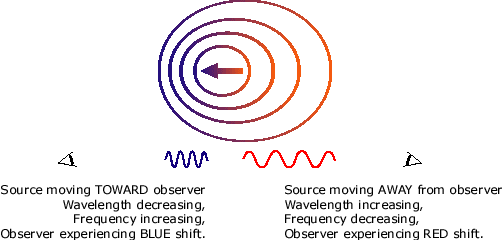
The doppler effect is the change in the wavelength/frequency of a wave as it moves towards and away from an object.
As the wave moves towards you, the frequency increases, the wavelength decreases.
As the wave moves away from you the frequency decreases, the wavelength increases.
The doppler effect can be used to determine how fast stars move.
Red/blue shift -
Red stars are further away from blue stars. yellow stars are not as far away as red stars but not as close as blue stars.
Red and blue shift on the spectrum: 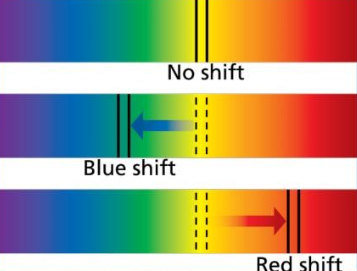
Electromagnetic spectrum - the range of all types of radiation that has both electric and magnetic fields and travels in waves.
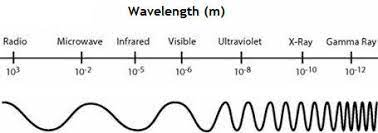
Doppler effect - the apparent change in wave length or frequency when the source of waves or the observer is moving responsible for the red shift of distant stars.
Oxford extended understanding
Difference between galaxies and constellations - galaxies are the collection of stars and constellations are the collection of a few stars.
Indigenous people using the sky as a calendar - indigenous people used the sky as a calendar by marking out different shapes and referring to them as different animals. As the animal moves, it indicates to the indigenous people the time of year like a calendar.
Orion - Orion is a constellation. To find Orion, look for 3 big stars close together. They are Orion’s belt. 2 brighter stars North of it represent the shoulders and the 2 stars to the south are feet.
Different stories of Orion - there may be different stories about Orion from indigenous people across Australia because there are many indigenous people spread across Australia and they all have different perspectives of Orion which they might associate with different objects.
Individual stars - we can’t see individual stars because they are too distant to be seen individually.
Main sequence star - any star that has a hot core which fuses H into He to produce energy.
Parallax - change in an object’s position when viewed from 2 different points of view.
After a supernova - after a supernova happens, a neutron star remains. When the neutron star collapses, the gravitational pull and density is so great that it creates a black hole.
Objects that pass by a black hole - objects that bass by black holes will be sucked spirally into the black hole. If you get sucked in a black hole, the closer you get to the black hole, you will be pulled in and you won’t be able to escape. If you were to go into a black hole, you will be stretched while also being compressed. You will be ripped apart when you are stretched and look like “spaghetti”, this is known as spaghettification. The closer you get to the black hole, time gets slower (gravitational time dilation). The event horizon has infinite time dilation because any object that goes in doesn’t pass the horizon (doesn’t come out).
Accretion disc - flattened astronomical objects made of rapidly rotating gas which slowly spirals onto a central gravitating body.
Location of black holes - black holes can be located anywhere.
The sun’s progress in the cycle - when the sun reaches the end of the main sequence, it will expand into a red giant. Helium is dumped in the core. There is a shell of hydrogen around the helium-rich core. Fusion causes it to expand. Hydrogen cools and rises to create a rich outer envelope. Gravity is still pushing inwards.
Comparison of the life cycle of our sun with another star that has 10 times the mass of the sun - since our sun is smaller, it won’t have the ability to create a black hole like other bigger stars. Our sun will become a red giant after the main sequence, after it runs out of He, only its hot, white, and dense core is left, creating a white dwarf. After the white dwarf dies, it becomes a black dwarf. If it were a bigger star than our sun, after the main sequence it will become a super red giant. After the super red giant has run out of He, it will become a supernova. It can either become a neutron star or a black hole after. A neutron star and a black hole is both very dense.
Big bang led to the structure of the universe - The big bang is said to have been extremely hot at the beginning. It expanded extremely condensed material in a ball called a singularity. The singularity started expanding which became our universe. People believed the bid bang had radiation. In 1965 scientists discovered left over energy from the big bang. The energy existed as background radiation (CMB) which is evidence that the big bang happened.
Earth science
The four global spheres -
lithosphere (solid earth)
atmosphere (in the air)
hydrosphere (water)
biosphere (life)
Interactions and relations between the spheres -
the biosphere gives material to the lithosphere to be decayed.
the lithosphere gives a habitat to life in the biosphere.
the atmosphere affects the lithosphere with things like wind to move dirt, sand etc to different locations over time which changes the topography of the land.
the atmosphere causes combustion, respiration and photosynthesis in the biosphere.
the hydrosphere can be a habitat for the biosphere.
the hydrosphere and atmosphere can create rain.
The carbon cycle - 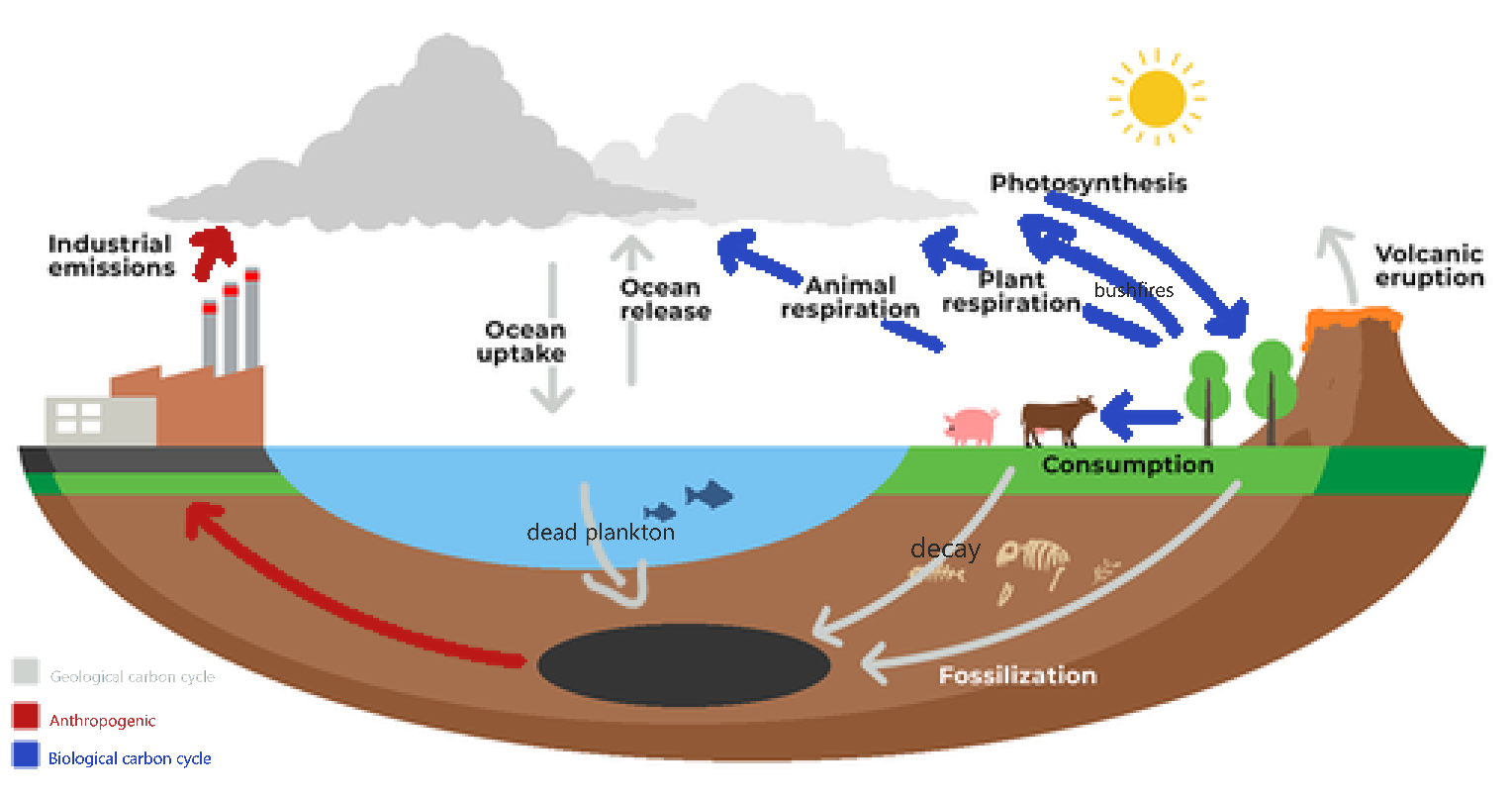
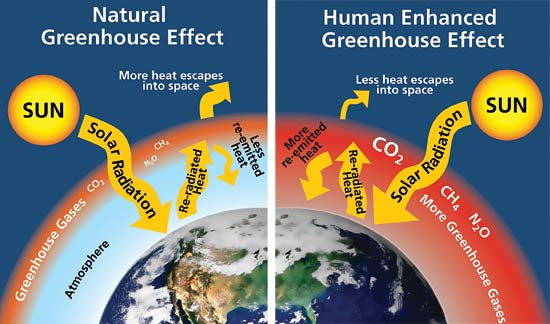
Greenhouse effect - the greenhouse effect is trapping some of the sun’s radiation naturally to keep the planet at a moderate temperature.
Enhanced greenhouse effect - the enhanced greenhouse effect is where the greenhouse gases in our atmosphere keeps too much of the sun’s heat and causes global warming. This extra gas is created by humans.
Long term carbon sinks - long term carbon sinks are carbon reservoirs that store carbon for a significantly longer than a human life span. Carbon will be slowly released back into the atmosphere/surface environment over many years. Examples of carbon sinks include: the ocean (hydrosphere), trees (biosphere), fossil fuels (lithosphere). These carbon sinks are being impacted by humans. Trees are being wiped out (deforestation) so less trees are able to store carbon. Fossil fuels are being used so we’re releasing more carbon in the air. These are negative impacts as we are filling the atmosphere with carbon which causes global warming because it keeps in more heat/solar radiation from the sun.
Chemistry
Atoms and the Periodic Table
An atom is a small unit of matter.
An element is a pure substance.
Subatomic particles:
Mass | Charge | |
|---|---|---|
Proton | 1 | positive |
Neutron | 1 | neutral |
Electron | 0 | negative |
The atomic number represents the number of protons in a nucleus.
The number of protons and neutrons control the atomic mass of an atom. It is also the weight of the nucleus.
The atomic number is a whole number and the atomic mass is a number with a decimal.
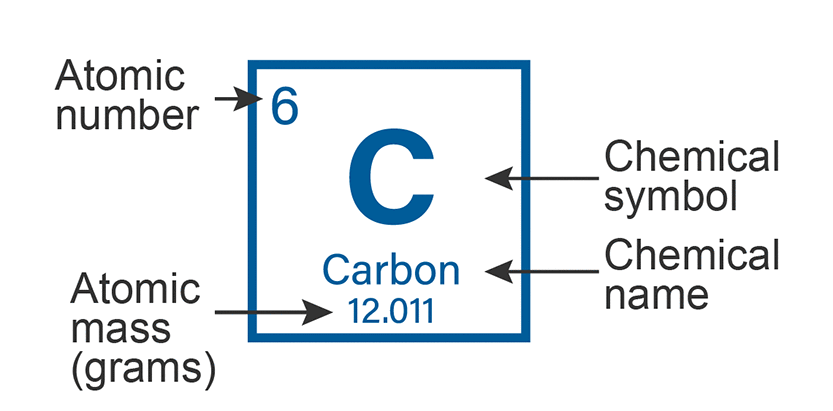
To calculate the number of neutrons in the atom: atomic mass - atomic number.
E.g Carbon, 12.011 (at. mass) - 6 (at. number) = 6
The atomic mass is not always a whole number because it is the average of its isotopes.
Electrons tell us about the chemical reactions, the atomic mass tell us how heavy the nucleus is.
Isotopes are the many forms of the same element, containing the same number of protons but different numbers of neutrons.
The atomic number is a whole number, not like the atomic mass because the number is the number of protons and they are countable.
Examples of the atomic number, atomic mass, # of neutrons, electrons and protons of an element.
Element name | Element symbol | Atomic number | Atomic mass | # of protons | # of electrons | # of neutrons |
|---|---|---|---|---|---|---|
Fluorine | F | 9 | 19 | 9 | 9 | 10 |
Potassium | K | 19 | 39 | 19 | 19 | 20 |
Aluminium | Al | 13 | 27 | 13 | 13 | 14 |
Strontium | Sr | 38 | 88 | 38 | 38 | 50 |
Atomic structure and the Periodic Table
The periodic table as we know hasn’t looked like it does now. It has developed over time as we have learnt more about chemistry.
Some early periodic tables:


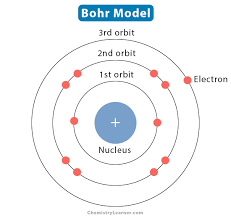
The Bohr model is the description of the structure of atoms. It consists of a small nucleus (no protons/neutrons, one nucleus as a whole), with electrons in orbits, placed on the electron shells.
On the periodic table there are periods and groups.
periods - periods are rows on the the periodic table. The numbers for periods are on the left side of the table. Period numbers represent the number of electron shells.
groups - groups are columns on the periodic table. The numbers for groups are at the top of the table. Group numbers represent the number of electrons on the valence shell.
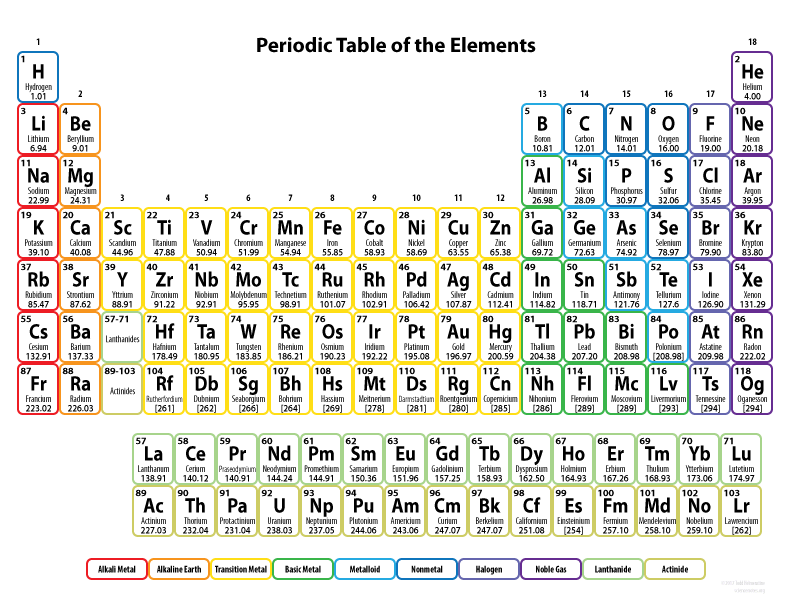
group 1 are alkali metals.
group 2 are alkaline Earth metals.
group 3 to group 12 are Transition metals.
group 17 are halogens.
group 18 are noble gases.
Electron configuration
Electron configuration refers to the arrangement of electrons - how many are in each shell.
Rules for determining electron configuration:
determine the period the element is in. This gives the number of occupied electron shells.
determine the group the element is in. This gives the number of valence electrons.
all shells, except the outer one, will have the, maximum number possible.
Examples:
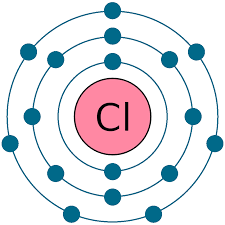
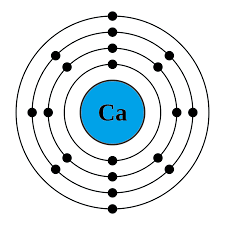
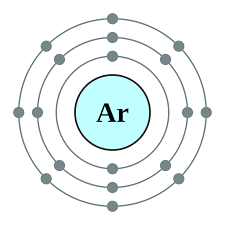
The purpose for knowing the electron configuration of an element gives us information about the chemistry or chemical reactions it may be in.
Ions
The term ‘neutral atom’ means that an atom has the same amount of electrons and protons and therefore is neither positive or negative.
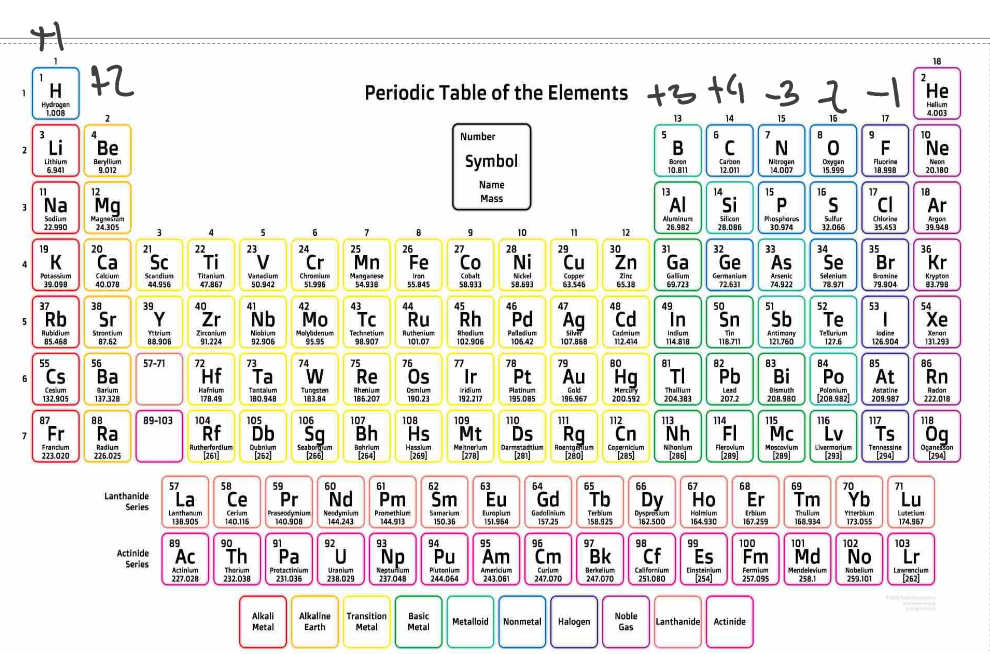
** Metals form anions.
Comparison of the number of protons in an atom to the number of electrons in an atom - the number of protons is the same as the number of electrons in an atom.
If an atom:
gains an electron - the charge becomes negative.
loses an electron - the charge becomes positive.
Charge on ion | Name of type of ion |
|---|---|
Positive | Cations |
Negative | Anions |
The difference between an atom and an ion is that an atom is neutral, whereas, an ion has either lost or gained one or more electrons so they are not neutral.
The atomic structure of all ions are the same because they will always have full valence shells.
If the atom has less electrons on the valence shell, it will lose electrons. If an atom has more electrons on the valence shell, it will gain more electrons. (Remember: the group they are in represents the number of electrons they have.)
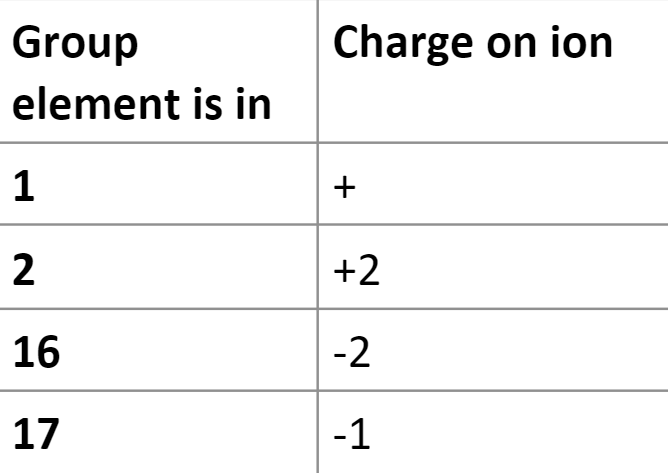
Elements in group 18 do not form ions because their valence shells are full and do not need to lose or gain electrons.
Elements in group 2 all form an ion with a charge
Metals
The atomic structure of metals explains the formation of metal ions and the reactivity of metals.
Groups 1 and 2 form cations by losing 1 or 2 electrons.
Group 1 is more reactive than group 2 because it only has to lose 1 electron while group 2 has to lose 2 electrons.
Alkali metals are more reactive, particularly with water.
As you go down the groups, metals become more reactive. They have more electrons in the shells and may need to lose more or gain more electrons.
Four common properties of metals
Property | Description |
|---|---|
Lustrous | shiny |
Conductive | able to conduct electricity and heat |
Malleable | Can change into a new shape (like foil) |
Ductile | Can be drawn into a wire |
3 important groups of metals:
Periodic table group(s) | Group name | Properties |
|---|---|---|
1 | alkali metals | low melting points, soft and highly reactive |
2 | alkaline earth metals | low melting points, relatively soft, and very reactive |
3-12 | transition metals | a small number of transition metals are magnetic. Gold and copper are the only metals that are not silvery in colour. Many form coloured compounds many form more than one compound with a non-metal, such as chlorine |
A chemical reaction is when reactants are converted to substances.
Reactivity refers to how easily a substance reacts with other substances (the ability to go under chemical change/reaction).
(Reactivity) How easily they lose electrons when they come in contact with other substances determines the reactivity of a metal. The more electron shells, the more reactive it is.
Metallic bonding
Bonding between metal and metal.
Metallic bonding has a structure that features many ions in a "sea" of electrons. They are cations, arranged geometrically, surround by delocalised elections.
Property | Elaboration | Picture |
|---|---|---|
Thermal and electrical conductivity | The electrons move easily through the solid. These moving electrons are able to transfer heat and electricity. There is electrostatic energy around the ions. |
|
Malleability and ductility | These cations can slide past each other but the electrostatic forces between the cations and the electrons stop the metal from breaking. |
|
Lustrous | The delocalised electrons in the surface of a metal reflect light and cause it to be lustrous. |
|
Melting/boiling points | Lots of heat and energy is needed to melt a solid metal and boil a liquid metal because of the very strong electrostatic forces. |
|
An alloy is a mixture of two or more metals. They may contain non-metals. 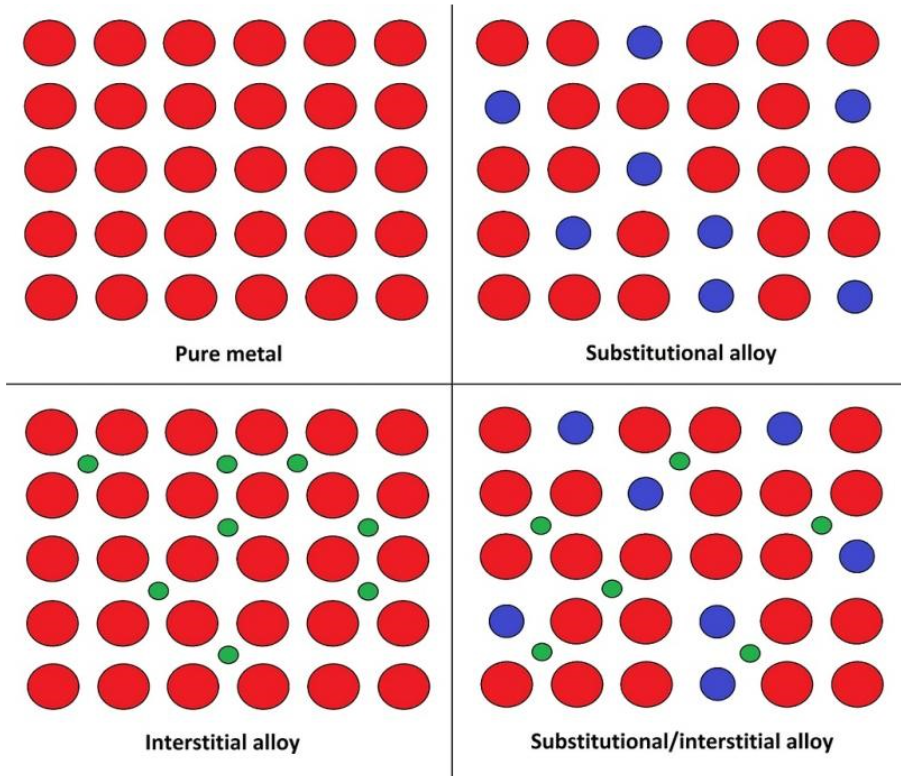
Non-metals
Non-metals on the periodic table:
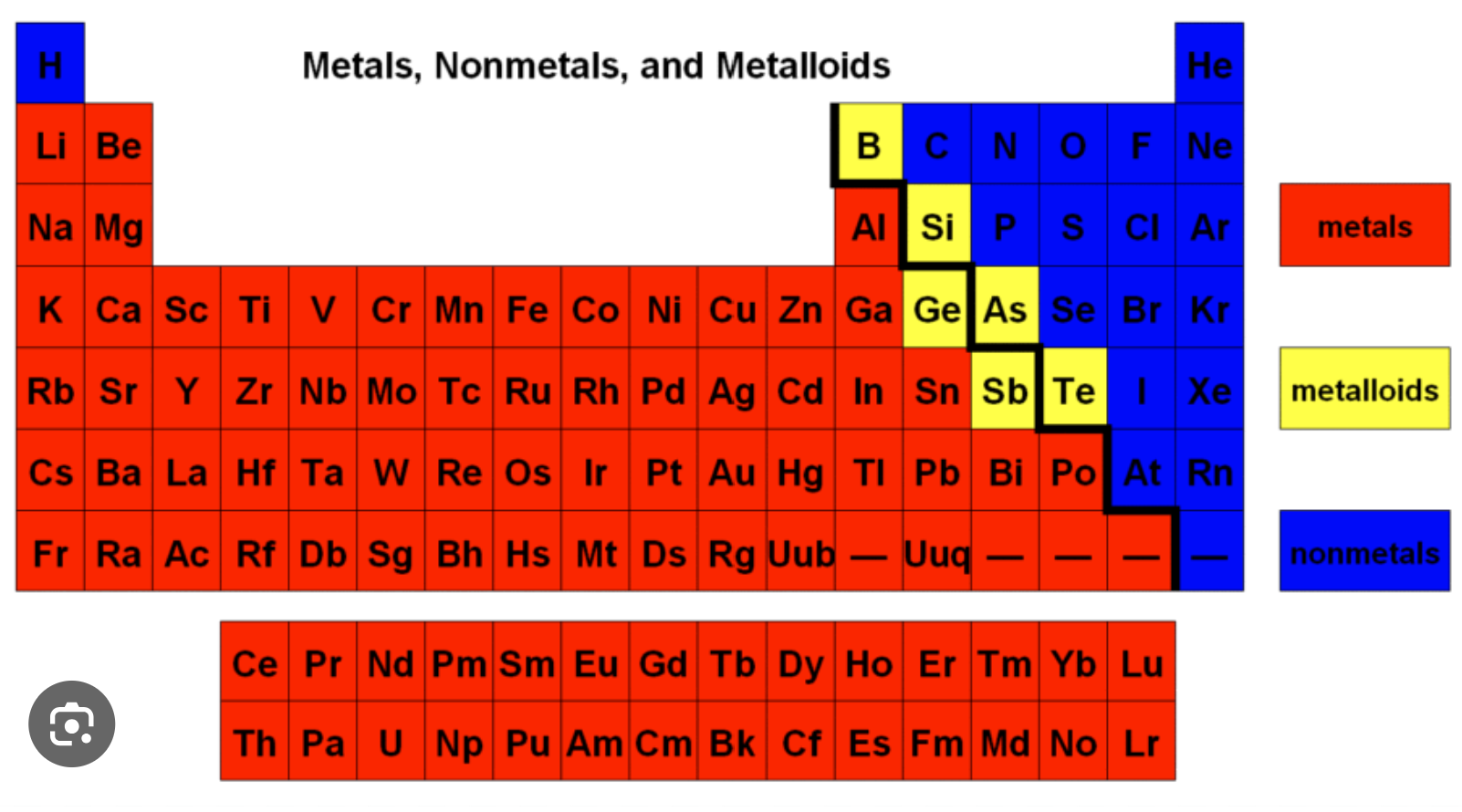
Non-metals form anions except noble gases, hydrogen and calcium.
The reactivity of non-metals decrease as the groups descend because the valence shells are closer to the nucleus which is positive. (A stronger attraction to electrons that are closer to the nucleus, therefore increasing reactivity).
Metalloids have properties of metals and non-metals.
The two important groups of non-metals:
Periodic table group(s) | Group name | Properties |
|---|---|---|
17 | halogens | all gases are diatomic (two atoms to each molecule), react with metals to form salts, all have 7 valence electrons, range from gases to solids. |
18 | noble gases | unreactive, don’t form compounds, all gases. |
Noble gases are described as ‘inert’ (unreactive), this is because their valence shells are full.
‘Semi-conductors’ only conduct electricity under certain conditions.
Metalloids:
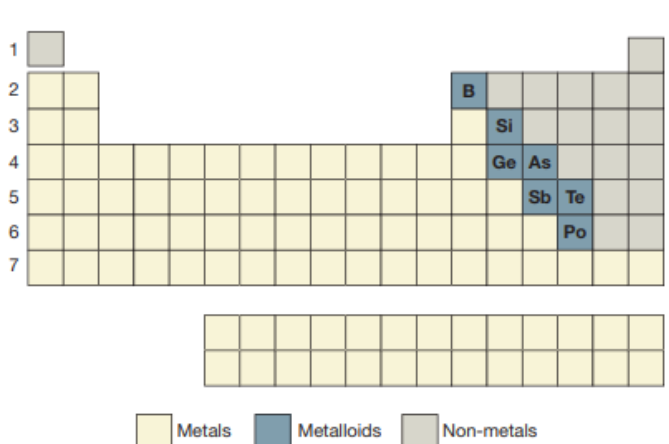
Similarities of metalloids, metals, and non-metals:
Metals | Conduct electricity, lustrous |
|---|---|
Non-metals | Not malleable or ductile |
Covalent bonding
Bonds between two non-metals.
Low melting points
covalent bonds within molecules are strong BUT there are very weak forces between molecules that don’t require much energy to break or separate them
Two non-metal atoms each share one or more of the unpaired electrons with each other to create covalent bonds that contain two electrons (one from each atom) so that all atoms have full valence shells.
Example: 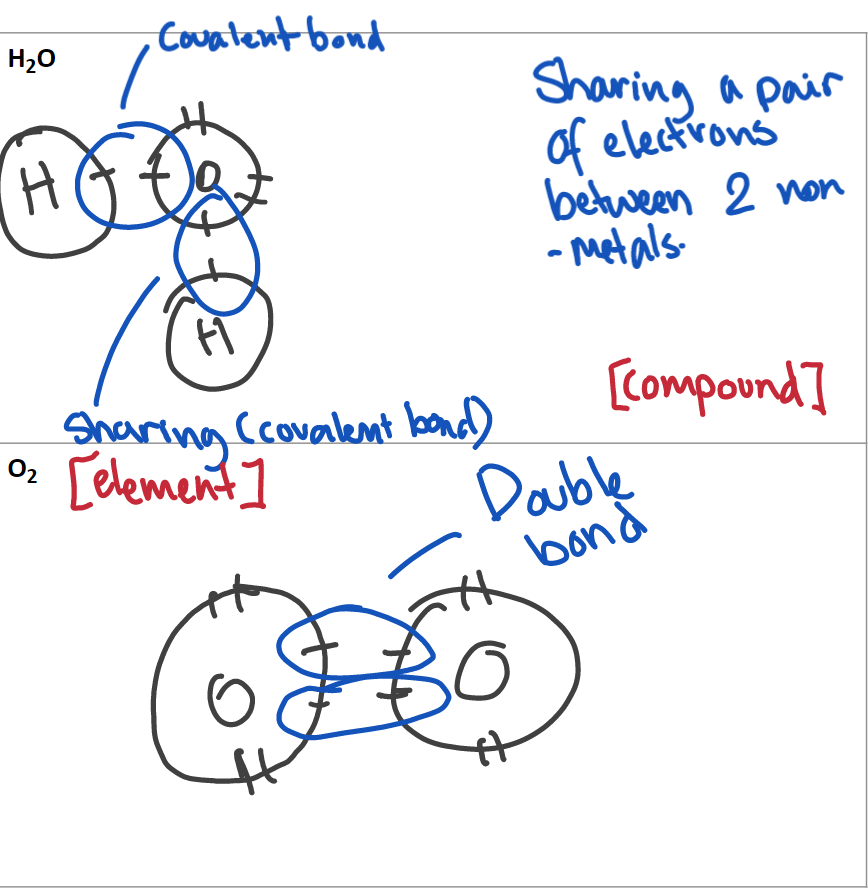
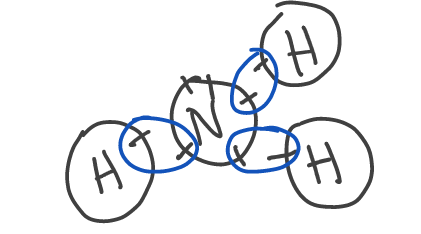
Covalent molecule (ammonia (NH3)) example:
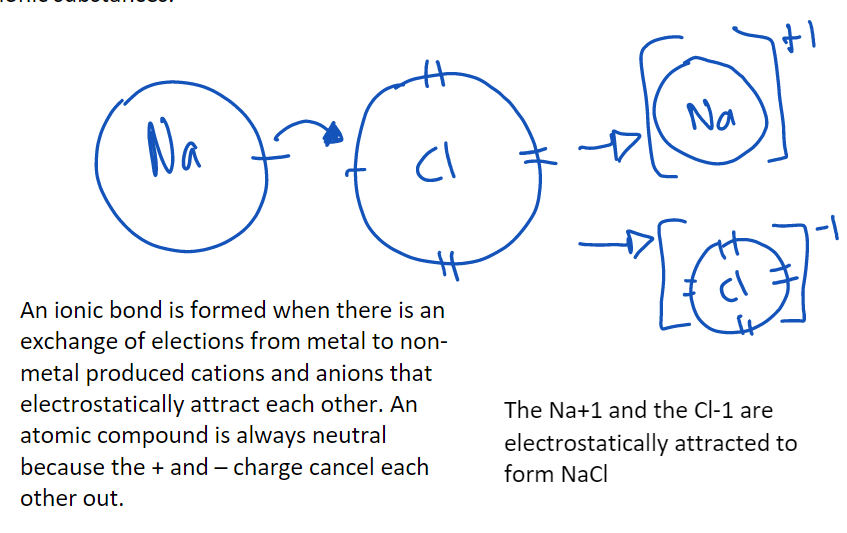
Ionic bonding
Bonds between non-metals and metals.
Properties of ionic compounds:
non-conductive as a solid.
conductive as a liquid because the anions and cations become free to move.
hard and brittle.
Properties of ionic substances
Property | Explanation |
|---|---|
No thermal and electrical conductivity when solid, but is when liquid | Ions in the solid can’t move past each other to conduct electricity and heat. They are locked in. |
High melting/boiling point | The electrostatic attraction between the cations and anions is to strong. Lots of energy is needed to separate. |
Hard and brittle | When you hit an ionic solid, you move the particles around. The anions end up next to each other, as do the cations. The similar ions repel each other and break the solid. |
Ionic compounds swap and drop method:
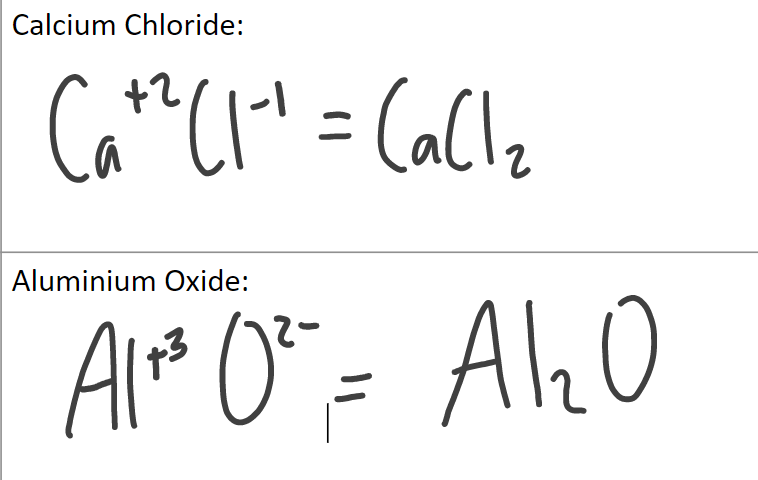
Only bracket polyatomic ions if you swap to make 2 or more
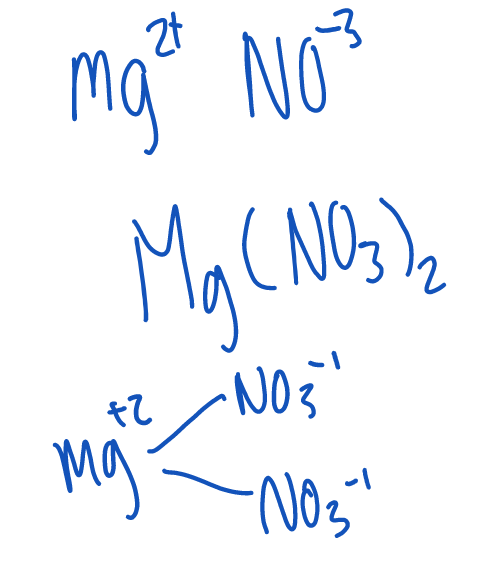
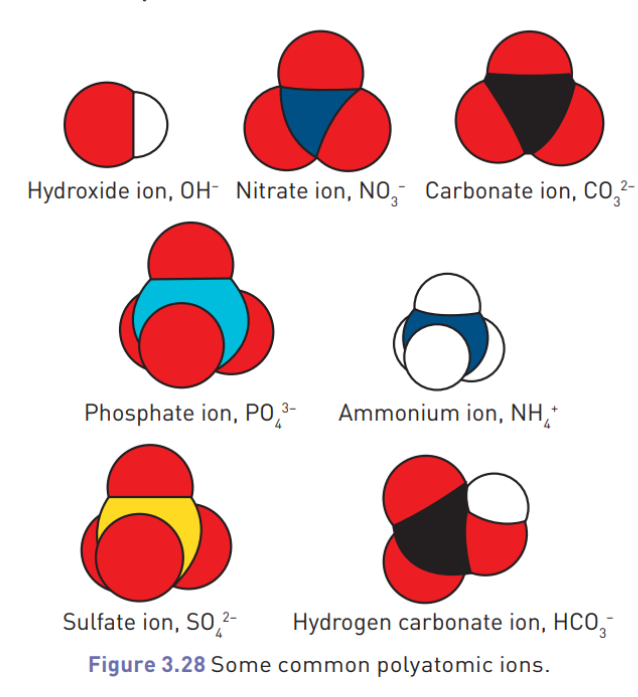
Polyatomic atoms (molecules of multiple atoms)
Ionic substances typically have much higher melting and boiling points than covalent substances because ionic bonds (electrostatic bonds) take a lot of energy to break.
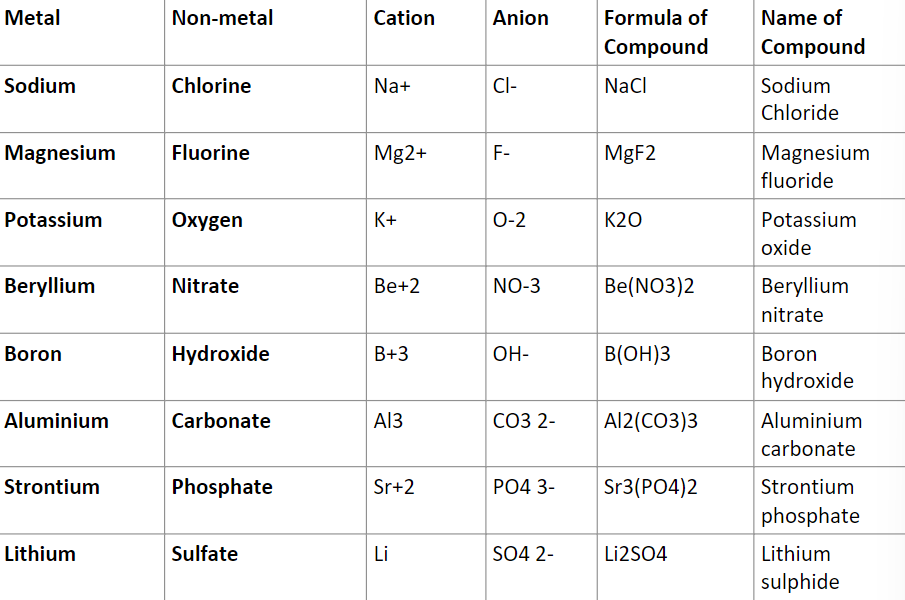
Examples of ionic compounds
Balanced chemical reactions
Remember: the number of atoms of an element on one side of the equation MUST equal the number of atoms of that element on the other side of the equation.
You CANNOT change any subscript numbers. Changing the subscript number changes the formula of the compound.
You CAN change any large numbers at the start of a compound formula.
Advice for balancing equations:
draw a table to count up the number of each type of atom/ion on each side of the equation.
start with elements that only appear in ONE compound on either side.
balance one element at a time. Once one is balanced, move on the next. You may need to revisit earlier elements multiple times.
Synthesis and decomposition
Synthesis reactions - the building up of compounds by combining simple substances, normally elements: A+B=AB
Example: Calcium (2Ca) + Oxygen (O2) → Calcium oxide (2CaO)
Decomposition reactions are the breakdown of compounds into simpler substances, either elements or more simple compounds. These reactions often require energy in the form of electricity or heat. Electrolytic decomposition is the breakdown of a compound as a result of an electric current passing through a solution. In decomposition reactions, only a single substance can be represented on the left side of the arrow.
States of matter:
solid - (s)
liquid - (l)
gas - (g)
aqueous - (aq)
Acids and bases
An acid is a substance that releases hydrogen ions (+) and forms salts by combining with certain metals.
An acid is a substance that releases hydroxide ions (-) and can neutralise acids.
Dissociation: to break up into ions.
Example of dissociation: H2SO4 -in water→ 2H + SO4-2
The strength of an acid/base refers to a measure of how easily an acid/base will dissociate (break up into ions). It is measured as pH 1-14; a pH of 1 is a strong acid and a pH of 14 is a strong base. (How many particles of THAT substance)
Concentration is how much acid/base is in the solution. (How many particles)
Examples
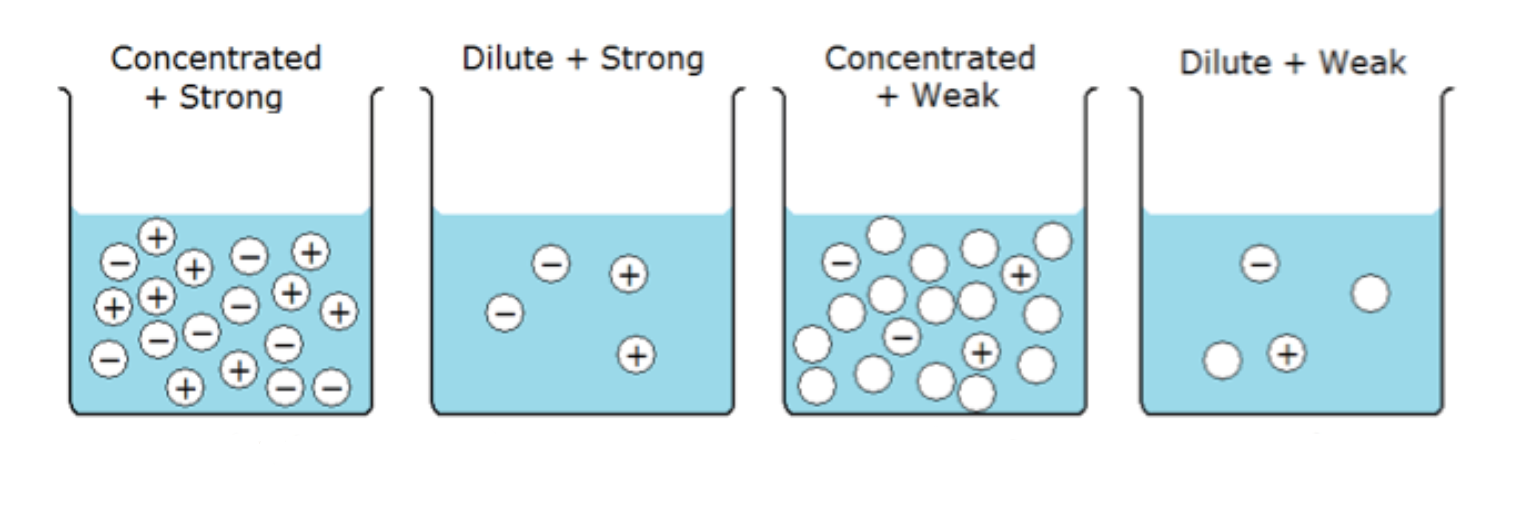
Dissociation tells the difference between a strong acid and a weak acid. If an acid dissociates 100% into ions, it is a strong acid. If the acid does not dissociate 100%, it is a weak acid.
pH is a measure of concentration of H+ ions in a solution, lower pH means a high concentration of H+. In what case might a strong acid have a higher pH (less H+) than a weak acid? A highly dilute strong acid can have a higher pH than a weak acid of sufficient concentration. (Diluting it lowers the pH)
Acid reactions
Acid base reactions are called neutralisation reactions because when they are mixed, they neutralise each other, becoming neither acidic or basic.
You must react an acid with carbonate to produce carbon dioxide gas.
Rates of chemical reactions
For a chemical reaction to occur, particles must collide.
Not all collisions between reactant molecules/ions result in a chemical reaction because for a reaction to occur, the particles must be in the right orientation, have the right amount of energy and actually collide.
Collision theory - this theory describes how and why substances react with each other. Collision theory provides three requirements for chemical reactions to occur;
correct orientation
reactants must collide
sufficient energy to overcome the activation energy required
Rate of reaction - refers to the change in mass of the reactants and products in a given amount of time (usually seconds.
Increasing the rate of reaction can be done with 5 things:
surface area - increasing the surface area by breaking the reactants into smaller pieces exposes the reactants to more collisions.
temperature - increasing the temperature increases the kinetic energy so that particles will collide more often and increasing the rate of reaction.
concentration - increasing concentration (amount of particles) the particles are more likely to collide. Using more concentrated acid will increase the chemical reaction with a carbonate, producing more CO2.
stirring - stirring keeps reactants moving and they are more likely to collide.
catalyst - a catalyst is a chemical that increases the rate of reaction but does not get used in the chemical reaction. A catalyst reduces the activation energy needed to start the chemical reaction.
Evolution
Natural selection
Natural selection:
natural selection is when organisms best suited to their environment survive and reproduce more offspring than those are not suited. Variation, selection, inheritance, time.
Fitness:
fitness refers to how many offspring an organism produces. An organism with greater fitness produces more off spring.
Survival of the fitness:
continued existence of a type of organism because that type continues to reproduce.
Process of natural selection:
variation:
gene | a gene is a section of DNA that codes for a certain protein/characteristic |
|---|---|
allele | if a gene codes for a particular characteristic, then the alleles are the alternatives for that gene. For example, if the gene is for eye colour, the alleles are alternatives (green, blue, brown and other variations). |
gene pool | all the genes and their alleles for a population. |
allele frequency | the amount of an allele for a population. |
mutations | random change in the DNA that may delete, add or rearrange genetic material. Maybe obvious or not. If it is a good mutation, then it will be passed onto the offspring. |
selection:
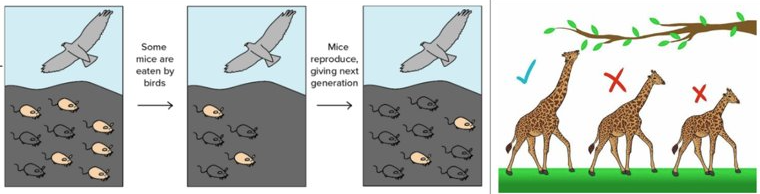
Selection pressure | an environmental factor that affects an organisms ability to survive. (e.g height of vegetation or the predation |
|---|---|
Effect of selection pressure on allele frequency | according to source 1, in the mice coat colour, there are two alleles: pink and grey. before selection, the allele frequency is 6 pink to 3 grey. After selection the allele frequency is 2 pink to 7 grey. selection pressures change the allele frequency. |
inheritance:
genetic influence | passing of genes/alleles onto offspring. |
|---|---|
effect of genetic inheritance on variation | the major source of variation. |
effect of genetic inherit | if some organisms survive and mate then those alleles will be passed on and increase allele frequency |
time:
Individuals vs populations | individuals do not evolve, populations do. |
|---|---|
Role of time in natural selection and evolution | natural selection can occur in a relatively small number of generations, but evolution is natural selection over millions of years. |
Examples of natural selection
Examples:
Cacti are a group of plants that typically live in very hot and dry locations. Their spines are actually highly modified leaves (thicker and curled up) and are shaped that way to help prevent loss of water by transpiration.
variation: cacti with different six leaves from large to very thin/spine like.
selection pressure: temperature and the amount of water.
time: over time the population changes to be higher frequency in the alleles for small leaves and low frequency for large leaves.
whales evolved from earlier land-dwelling mammals that started spending more time back in the ocean and now have limbs (tail and fins) that are suited to efficient movement through the water.
variation: some of the land-dwelling whales with fin like limbs (probably a mutation).
selection pressure: water environment (select some to survive).
inheritance: the ones with finds survived and reproduced so over time the whale population had fins.
time: over time the population changes to be a higher frequency in the alleles for fins and low frequency for limbs.
Speciation
new species are formed by speciation.
speciation: formation of a new and distinct species in the course of evolution.
the continents that are now separated were once joined together known as Pangea.
isolation is the first step in the formation of new species.
a place like Australia has a barrier of se meaning each gene pool was now exposed to different conditions like climate, food and competition.
those best adapted survived through process of natural selection.
gene pool changed and organisms became more and more different from those left behind.
those differences became so great that isolated populations are unable to interbreed and a new species is formed.
if environment changes due to human or natural influence they may become extinct like the Tasmanian tiger.
the variety of animals we have in our world is due to speciation.
Isolation:
environmental barriers
geographical
reproduction times
Human biology
DNA
DNA:
carries the code for protein and characteristics.
deoxyribonucleic acid.
is made of nucleotides
→ nucleotides are composed of a sugar molecule, phosphate, and nitrogenous base.
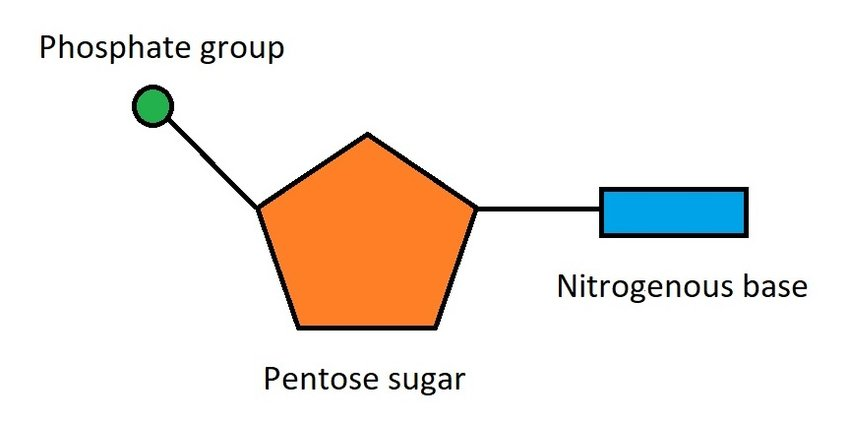
double helix or twisted-ladder-shaped.
Nitrogenous bases:
adenine.
thymine.
cytosine.
guanine.
Gene:
a short section of your DNA. Carry instructions for cells to make proteins.
Chromosomes:
condensed, coiled DNA into thread-like structures. DNA coils around histones.
our chromosomes come from our parents.
there are 23 pairs of chromosomes in each human cell.
the first 22 pairs of chromosomes are called autosomes.
the twenty-third pair is called the sex chromosome. Females have (XX), males have (XY).
human sex cells are called gametes. There are 23 chromosomes in each gamete. It is this number because at fertilisation, the zygote only has 46.
23 chromosomes are called haploids.
Protein:
the order and the number of bases in the gene determines the size and shape of the protein.
the size and shape of the protein determines its function.
PROTEINS → CELLS → TISSUES → ORGANS
DNA replication:
An enzyme breaks the H bonds between the base pairs
the DNA is now exposed as 2 single strands
Another enzyme attaches new nucleotides to the 2 exposed strands
Each single strand now becomes a double strand and the DNA has been replicated/doubled
Karyotypes
A karyotype is a photo of a cell set of chromosomes that have been ordered inter their parts.
They are used to check if a person’s chromosomes are correct.
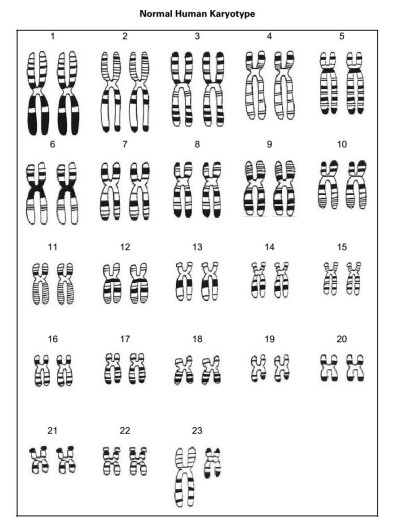
there are 23 pairs of chromosomes in each human cell.
the first 22 pairs of chromosomes are called autosomes.
to check that each chromosome is complete, check that there are no added/missing parts.
Protein
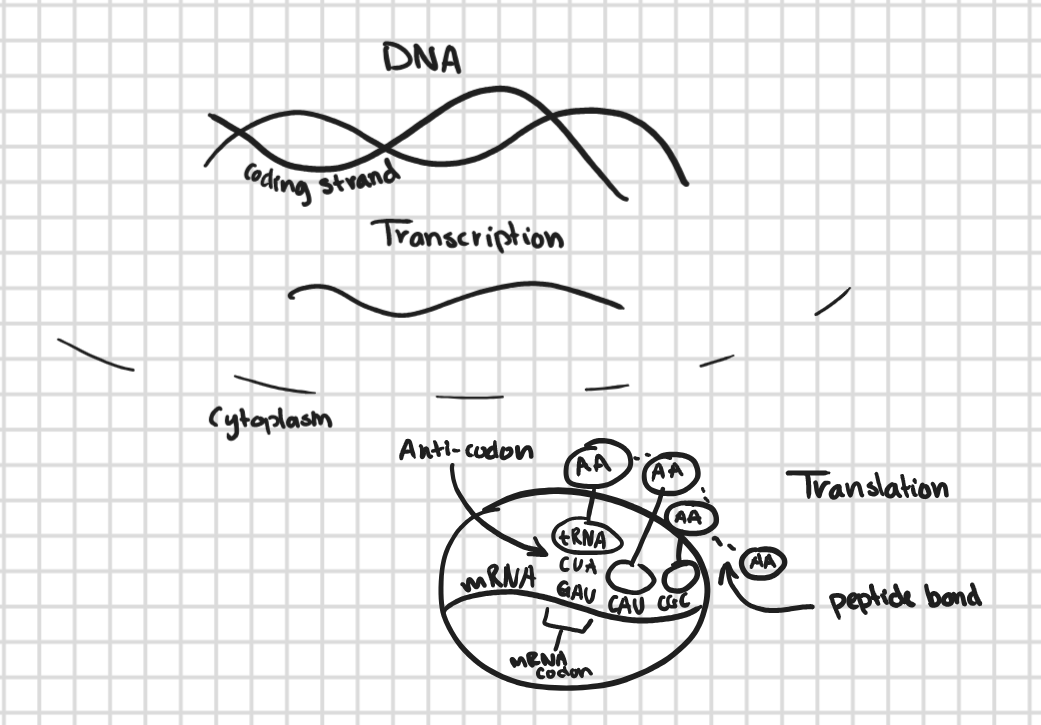
Transcription
RNA polymerase will connect complimentary bases to the DNA in the nucleus.
the bases bond together and for a single stranded mRNA.
mRNA goes out the nucleus and attaches to a ribosome.
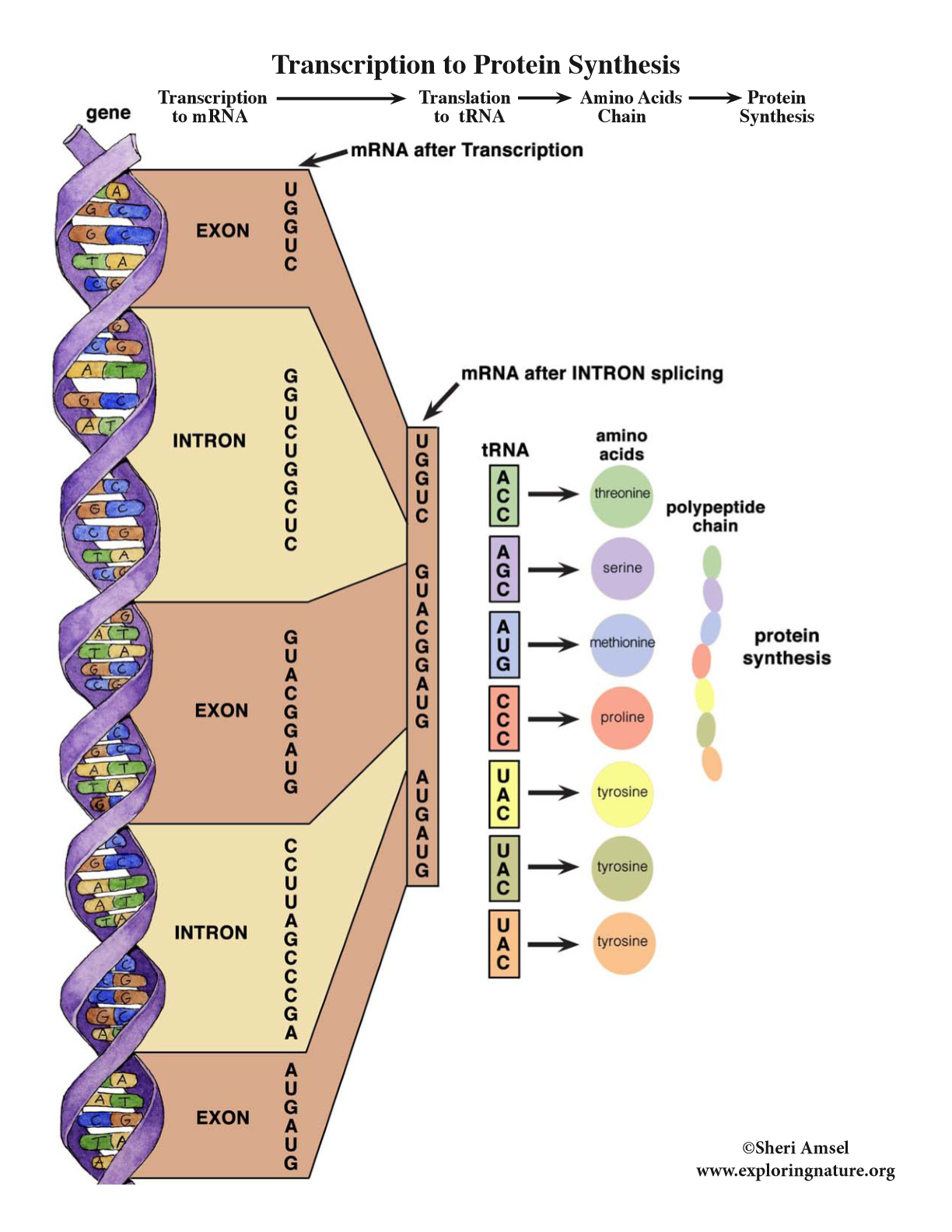
Translation
in the cytoplasm, there are tRNA molecules (they carry amino acids on them).
the mRNA directs the tRNA which amino acids to bring.
the tRNA looks for its complimentary base and transfers their amino acid.
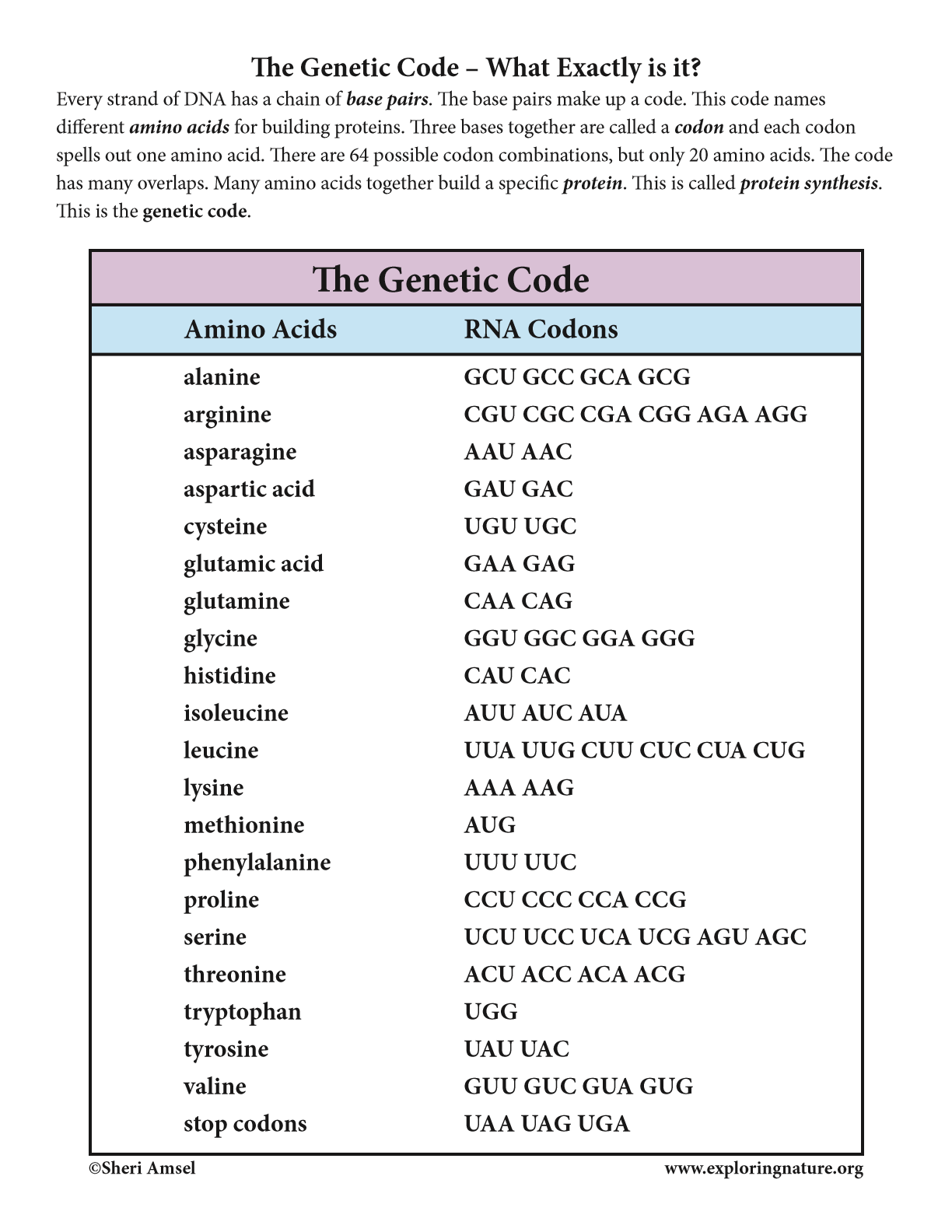
when tRNA is bringing in the amino acids it reads the bases in threes (codons).
the amino acids start building up and eventually a STOP codon is read and the chain stops.
once the chain stops, the protein is finished.
DNA/RNA
DNA has a double helix structure.
DNA replicates itself.
DNA has deoxyribose sugar.
DNA have the bases: adenine, thymine, cytosine, guanine.
RNA is single stranded.
RNA cannot replicate itself.
RNA has ribose sugar.
RNA have the bases: adenine, uracil, cytosine, guanine.
Mitosis
Mitosis is the division of the genetic material into two new identical daughter cells.
The cell cycle:
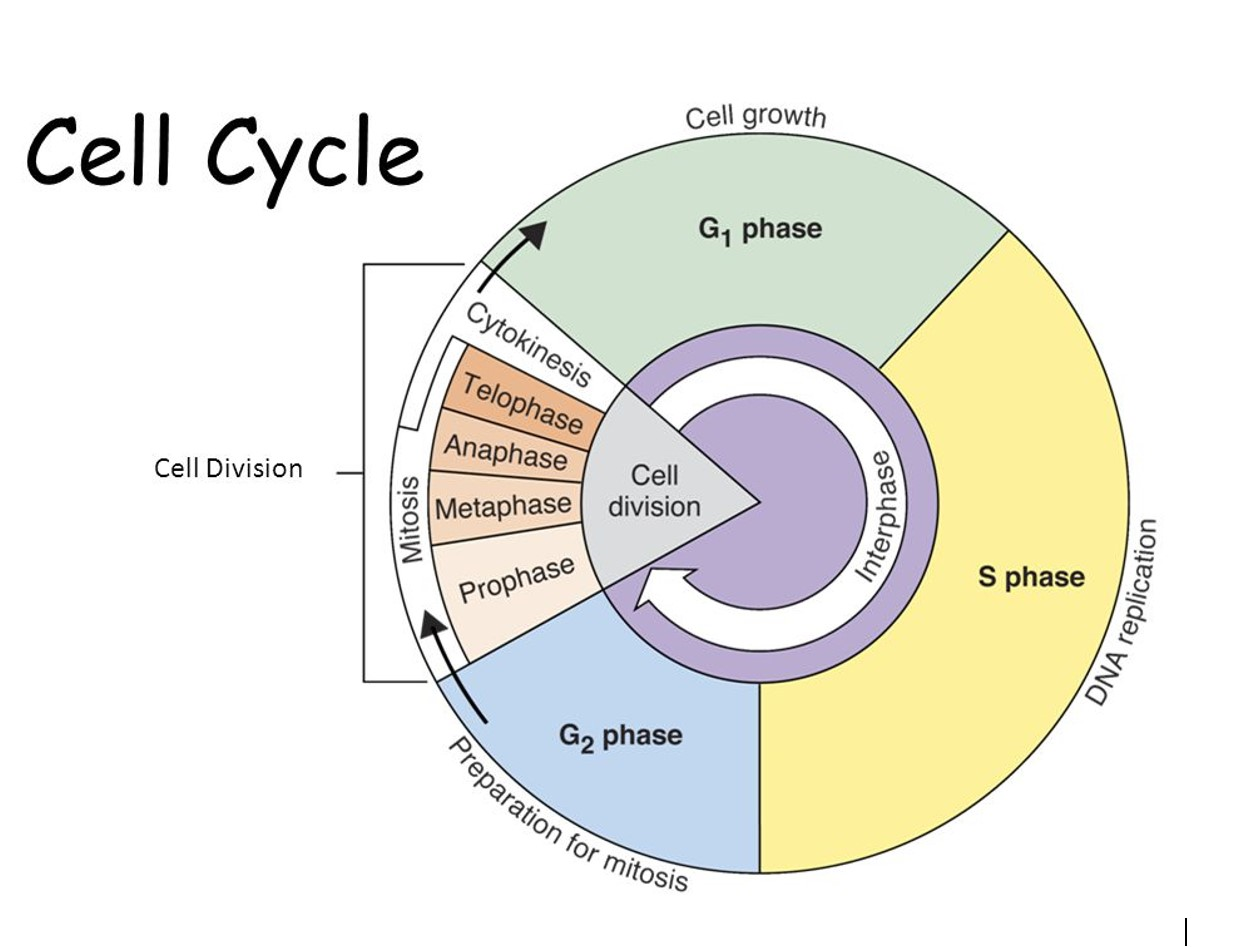
Cells in our body are consistently dividing to make new cells.
Cells communicate with each other using chemicals which signals when the cells should start and stop dividing.
Sometimes when the cell has abnormalities, they don’t know when to stop dividing.
As the cells grow and divide, they form an abnormal growth called a tumour.
G1 - new cells grow to full size.
S - the genetic material doubles or replicates.
G2 - the cell getting ready to divide.
Cell division:
mitosis: the division of the nucleus.
cytokinesis: division of the cytoplasm.
Mitosis process:
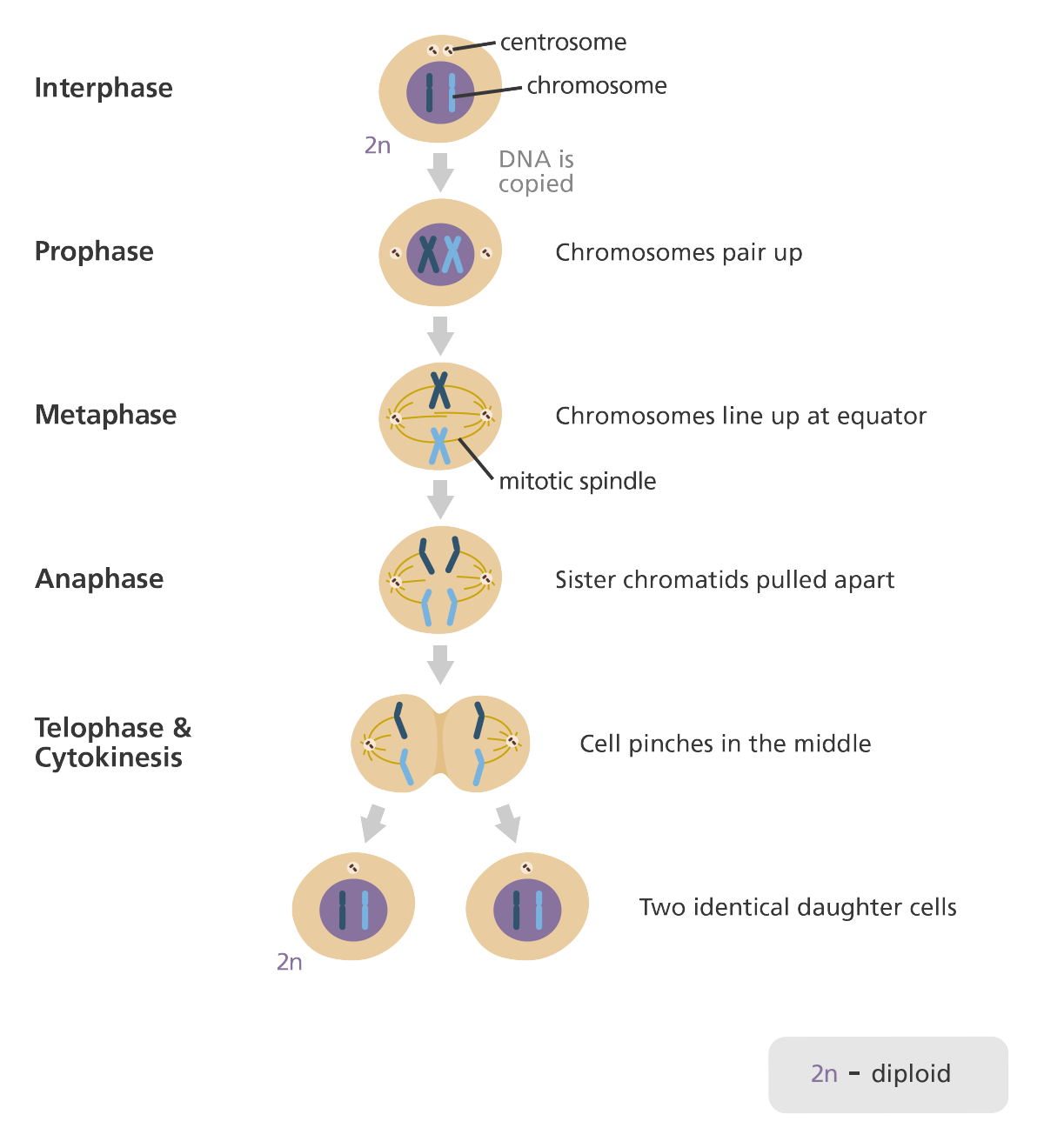
interphase
DNA in the cell is copied in preparation for cell division.
prophase
chromosomes condense into X shaped structures, composed of two sister chromatids containing identical genetic information.
chromosomes move to the equator in a line.
metaphase
chromosomes completely lined up at the equator, end-to-end.
the centrioles are now at opposite poles of the cell.
mitotic spindle fibres extending from the poles attaching to sister chromatids.
anaphase
the sister chromatids are pulled apart by mitotic spindles.
each chromatid at each pole.
telophase + cytokinesis
at each end of the poles, chromosomes are gathered together.
membrane forms around each set of chromosomes, creating two new nuclei.
the middle pinches, divides the cell into two daughter cells.
(cytokinesis) completion of cell division.
Meiosis
Meiosis is the process in formation of gametes.
It is 2x the process of mitosis, creates four daughter cells, not genetically identical.
interphase I
DNA in the cell is copied, making two sets of chromosomes.
each chromosome is duplicated, forming 2 identical chromatids held at the centromere.
prophase I
chromosomes condense into X shaped structures.
they pair up.
membrane around nucleus dissolves, releasing chromosomes.
[DELETED SECTION]
metaphase I
chromosome pairs line up at the equator.
genetic crossover occurs.
the centrioles are now at opposite poles of the cell.
meiotic spindle fibres extending from the poles attaching to sister chromatids.
anaphase I
cells divide to form gametes.
homologous chromosomes (from each parents), pulled to opposite ends by spindles.
telophase I
nuclear membrane develops around each set of of homologous chromosomes.
forms two haploid daughter cells with half as many chromosomes in the original cell.
cytokinesis I
cell divides into two daughter cells.
each cell has half as many chromosomes as parent cell.
SECOND ROUND
prophase II
membrane in each daughter cell dissolves away, releasing chromosomes.
meiotic spindle fibres form again.
metaphase II
chromosomes line up at equator and split.
no genetic crossover or pairs.
anaphase II
sister chromatids are divided and pulled to each pole.
four haploid daughter cells are created, each with different genetic makeup.
telophase II
separated sister chromatids reach opposite ends of the poles.
nuclear membrane develops around each set.
each daughter cell has one set of chromosomes.
cytokinesis II
the two daughter cells created during telophase II physically divide into four haploid cells.
each cell is distinct.
Important terms:
haploid - a cell with a single set of unpaired chromosomes.
diploid - a cell with two complete sets of chromosomes (homologous).
somatic cell - any cell of a living organism (not gametes).
sex cell - organism’s reproductive cells.
Apoptosis
Apoptosis is the process of programmed cell death.
It is used to eliminate unwanted cells.
Relation to cancer
apoptosis is when cells are programmed to die at a certain time.
in cancer, apoptotic control is lost, meaning cancer cells are able to survive longer.
Alleles
Alleles are variations/alternatives of a gene.
Alleles, genes, and chromosomes:
an allele is a variant form of a gene.
a gene is contained in a chromosome.
Autosomes and sex cells
autosomes are the first 22 chromosomes.
gametes are the 23rd pair.
in an autosomal pedigree, both parents can be carriers.
in a x-linked pedigree, only the mother can be a carrier.
Incomplete and co-dominance
Incomplete dominance:
one allele is not dominant over another.
for an allele to be expressed, both has to be homozygous.
for example - in snapdragon flowers, RR is red, rr is white, Rr, is pink.
Co-dominance:
both colours are dominant.
for example - in chickens, BB is black, WW is white, BW is black/white speckled.
Phenotype/Genotype
A phenotype is the physical appearance.
A genotype is the written symbol of that allele.
Haemophilia and red-green colour blindness
Haemophilia
A father who has haemophilia passes his only X chromosome down to all of his daughters, so they will always get his haemophilia allele and be heterozygous (carriers).
A father passes down his Y chromosome to his sons; thus, he cannot pass down a haemophilia allele to them.
Physics
Distance/Displacement
Distance - how far an object travels in a set time.
Displacement - the final distance and direction from the starting point of an object.
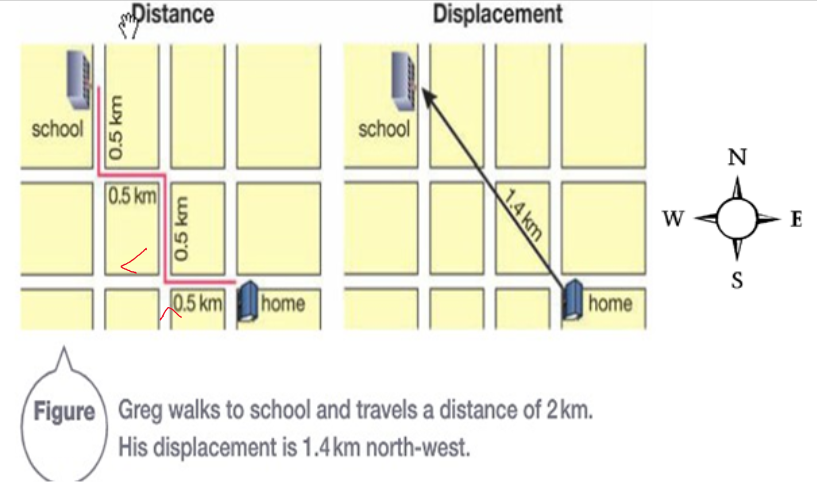
Quantities:
Scalar - measures magnitude in numbers and units, e.g. 9km, 60kg, 50 mins.
Vector - measures magnitude with numbers, units AND direction, e.g. 9km North, 600 Newtons down.
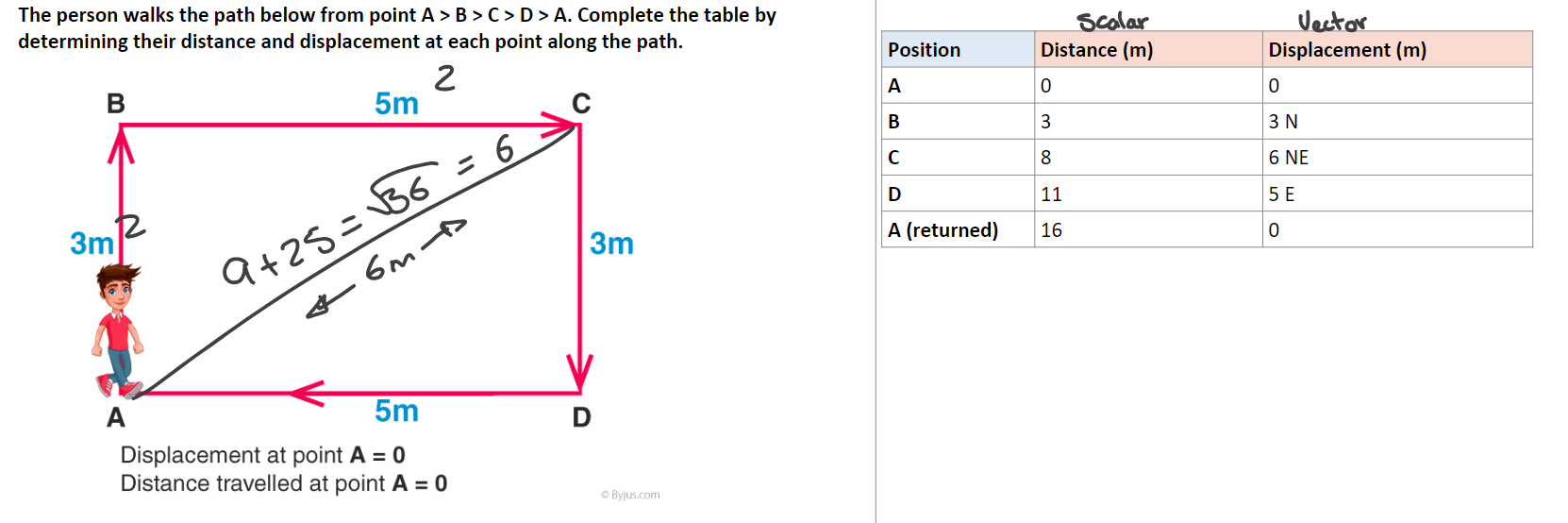
Example questions:
an object moves 14 m North and the 14 m south. What distance has it covered? What is its displacement?
distance is 28m.
displaced 0m.
a person runs 50 m north, the 20 m south and the 30 m west. What is the total distance covered? What is the person's displacement?
distance is 100m.
displacement is 42.42m North.
Explanation:
Speed/Velocity
Speed (scalar) - distance travelled per unit of time. m/s or ms^-1.
Velocity (vector) - distance travelled per unit of time with direction. m/s or ms^-1 with direction.
Formulae:
distance = speed*time
speed = distance/time
time = distance/speed
Example questions:
If a car travels 400 m in 20 seconds, how fast is it going?
Speed = 400/20 = 20 m/s.
How much time will it take for a bug to travel 5 metres across the floor if it is travelling at 0.5 m/s?
Time = 5/0.5 = 10s.
How far can you get away from your little brother with the squirt gun filled with paint if you travel at 3 m/s and you have 15 seconds before he sees you?
Distance = 3*15 = 45m
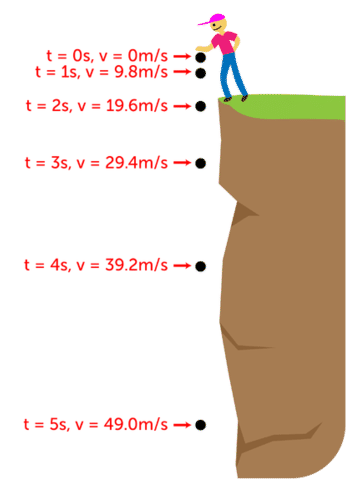
Acceleration
If you drop a bowling ball and a feather from the same height, which will hit the ground first?
the bowling ball will hit the ground first because the feather has more air resistance.
What if it was on the moon?
both would land at the same time because the moon does not have air.
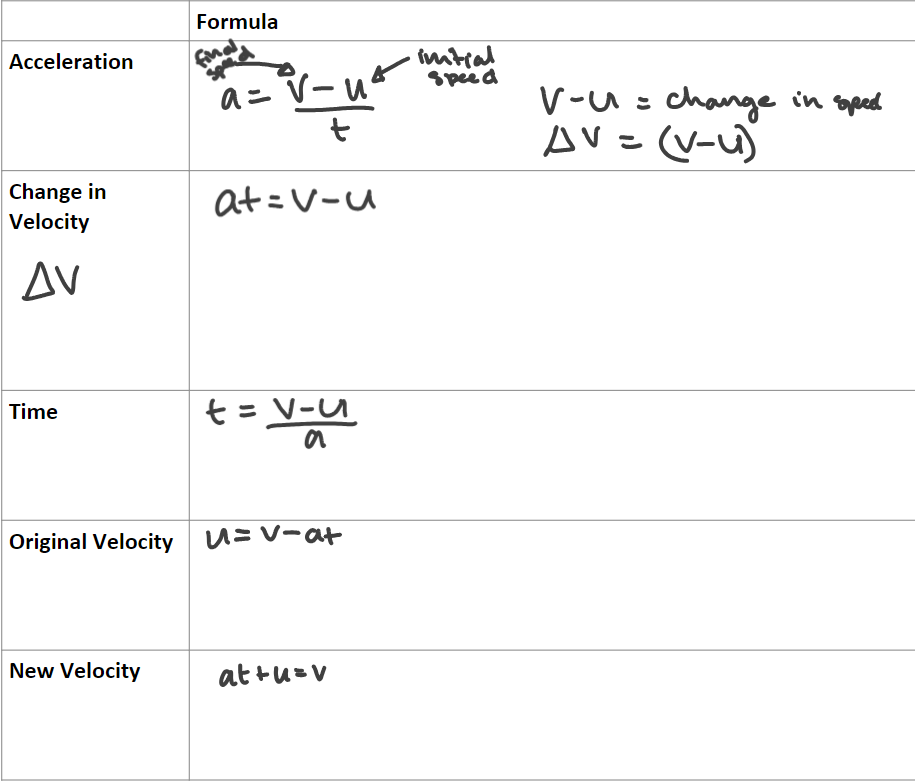
Formulae:
(a - acceleration, v - final velocity, u - initial speed)
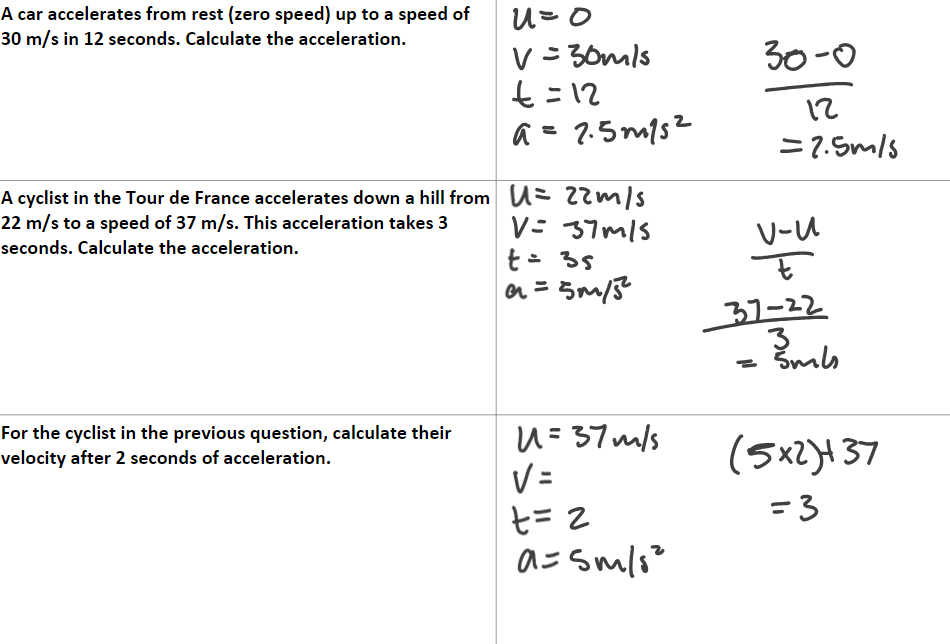
Example questions:
Graphing motion
Example:
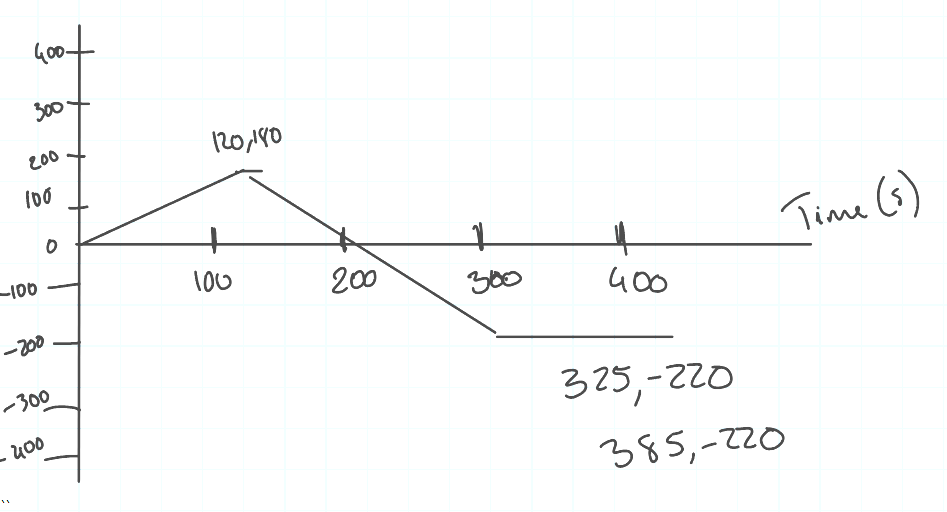
Bruce starts walking from his home eastwards towards school.
It takes him two minutes (120 seconds) to cover 180 m.
He then gets a call to meet his friend at the shops, so after a 5 second pause, he turns around and walks 400 m in the opposite direction, which takes him 200 seconds.
They are both in the shop for 1 minute (60 seconds) before leaving again.
Newton’s first law
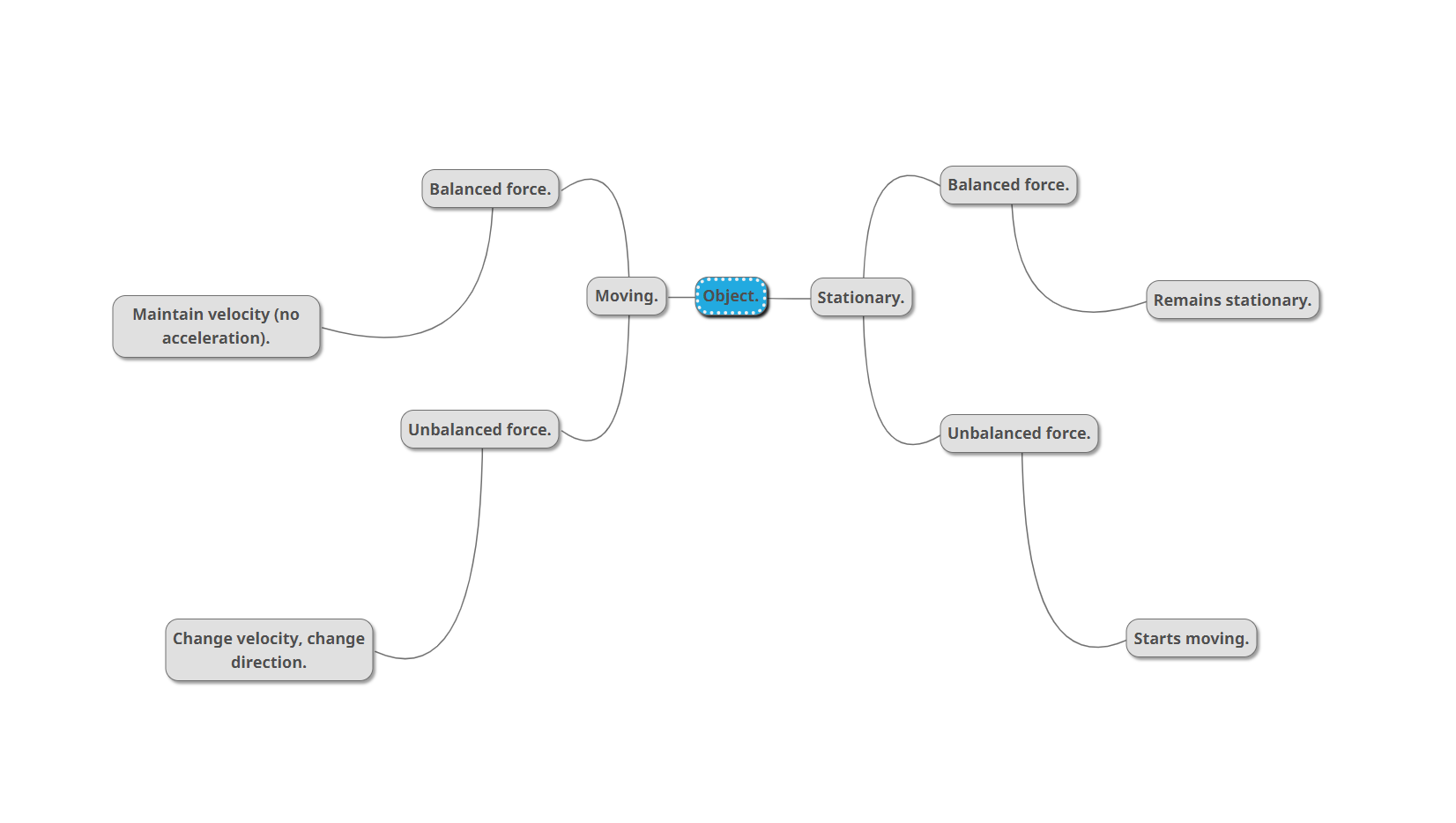
An object remains at rest or in constant motion in a straight line unless acted on by a net unbalanced force.
What is inertia?
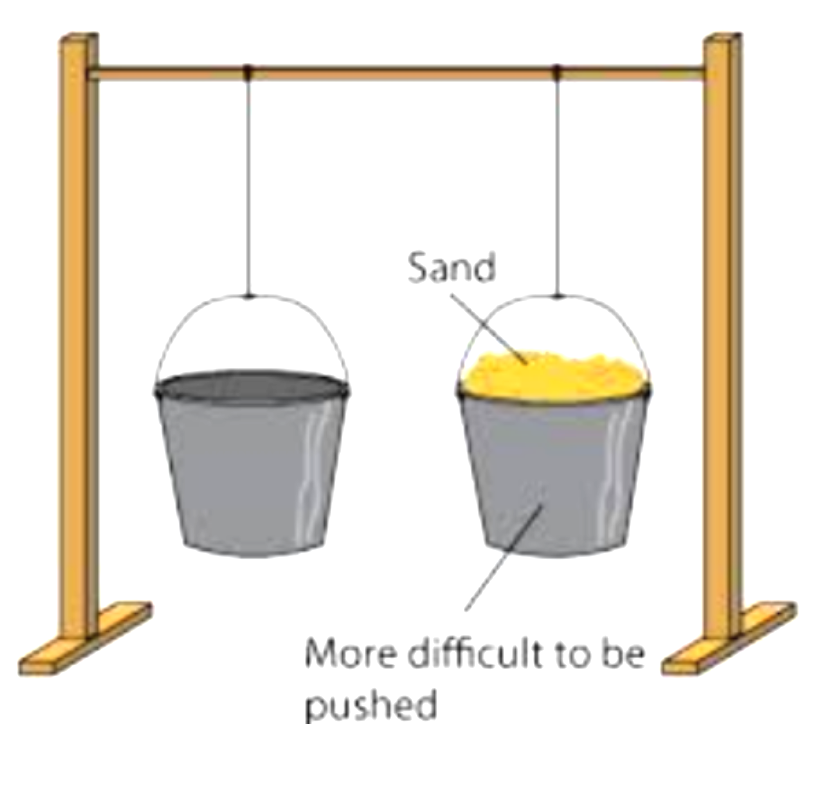
inertia is the need for an object to keep on doing what it was already doing.
The larger the mass of an object, the greater it’s inertia
Force on an object:
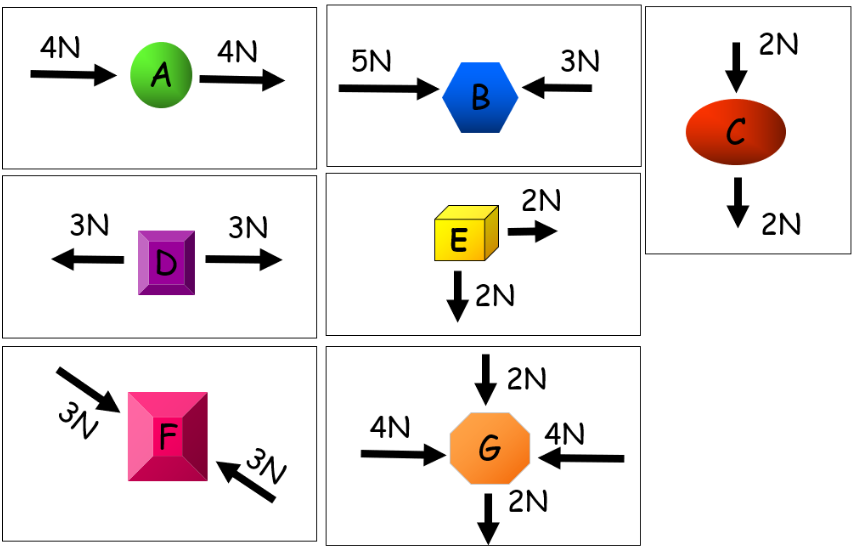
Object | Balanced/Unbalanced | Direction of Movement |
A | Unbalanced | East |
B | Unbalanced | East |
C | unbalanced | South |
D | Balanced | Stationary |
E | Unbalanced | South East |
F | Balanced | Stationary |
G | Unbalanced | South |
Newton’s second law
Newton’s second law states that acceleration is directly proportional to force and indirectly related to mass.
Formulae:
force = mass*acceleration.
mass = force/acceleration.
acceleration = force/mass.
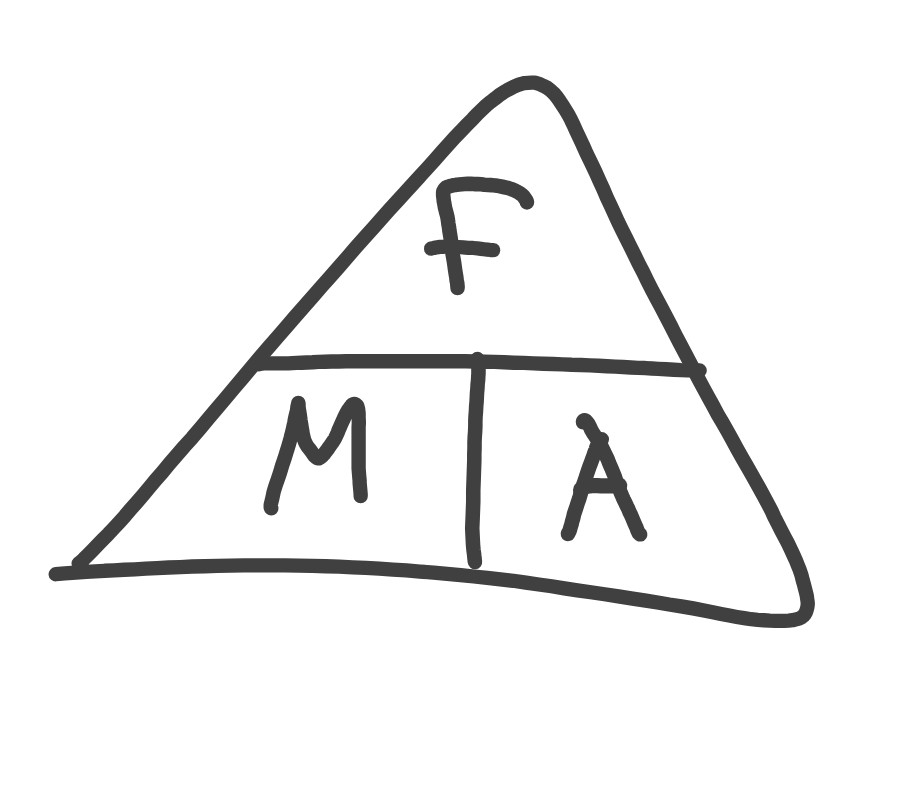
What’s the difference between mass and weight?
mass is the amount of matter measured in kilograms.
weight is the force of gravity. Mass*gravity.
Newton’s third law
Whenever two object interact, they exert equal and opposite forces on each other.
Examples:
Scenario | Action | Reaction |
A tennis racquet hitting a ball. | Tennis racquet pushes on the ball | Ball pushes back on the racquet |
Your foot and the floor when you are walking. | Foot pushes on the floor | Floor pushes back on foot |
Your hand and a brick wall attached to the ground when you push on it. | Hand pushes on wall | Wall pushes on hand |
A rocket and its exhaust gas during lift off. | The rocket pushes on the gas | The gas pushes on the rocket |
A sprinter pushing off from the start blocks. | Foot pushes off start block | Start block pushes on foot |
The Moon in orbit around the Earth. | Earth is pulling on moon | Moon is pulling on earth |
Weight and mass
Mass - measure of how much matter there is in an object (Kg).
Weight - force oh how much gravity pulling on an object (N).
Formulae:
w = m*g
m = w/g
g = w/m

Examples:
Question: | Answer: |
What is the weight of a small car if it's mass is 20,000g? | W = 20kg * 9.8
= 196 N |
What is the mass of an item if it's weight is 65 N on Earth? | M = w/g M = 65N/9.8
M = 6.63kg |
If a person weighs 78N on Earth, what is their mass? | M = 78/9.8
M = 7.95kg |
What is Jupiter's gravitational pull if the same person from the scenario above weighs 197.3N on Jupiter? | G = w/m G = 197.3/7.95 G = 24.79m/s |
Mercury has 0.37 g's of Earth. What is Mercury's gravitational pull? | G=9.8*0.37 = 3.63 ms-2 |
What is the weight of a person with a mass of 45kg on the moon, if the moon's gravitational pull is 1.6 m/s2? | W=mg
45*1.6 = 72 N |
Work and energy
Energy - the ability to do work (J)/(kJ).
Main types of energy:
kinetic - energy associated with movement.
potential - energy associated with position (stored energy).
Law of conservation of energy:
energy cannot be created nor destroyed.
the total energy of a system remains constant.
Example:
Consider two identical, large boxes placed on the floor. Both of them are heavy and are difficult for you to move.
You are challenged to EITHER move both boxes 1 m each, OR to move just one box 10 m.
Intuitively, which challenge would you accept? | Two boxes x2 |
Explain your choice. (You don't need to use physics principals yet). | 2m is less than 10m so you need less energy |
Which of the two challenges would make you the most 'tired'? In other words, which challenge would require you to expend the most energy? | The second one |
Moving the box requires you to expend energy? Where does that energy go? | Kinetic energy Thermal energy Sound energy |
What do you need to apply to the boxes to actually get them to move at all? (This is a physics term). | Force |
Work - measure of the amount of energy transferred into moving or rearranging an object.
The relationship between force and work - they are directly proportionate to one another, and as distance increases, work also increases.
The relationship between distance and work - they are directly proportionate to one another, and as distance increases, work also increases.

Examples:
How much work is done if a force of 200 N moves an object 6 m? | W=fd 200*6 = 1200J |
How far does an object move is 500 J of work is done on it by a constant force of 75 N? | D=w/f 500*75 = 6.67m |
How much work is done by a student who pushes a backpack with a force of 20 N for 5 m along the hallway? | W = f*d W = 20*5 = 100 j |
A soccer player kicks a ball with a force of 100 N for 0.1 m. How much work does the player do on the ball? | W = fd W = 100*0.1 = 100 j |
A cyclist travels 20 m and does 3000 J of work on their bike. What force did the cyclist apply to the bike pedals? | F = w/d F= 3000/20 = 150 N |
Gravitational potential energy
Formulae:
G = 9.8
M = GPE/(g*h)
GPE = m*g*h
H = GPE/(m*g)
G = GPE/(m*h)
Examples:
Consider a person at the gym lifting weights from the floor.
Mass (kg) | Exercise | Mass lifted to height of (m) |
30 |
| 0.75 |
30 |
| 2 |
60 |
| 2 |
Exercise | Force (N) | Work (J) |
1 | 294 | 220.5 |
2 | 294 | 588 |
3 | 588 | 1176 |
A person with a mass of 60 kg climbs up a ladder that is 5 m high. How much work does the person do on their body? | M= 60 kg H = 5 m G= 9.8 m/s/s GPE = m. g. h 60x9.8x5 = 2940 J 2.94 kJ |
A roller coaster with a mass of 500 kg starts at a height of 10 m. As it is winched up the start ramp, 196 kJ of work is done on it by the motor. What height does the rollercoaster reach? | M = 500 kg W = 196 000 J H =?
GPE/ m x g = h 196 000/ 500 x 9.8 = 40 m
H= 40 -10 m = 30 m |
Kinetic energy
What type of energy does a barbell have at the top of the lift? | Gravitational potential |
What type of energy does a barbell have just before it hits the ground? | Kinetic energy |
Qualitatively describe the change in the barbells energy up until it hits the ground. | The gravitational potential energy is transforming into kinetic energy |
Can you use this to relate kinetic and gravitational potential energy. | Total energy= GPE + KE At the top total energy = GPE ( KE =0) At the bottom total energy = KE (GPE = 0) In the middle total energy = KE + GPE |
Define the formula for Kinetic Energy. | KE = 1/2 mv2 |
A 100 kg cyclist on a bike is travelling at 15 m/s. Calculate the total kinetic energy of the cyclist and bike.
| KE = 1/2 mv2 KE = 1/2 x 100 x 15 x 15 = 11,250 J |
A force of 900 N acts on a car with a mass of 1 tonne over 0.5 km.
|
V = square root of 2 KE/m = square root of 2 x 450000/1000 = 30 m/s |
Power stations: thermal energy
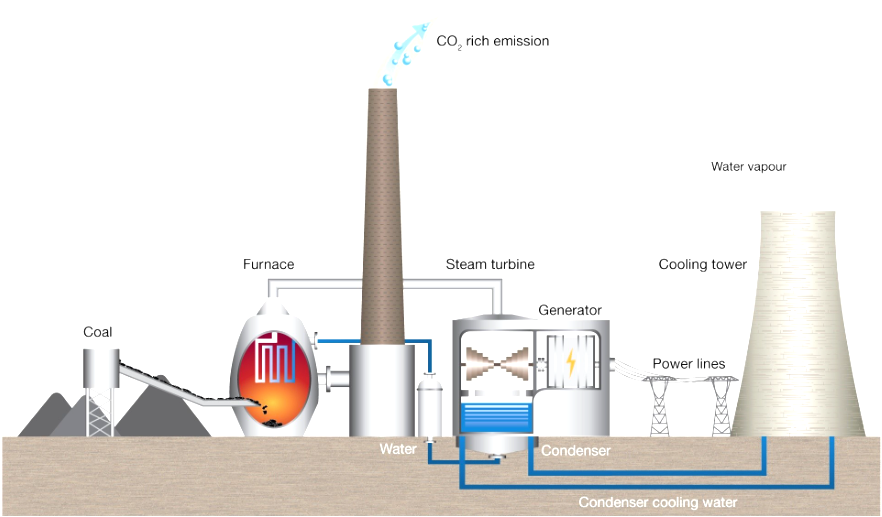
Steps involved in the generation of electricity in a power station:
burn coal/gas to release heat.
heat turns water in boiler into steam.
steam turns turbine to generate electricity.
steam condensed back into water, and fed back into boiler
Temperature:
a measure of the average kinetic of the particles in a substance.
Heat:
heat is an energy type that transfers from an object of higher temperature to an object of lower temperature.
Which has more energy, an ice-burg or a cup of coffee?
ice-burg has higher energy because it has more particles.
Which has a higher temperature?
the cup of coffee has a higher temperature because the particles are moving faster.
Specific heat capacity:
Consider two saucepans of water sitting on a stove. One is large and contains 5 L of water, the other small and contains only 1 L. The stove applies heat to the saucepans at the same rate.
Which saucepan would take longer to start boiling? | The larger one. |
Why is this? | The larger one has more particles so more total energy is required to increase by the same amount. |
What does this mean about the relationship between heat transfer and mass? | As mass increases, the heat required also increases. |
Formula:
Q = mcΔT
Units used:
J/kgoC
Example:
Water is fed into the boiler of a thermal power station at a temperature of 14 oC. A particular boiler has a volume of 9000 L of water. Calculate the energy required to start boiling all of the water in the boiler. The specific heat capacity of water is 4180 J/kg oC | Q = mcΔT
m = 9000 L = 9000 kg c = 4180 J/kg oC ΔT = 100 - 14 = 86 oC
Q = 9000 x 4180 x 86 = 3,235,320,000 J |
1155 J of energy is required to heat 50 g of copper metal from 20 oC to 80 oC. Calculate the specific heat capacity of copper. | Q = mcΔT
Q = 1155 J m = 50 g = 0.05 kg ΔT = 80 - 20 = 60 oC
c = Q / (mΔT) = 1155 / (0.05 x 60) = 385 J/kg oC |
Space science
Structure of the Universe
Emu in the sky
Emu is running - April and May
Time to collect eggs
Emu sitting - June and July
Still time to collect eggs
Eggs start to hatch - August
No collecting of eggs
Emu drinking - November
Water holes are full
Uses of emu in the sky
ceremonies
time to hunt
The brothers of Kingfish clan where forbidden to eat any kingfish. The brothers caught kingfish after kingfish but had to throw them all back. Eventually one of the brothers became so hungry, he ate the kingfish. The sun women, Walu, saw him and in anger, created a water sprout that lifted the brothers up into the sky.
Cosmic background noise - CMB is the ‘noise’ (radiation) left over from the big bang and represents the temperature of space in the whole universe. The temperature is not the same everywhere, therefore density is not the same everywhere. Discovered May 20, 1964.

Cosmic microwave background - cosmic background microwave background is a form of electromagnetic radiation left over from the Big Bang. As the universe expands, the wavelength of the CMB radiation is stretched, causing it to shift towards longer wavelengths, or lower frequencies, this is known as redshift.
Cosmic background noise provides evidence for the big bang - the fact that we can see the microwaves expand means the waves started at a single point.
The Milky Way - the Milky Way is a galaxy that includes the Solar System. It is a huge collection of stars, dust and gas in a spiral system. Our sun is 25,000 light years away from the centre of the galaxy. We can see the stars and gas clouds of the Milky Way across the skies on Earth. We can also see other galaxies from Earth.
Universe mind map -

Galaxies - galaxies are a collection of gas, dust and billions of stars and their solar system.
Stars - a star is a luminous ball of gas held together by its own gravity. Stars are mostly made of hydrogen and helium.
Difference between a star, a planet, a moon and an asteroid - Stars have nuclear fusion, and they produce light. A moon has a body that orbits a planet, an asteroid orbits a star. A planet is a body that orbits the sun.
Role of gravity in the universe - gravity is the force which holes the universe together. It creates a path of objects around another body.
Unit of measurement in the universe - the unit of measurement used in space is light years. Light years is the distance light travels in one year. One light year equals 9.46 trillion kilometres. For example, if a star that was 20 light years away exploded right now, we will see the explosion 20 years from that time it exploded.
Life cycle of a star
Stars can appear in different colours such as orange, yellow, blue and white and they can vary in sizes. Our sun is small compared to many stars. It is still huge compared to the Earth and other planets.
Hertzprung Russell diagram -

The Hertzsprung-Russell diagram is a graph that plots the luminosity of stars against their surface temperature. It is used to classify stars and understand their evolution (cycle).
Hot, bright stars are located in the upper left corner, while cool, dim stars are located in the lower right corner.
The main sequence, where most stars spend the majority of their lives, runs diagonally from the upper left to the lower right.
The cycle:
Nebula - stars come from clouds of dust and gas called nebulae which are mainly of hydrogen. All star begin their life as part of a nebula.
Protostar - gravity causes the dust and gas in nebulae to become denser and more concentrated. The temperature increases until hydrogen and other light elements undergo nuclear fusion. This produces energy and the core get brighter and hotter until a protostar is born.
Main sequence star - nuclear fusion of hydrogen atoms is a now in full flow and a protostar becomes a main sequence star. This star is much hotter and brighter then before, releasing energy and a flow of radiation from its core. A main sequence star is in equilibrium: it is stable because the forces within it are balanced. Our sun is currently in its main sequence stage… but we are safe. These processes take billions of years.
Cycle of smaller (low mass) stars after the main sequence:
Red giant - helium and other light elements in the star’s core fuse to form heavier elements. This process releases even more energy, causing the star to expand. The star gives off a red light, hence the name “red giant”.
White dwarf - a red giant will eventually run out of its “fuel” of light elements. Nothing else in it will fuse. It will lose its outer layers leaving behind the core of the star. This is so dense and hot that it glows white.
Black dwarf - a white dwarf will eventually cool down enough that it stops glowing white. Stars like the sun then fade out, go cold and enter the stage of a black dwarf.
Cycle of bigger (high mass) stars after the main sequence:
Red super giant - following the main sequence, a star begins to fuse together heavier elements. As it has more fuel, it swells out to a much larger size, giving off a lot more energy.
Supernova - a red supergiant will start to collapse in on itself. This compresses its core more until it reaches a critical point. This causes a massive shockwave or a cataclysmic explosion called a supernova. A supernova event can outshine an entire galaxy for several weeks. The explosion only lasts seconds, in those seconds it can release as much energy as the star has released up to that point. It can be as bright as the light from 10 billion stars
THEN
Neutron star - after a supernova, only the star’s compressed core is left behind. The core has turned into lots of neutrons. The neutron star is extremely dense. A small amount of the neutron star would way more than the earth.
OR
Black hole - the core left from the supernova will continue to collapse and keep getting smaller until the entire star collapses into an infinitely small point. The gravitational field is now so strong, nothing can escape from it, not even light of any electromagnetic radiation.
Visual map of cycle:

More information:
Stellar nebula - stellar nebula are clouds that form new stars. Gravity causes the hydrogen to fuse into helium.
Stars turn into giants/supergiants as they get older - stars turn into giants/supergiants as they get older because helium and other elements in the star’s core fuse to form heavier elements. This process releases even more energy, causing the star to expand.
Larger stars are hotter but live shorter. - larger stars are shorter lived because
they burn more fuel, so they run out of hydrogen sooner than smaller stars do.
Likely progress of our sun through the cycle - it will expand and run out of energy, becoming a white dwarf, then a black dwarf.
Supernova - the size of the red giant depends if the star will go into a supernova or not.
Difference between a neutron star and a white dwarf - a white dwarf is hotter and it is remains of a red giant. A neutron star is full of neutrons and comes from a super red giant.
Black hole - a black hole is remnants of super red giants. When gravity is so string it collapses and becomes a black hole.
The big bang
The big bang - how astronomers explain the way the universe began at a hot, dense, single point, then expanded and stretched into the universe we know today. It is still expanding.
The Doppler effect - 
The doppler effect is the change in the wavelength/frequency of a wave as it moves towards and away from an object.
As the wave moves towards you, the frequency increases, the wavelength decreases.
As the wave moves away from you the frequency decreases, the wavelength increases.
The doppler effect can be used to determine how fast stars move.
Red/blue shift -

Red stars are further away from blue stars. yellow stars are not as far away as red stars but not as close as blue stars.
Red and blue shift on the spectrum: 
Electromagnetic spectrum - the range of all types of radiation that has both electric and magnetic fields and travels in waves.

Doppler effect - the apparent change in wave length or frequency when the source of waves or the observer is moving responsible for the red shift of distant stars.
Oxford extended understanding
Difference between galaxies and constellations - galaxies are the collection of stars and constellations are the collection of a few stars.
Indigenous people using the sky as a calendar - indigenous people used the sky as a calendar by marking out different shapes and referring to them as different animals. As the animal moves, it indicates to the indigenous people the time of year like a calendar.
Orion - Orion is a constellation. To find Orion, look for 3 big stars close together. They are Orion’s belt. 2 brighter stars North of it represent the shoulders and the 2 stars to the south are feet.
Different stories of Orion - there may be different stories about Orion from indigenous people across Australia because there are many indigenous people spread across Australia and they all have different perspectives of Orion which they might associate with different objects.
Individual stars - we can’t see individual stars because they are too distant to be seen individually.
Main sequence star - any star that has a hot core which fuses H into He to produce energy.
Parallax - change in an object’s position when viewed from 2 different points of view.
After a supernova - after a supernova happens, a neutron star remains. When the neutron star collapses, the gravitational pull and density is so great that it creates a black hole.
Objects that pass by a black hole - objects that bass by black holes will be sucked spirally into the black hole. If you get sucked in a black hole, the closer you get to the black hole, you will be pulled in and you won’t be able to escape. If you were to go into a black hole, you will be stretched while also being compressed. You will be ripped apart when you are stretched and look like “spaghetti”, this is known as spaghettification. The closer you get to the black hole, time gets slower (gravitational time dilation). The event horizon has infinite time dilation because any object that goes in doesn’t pass the horizon (doesn’t come out).
Accretion disc - flattened astronomical objects made of rapidly rotating gas which slowly spirals onto a central gravitating body.
Location of black holes - black holes can be located anywhere.
The sun’s progress in the cycle - when the sun reaches the end of the main sequence, it will expand into a red giant. Helium is dumped in the core. There is a shell of hydrogen around the helium-rich core. Fusion causes it to expand. Hydrogen cools and rises to create a rich outer envelope. Gravity is still pushing inwards.
Comparison of the life cycle of our sun with another star that has 10 times the mass of the sun - since our sun is smaller, it won’t have the ability to create a black hole like other bigger stars. Our sun will become a red giant after the main sequence, after it runs out of He, only its hot, white, and dense core is left, creating a white dwarf. After the white dwarf dies, it becomes a black dwarf. If it were a bigger star than our sun, after the main sequence it will become a super red giant. After the super red giant has run out of He, it will become a supernova. It can either become a neutron star or a black hole after. A neutron star and a black hole is both very dense.
Big bang led to the structure of the universe - The big bang is said to have been extremely hot at the beginning. It expanded extremely condensed material in a ball called a singularity. The singularity started expanding which became our universe. People believed the bid bang had radiation. In 1965 scientists discovered left over energy from the big bang. The energy existed as background radiation (CMB) which is evidence that the big bang happened.
Earth science
The four global spheres -
lithosphere (solid earth)
atmosphere (in the air)
hydrosphere (water)
biosphere (life)
Interactions and relations between the spheres -
the biosphere gives material to the lithosphere to be decayed.
the lithosphere gives a habitat to life in the biosphere.
the atmosphere affects the lithosphere with things like wind to move dirt, sand etc to different locations over time which changes the topography of the land.
the atmosphere causes combustion, respiration and photosynthesis in the biosphere.
the hydrosphere can be a habitat for the biosphere.
the hydrosphere and atmosphere can create rain.
The carbon cycle - 
Greenhouse effect - the greenhouse effect is trapping some of the sun’s radiation naturally to keep the planet at a moderate temperature.
Enhanced greenhouse effect - the enhanced greenhouse effect is where the greenhouse gases in our atmosphere keeps too much of the sun’s heat and causes global warming. This extra gas is created by humans.

Long term carbon sinks - long term carbon sinks are carbon reservoirs that store carbon for a significantly longer than a human life span. Carbon will be slowly released back into the atmosphere/surface environment over many years. Examples of carbon sinks include: the ocean (hydrosphere), trees (biosphere), fossil fuels (lithosphere). These carbon sinks are being impacted by humans. Trees are being wiped out (deforestation) so less trees are able to store carbon. Fossil fuels are being used so we’re releasing more carbon in the air. These are negative impacts as we are filling the atmosphere with carbon which causes global warming because it keeps in more heat/solar radiation from the sun.
Chemistry
Atoms and the Periodic Table
An atom is a small unit of matter.
An element is a pure substance.
Subatomic particles:
Mass | Charge | |
|---|---|---|
Proton | 1 | positive |
Neutron | 1 | neutral |
Electron | 0 | negative |
The atomic number represents the number of protons in a nucleus.
The number of protons and neutrons control the atomic mass of an atom. It is also the weight of the nucleus.
The atomic number is a whole number and the atomic mass is a number with a decimal.

To calculate the number of neutrons in the atom: atomic mass - atomic number.
E.g Carbon, 12.011 (at. mass) - 6 (at. number) = 6
The atomic mass is not always a whole number because it is the average of its isotopes.
Electrons tell us about the chemical reactions, the atomic mass tell us how heavy the nucleus is.
Isotopes are the many forms of the same element, containing the same number of protons but different numbers of neutrons.
The atomic number is a whole number, not like the atomic mass because the number is the number of protons and they are countable.
Examples of the atomic number, atomic mass, # of neutrons, electrons and protons of an element.
Element name | Element symbol | Atomic number | Atomic mass | # of protons | # of electrons | # of neutrons |
|---|---|---|---|---|---|---|
Fluorine | F | 9 | 19 | 9 | 9 | 10 |
Potassium | K | 19 | 39 | 19 | 19 | 20 |
Aluminium | Al | 13 | 27 | 13 | 13 | 14 |
Strontium | Sr | 38 | 88 | 38 | 38 | 50 |
Atomic structure and the Periodic Table
The periodic table as we know hasn’t looked like it does now. It has developed over time as we have learnt more about chemistry.
Some early periodic tables:


The Bohr model is the description of the structure of atoms. It consists of a small nucleus (no protons/neutrons, one nucleus as a whole), with electrons in orbits, placed on the electron shells.

On the periodic table there are periods and groups.
periods - periods are rows on the the periodic table. The numbers for periods are on the left side of the table. Period numbers represent the number of electron shells.
groups - groups are columns on the periodic table. The numbers for groups are at the top of the table. Group numbers represent the number of electrons on the valence shell.

group 1 are alkali metals.
group 2 are alkaline Earth metals.
group 3 to group 12 are Transition metals.
group 17 are halogens.
group 18 are noble gases.
Electron configuration
Electron configuration refers to the arrangement of electrons - how many are in each shell.
Rules for determining electron configuration:
determine the period the element is in. This gives the number of occupied electron shells.
determine the group the element is in. This gives the number of valence electrons.
all shells, except the outer one, will have the, maximum number possible.
Examples:



The purpose for knowing the electron configuration of an element gives us information about the chemistry or chemical reactions it may be in.
Ions
The term ‘neutral atom’ means that an atom has the same amount of electrons and protons and therefore is neither positive or negative.

** Metals form anions.
Comparison of the number of protons in an atom to the number of electrons in an atom - the number of protons is the same as the number of electrons in an atom.
If an atom:
gains an electron - the charge becomes negative.
loses an electron - the charge becomes positive.
Charge on ion | Name of type of ion |
|---|---|
Positive | Cations |
Negative | Anions |
The difference between an atom and an ion is that an atom is neutral, whereas, an ion has either lost or gained one or more electrons so they are not neutral.
The atomic structure of all ions are the same because they will always have full valence shells.
If the atom has less electrons on the valence shell, it will lose electrons. If an atom has more electrons on the valence shell, it will gain more electrons. (Remember: the group they are in represents the number of electrons they have.)

Elements in group 18 do not form ions because their valence shells are full and do not need to lose or gain electrons.
Elements in group 2 all form an ion with a charge
Metals
The atomic structure of metals explains the formation of metal ions and the reactivity of metals.
Groups 1 and 2 form cations by losing 1 or 2 electrons.
Group 1 is more reactive than group 2 because it only has to lose 1 electron while group 2 has to lose 2 electrons.
Alkali metals are more reactive, particularly with water.
As you go down the groups, metals become more reactive. They have more electrons in the shells and may need to lose more or gain more electrons.
Four common properties of metals
Property | Description |
|---|---|
Lustrous | shiny |
Conductive | able to conduct electricity and heat |
Malleable | Can change into a new shape (like foil) |
Ductile | Can be drawn into a wire |
3 important groups of metals:
Periodic table group(s) | Group name | Properties |
|---|---|---|
1 | alkali metals | low melting points, soft and highly reactive |
2 | alkaline earth metals | low melting points, relatively soft, and very reactive |
3-12 | transition metals | a small number of transition metals are magnetic. Gold and copper are the only metals that are not silvery in colour. Many form coloured compounds many form more than one compound with a non-metal, such as chlorine |
A chemical reaction is when reactants are converted to substances.
Reactivity refers to how easily a substance reacts with other substances (the ability to go under chemical change/reaction).
(Reactivity) How easily they lose electrons when they come in contact with other substances determines the reactivity of a metal. The more electron shells, the more reactive it is.
Metallic bonding
Bonding between metal and metal.
Metallic bonding has a structure that features many ions in a "sea" of electrons. They are cations, arranged geometrically, surround by delocalised elections.
Property | Elaboration | Picture |
|---|---|---|
Thermal and electrical conductivity | The electrons move easily through the solid. These moving electrons are able to transfer heat and electricity. There is electrostatic energy around the ions. |
|
Malleability and ductility | These cations can slide past each other but the electrostatic forces between the cations and the electrons stop the metal from breaking. |
|
Lustrous | The delocalised electrons in the surface of a metal reflect light and cause it to be lustrous. |
|
Melting/boiling points | Lots of heat and energy is needed to melt a solid metal and boil a liquid metal because of the very strong electrostatic forces. |
|
An alloy is a mixture of two or more metals. They may contain non-metals. 
Non-metals
Non-metals on the periodic table:

Non-metals form anions except noble gases, hydrogen and calcium.
The reactivity of non-metals decrease as the groups descend because the valence shells are closer to the nucleus which is positive. (A stronger attraction to electrons that are closer to the nucleus, therefore increasing reactivity).
Metalloids have properties of metals and non-metals.
The two important groups of non-metals:
Periodic table group(s) | Group name | Properties |
|---|---|---|
17 | halogens | all gases are diatomic (two atoms to each molecule), react with metals to form salts, all have 7 valence electrons, range from gases to solids. |
18 | noble gases | unreactive, don’t form compounds, all gases. |
Noble gases are described as ‘inert’ (unreactive), this is because their valence shells are full.
‘Semi-conductors’ only conduct electricity under certain conditions.
Metalloids:

Similarities of metalloids, metals, and non-metals:
Metals | Conduct electricity, lustrous |
|---|---|
Non-metals | Not malleable or ductile |
Covalent bonding
Bonds between two non-metals.
Low melting points
covalent bonds within molecules are strong BUT there are very weak forces between molecules that don’t require much energy to break or separate them
Two non-metal atoms each share one or more of the unpaired electrons with each other to create covalent bonds that contain two electrons (one from each atom) so that all atoms have full valence shells.
Example: 
Covalent molecule (ammonia (NH3)) example:

Ionic bonding
Bonds between non-metals and metals.
Properties of ionic compounds:
non-conductive as a solid.
conductive as a liquid because the anions and cations become free to move.
hard and brittle.

Properties of ionic substances
Property | Explanation |
|---|---|
No thermal and electrical conductivity when solid, but is when liquid | Ions in the solid can’t move past each other to conduct electricity and heat. They are locked in. |
High melting/boiling point | The electrostatic attraction between the cations and anions is to strong. Lots of energy is needed to separate. |
Hard and brittle | When you hit an ionic solid, you move the particles around. The anions end up next to each other, as do the cations. The similar ions repel each other and break the solid. |
Ionic compounds swap and drop method:
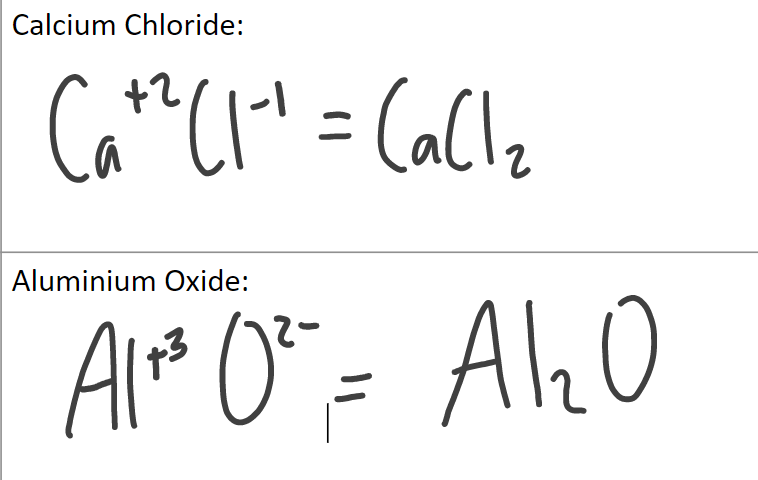
Only bracket polyatomic ions if you swap to make 2 or more
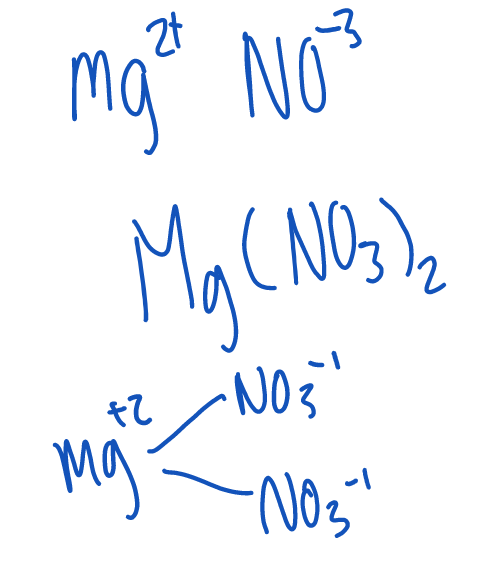
Polyatomic atoms (molecules of multiple atoms)

Ionic substances typically have much higher melting and boiling points than covalent substances because ionic bonds (electrostatic bonds) take a lot of energy to break.
Examples of ionic compounds

Balanced chemical reactions
Remember: the number of atoms of an element on one side of the equation MUST equal the number of atoms of that element on the other side of the equation.
You CANNOT change any subscript numbers. Changing the subscript number changes the formula of the compound.
You CAN change any large numbers at the start of a compound formula.
Advice for balancing equations:
draw a table to count up the number of each type of atom/ion on each side of the equation.
start with elements that only appear in ONE compound on either side.
balance one element at a time. Once one is balanced, move on the next. You may need to revisit earlier elements multiple times.
Synthesis and decomposition
Synthesis reactions - the building up of compounds by combining simple substances, normally elements: A+B=AB
Example: Calcium (2Ca) + Oxygen (O2) → Calcium oxide (2CaO)
Decomposition reactions are the breakdown of compounds into simpler substances, either elements or more simple compounds. These reactions often require energy in the form of electricity or heat. Electrolytic decomposition is the breakdown of a compound as a result of an electric current passing through a solution. In decomposition reactions, only a single substance can be represented on the left side of the arrow.
States of matter:
solid - (s)
liquid - (l)
gas - (g)
aqueous - (aq)
Acids and bases
An acid is a substance that releases hydrogen ions (+) and forms salts by combining with certain metals.
An acid is a substance that releases hydroxide ions (-) and can neutralise acids.
Dissociation: to break up into ions.
Example of dissociation: H2SO4 -in water→ 2H + SO4-2
The strength of an acid/base refers to a measure of how easily an acid/base will dissociate (break up into ions). It is measured as pH 1-14; a pH of 1 is a strong acid and a pH of 14 is a strong base. (How many particles of THAT substance)
Concentration is how much acid/base is in the solution. (How many particles)
Examples

Dissociation tells the difference between a strong acid and a weak acid. If an acid dissociates 100% into ions, it is a strong acid. If the acid does not dissociate 100%, it is a weak acid.
pH is a measure of concentration of H+ ions in a solution, lower pH means a high concentration of H+. In what case might a strong acid have a higher pH (less H+) than a weak acid? A highly dilute strong acid can have a higher pH than a weak acid of sufficient concentration. (Diluting it lowers the pH)
Acid reactions
Acid base reactions are called neutralisation reactions because when they are mixed, they neutralise each other, becoming neither acidic or basic.
You must react an acid with carbonate to produce carbon dioxide gas.
Rates of chemical reactions
For a chemical reaction to occur, particles must collide.
Not all collisions between reactant molecules/ions result in a chemical reaction because for a reaction to occur, the particles must be in the right orientation, have the right amount of energy and actually collide.
Collision theory - this theory describes how and why substances react with each other. Collision theory provides three requirements for chemical reactions to occur;
correct orientation
reactants must collide
sufficient energy to overcome the activation energy required
Rate of reaction - refers to the change in mass of the reactants and products in a given amount of time (usually seconds.
Increasing the rate of reaction can be done with 5 things:
surface area - increasing the surface area by breaking the reactants into smaller pieces exposes the reactants to more collisions.
temperature - increasing the temperature increases the kinetic energy so that particles will collide more often and increasing the rate of reaction.
concentration - increasing concentration (amount of particles) the particles are more likely to collide. Using more concentrated acid will increase the chemical reaction with a carbonate, producing more CO2.
stirring - stirring keeps reactants moving and they are more likely to collide.
catalyst - a catalyst is a chemical that increases the rate of reaction but does not get used in the chemical reaction. A catalyst reduces the activation energy needed to start the chemical reaction.
Evolution
Natural selection
Natural selection:
natural selection is when organisms best suited to their environment survive and reproduce more offspring than those are not suited. Variation, selection, inheritance, time.
Fitness:
fitness refers to how many offspring an organism produces. An organism with greater fitness produces more off spring.
Survival of the fitness:
continued existence of a type of organism because that type continues to reproduce.
Process of natural selection:
variation:
gene | a gene is a section of DNA that codes for a certain protein/characteristic |
|---|---|
allele | if a gene codes for a particular characteristic, then the alleles are the alternatives for that gene. For example, if the gene is for eye colour, the alleles are alternatives (green, blue, brown and other variations). |
gene pool | all the genes and their alleles for a population. |
allele frequency | the amount of an allele for a population. |
mutations | random change in the DNA that may delete, add or rearrange genetic material. Maybe obvious or not. If it is a good mutation, then it will be passed onto the offspring. |
selection:

Selection pressure | an environmental factor that affects an organisms ability to survive. (e.g height of vegetation or the predation |
|---|---|
Effect of selection pressure on allele frequency | according to source 1, in the mice coat colour, there are two alleles: pink and grey. before selection, the allele frequency is 6 pink to 3 grey. After selection the allele frequency is 2 pink to 7 grey. selection pressures change the allele frequency. |
inheritance:
genetic influence | passing of genes/alleles onto offspring. |
|---|---|
effect of genetic inheritance on variation | the major source of variation. |
effect of genetic inherit | if some organisms survive and mate then those alleles will be passed on and increase allele frequency |
time:
Individuals vs populations | individuals do not evolve, populations do. |
|---|---|
Role of time in natural selection and evolution | natural selection can occur in a relatively small number of generations, but evolution is natural selection over millions of years. |
Examples of natural selection
Examples:
Cacti are a group of plants that typically live in very hot and dry locations. Their spines are actually highly modified leaves (thicker and curled up) and are shaped that way to help prevent loss of water by transpiration.
variation: cacti with different six leaves from large to very thin/spine like.
selection pressure: temperature and the amount of water.
time: over time the population changes to be higher frequency in the alleles for small leaves and low frequency for large leaves.
whales evolved from earlier land-dwelling mammals that started spending more time back in the ocean and now have limbs (tail and fins) that are suited to efficient movement through the water.
variation: some of the land-dwelling whales with fin like limbs (probably a mutation).
selection pressure: water environment (select some to survive).
inheritance: the ones with finds survived and reproduced so over time the whale population had fins.
time: over time the population changes to be a higher frequency in the alleles for fins and low frequency for limbs.
Speciation
new species are formed by speciation.
speciation: formation of a new and distinct species in the course of evolution.
the continents that are now separated were once joined together known as Pangea.
isolation is the first step in the formation of new species.
a place like Australia has a barrier of se meaning each gene pool was now exposed to different conditions like climate, food and competition.
those best adapted survived through process of natural selection.
gene pool changed and organisms became more and more different from those left behind.
those differences became so great that isolated populations are unable to interbreed and a new species is formed.
if environment changes due to human or natural influence they may become extinct like the Tasmanian tiger.
the variety of animals we have in our world is due to speciation.
Isolation:
environmental barriers
geographical
reproduction times
Human biology
DNA
DNA:
carries the code for protein and characteristics.
deoxyribonucleic acid.
is made of nucleotides
→ nucleotides are composed of a sugar molecule, phosphate, and nitrogenous base.

double helix or twisted-ladder-shaped.
Nitrogenous bases:
adenine.
thymine.
cytosine.
guanine.
Gene:
a short section of your DNA. Carry instructions for cells to make proteins.
Chromosomes:
condensed, coiled DNA into thread-like structures. DNA coils around histones.
our chromosomes come from our parents.
there are 23 pairs of chromosomes in each human cell.
the first 22 pairs of chromosomes are called autosomes.
the twenty-third pair is called the sex chromosome. Females have (XX), males have (XY).
human sex cells are called gametes. There are 23 chromosomes in each gamete. It is this number because at fertilisation, the zygote only has 46.
23 chromosomes are called haploids.
Protein:
the order and the number of bases in the gene determines the size and shape of the protein.
the size and shape of the protein determines its function.
PROTEINS → CELLS → TISSUES → ORGANS
DNA replication:
An enzyme breaks the H bonds between the base pairs
the DNA is now exposed as 2 single strands
Another enzyme attaches new nucleotides to the 2 exposed strands
Each single strand now becomes a double strand and the DNA has been replicated/doubled
Karyotypes
A karyotype is a photo of a cell set of chromosomes that have been ordered inter their parts.
They are used to check if a person’s chromosomes are correct.

there are 23 pairs of chromosomes in each human cell.
the first 22 pairs of chromosomes are called autosomes.
to check that each chromosome is complete, check that there are no added/missing parts.
Protein

Transcription
RNA polymerase will connect complimentary bases to the DNA in the nucleus.
the bases bond together and for a single stranded mRNA.
mRNA goes out the nucleus and attaches to a ribosome.


Translation
in the cytoplasm, there are tRNA molecules (they carry amino acids on them).
the mRNA directs the tRNA which amino acids to bring.
the tRNA looks for its complimentary base and transfers their amino acid.
when tRNA is bringing in the amino acids it reads the bases in threes (codons).
the amino acids start building up and eventually a STOP codon is read and the chain stops.
once the chain stops, the protein is finished.
DNA/RNA
DNA has a double helix structure.
DNA replicates itself.
DNA has deoxyribose sugar.
DNA have the bases: adenine, thymine, cytosine, guanine.
RNA is single stranded.
RNA cannot replicate itself.
RNA has ribose sugar.
RNA have the bases: adenine, uracil, cytosine, guanine.
Mitosis
Mitosis is the division of the genetic material into two new identical daughter cells.
The cell cycle:

Cells in our body are consistently dividing to make new cells.
Cells communicate with each other using chemicals which signals when the cells should start and stop dividing.
Sometimes when the cell has abnormalities, they don’t know when to stop dividing.
As the cells grow and divide, they form an abnormal growth called a tumour.
G1 - new cells grow to full size.
S - the genetic material doubles or replicates.
G2 - the cell getting ready to divide.
Cell division:
mitosis: the division of the nucleus.
cytokinesis: division of the cytoplasm.
Mitosis process:

interphase
DNA in the cell is copied in preparation for cell division.
prophase
chromosomes condense into X shaped structures, composed of two sister chromatids containing identical genetic information.
chromosomes move to the equator in a line.
metaphase
chromosomes completely lined up at the equator, end-to-end.
the centrioles are now at opposite poles of the cell.
mitotic spindle fibres extending from the poles attaching to sister chromatids.
anaphase
the sister chromatids are pulled apart by mitotic spindles.
each chromatid at each pole.
telophase + cytokinesis
at each end of the poles, chromosomes are gathered together.
membrane forms around each set of chromosomes, creating two new nuclei.
the middle pinches, divides the cell into two daughter cells.
(cytokinesis) completion of cell division.
Meiosis
Meiosis is the process in formation of gametes.
It is 2x the process of mitosis, creates four daughter cells, not genetically identical.
interphase I
DNA in the cell is copied, making two sets of chromosomes.
each chromosome is duplicated, forming 2 identical chromatids held at the centromere.
prophase I
chromosomes condense into X shaped structures.
they pair up.
membrane around nucleus dissolves, releasing chromosomes.
[DELETED SECTION]
metaphase I
chromosome pairs line up at the equator.
genetic crossover occurs.
the centrioles are now at opposite poles of the cell.
meiotic spindle fibres extending from the poles attaching to sister chromatids.
anaphase I
cells divide to form gametes.
homologous chromosomes (from each parents), pulled to opposite ends by spindles.
telophase I
nuclear membrane develops around each set of of homologous chromosomes.
forms two haploid daughter cells with half as many chromosomes in the original cell.
cytokinesis I
cell divides into two daughter cells.
each cell has half as many chromosomes as parent cell.
SECOND ROUND
prophase II
membrane in each daughter cell dissolves away, releasing chromosomes.
meiotic spindle fibres form again.
metaphase II
chromosomes line up at equator and split.
no genetic crossover or pairs.
anaphase II
sister chromatids are divided and pulled to each pole.
four haploid daughter cells are created, each with different genetic makeup.
telophase II
separated sister chromatids reach opposite ends of the poles.
nuclear membrane develops around each set.
each daughter cell has one set of chromosomes.
cytokinesis II
the two daughter cells created during telophase II physically divide into four haploid cells.
each cell is distinct.
Important terms:
haploid - a cell with a single set of unpaired chromosomes.
diploid - a cell with two complete sets of chromosomes (homologous).
somatic cell - any cell of a living organism (not gametes).
sex cell - organism’s reproductive cells.
Apoptosis
Apoptosis is the process of programmed cell death.
It is used to eliminate unwanted cells.
Relation to cancer
apoptosis is when cells are programmed to die at a certain time.
in cancer, apoptotic control is lost, meaning cancer cells are able to survive longer.
Alleles
Alleles are variations/alternatives of a gene.
Alleles, genes, and chromosomes:
an allele is a variant form of a gene.
a gene is contained in a chromosome.
Autosomes and sex cells
autosomes are the first 22 chromosomes.
gametes are the 23rd pair.
in an autosomal pedigree, both parents can be carriers.
in a x-linked pedigree, only the mother can be a carrier.
Incomplete and co-dominance
Incomplete dominance:
one allele is not dominant over another.
for an allele to be expressed, both has to be homozygous.
for example - in snapdragon flowers, RR is red, rr is white, Rr, is pink.
Co-dominance:
both colours are dominant.
for example - in chickens, BB is black, WW is white, BW is black/white speckled.
Phenotype/Genotype
A phenotype is the physical appearance.
A genotype is the written symbol of that allele.
Haemophilia and red-green colour blindness
Haemophilia
A father who has haemophilia passes his only X chromosome down to all of his daughters, so they will always get his haemophilia allele and be heterozygous (carriers).
A father passes down his Y chromosome to his sons; thus, he cannot pass down a haemophilia allele to them.
Physics
Distance/Displacement
Distance - how far an object travels in a set time.
Displacement - the final distance and direction from the starting point of an object.

Quantities:
Scalar - measures magnitude in numbers and units, e.g. 9km, 60kg, 50 mins.
Vector - measures magnitude with numbers, units AND direction, e.g. 9km North, 600 Newtons down.

Example questions:
an object moves 14 m North and the 14 m south. What distance has it covered? What is its displacement?
distance is 28m.
displaced 0m.
a person runs 50 m north, the 20 m south and the 30 m west. What is the total distance covered? What is the person's displacement?
distance is 100m.
displacement is 42.42m North.
Explanation:

Speed/Velocity
Speed (scalar) - distance travelled per unit of time. m/s or ms^-1.
Velocity (vector) - distance travelled per unit of time with direction. m/s or ms^-1 with direction.
Formulae:
distance = speed*time
speed = distance/time
time = distance/speed

Example questions:
If a car travels 400 m in 20 seconds, how fast is it going?
Speed = 400/20 = 20 m/s.
How much time will it take for a bug to travel 5 metres across the floor if it is travelling at 0.5 m/s?
Time = 5/0.5 = 10s.
How far can you get away from your little brother with the squirt gun filled with paint if you travel at 3 m/s and you have 15 seconds before he sees you?
Distance = 3*15 = 45m
Acceleration
If you drop a bowling ball and a feather from the same height, which will hit the ground first?
the bowling ball will hit the ground first because the feather has more air resistance.
What if it was on the moon?
both would land at the same time because the moon does not have air.

Formulae:
(a - acceleration, v - final velocity, u - initial speed)

Example questions:

Graphing motion
Example:
Bruce starts walking from his home eastwards towards school.
It takes him two minutes (120 seconds) to cover 180 m.
He then gets a call to meet his friend at the shops, so after a 5 second pause, he turns around and walks 400 m in the opposite direction, which takes him 200 seconds.
They are both in the shop for 1 minute (60 seconds) before leaving again.

Newton’s first law
An object remains at rest or in constant motion in a straight line unless acted on by a net unbalanced force.
What is inertia?
inertia is the need for an object to keep on doing what it was already doing.
The larger the mass of an object, the greater it’s inertia

Force on an object:


Object | Balanced/Unbalanced | Direction of Movement |
A | Unbalanced | East |
B | Unbalanced | East |
C | unbalanced | South |
D | Balanced | Stationary |
E | Unbalanced | South East |
F | Balanced | Stationary |
G | Unbalanced | South |
Newton’s second law
Newton’s second law states that acceleration is directly proportional to force and indirectly related to mass.
Formulae:
force = mass*acceleration.
mass = force/acceleration.
acceleration = force/mass.

What’s the difference between mass and weight?
mass is the amount of matter measured in kilograms.
weight is the force of gravity. Mass*gravity.
Newton’s third law
Whenever two object interact, they exert equal and opposite forces on each other.
Examples:
Scenario | Action | Reaction |
A tennis racquet hitting a ball. | Tennis racquet pushes on the ball | Ball pushes back on the racquet |
Your foot and the floor when you are walking. | Foot pushes on the floor | Floor pushes back on foot |
Your hand and a brick wall attached to the ground when you push on it. | Hand pushes on wall | Wall pushes on hand |
A rocket and its exhaust gas during lift off. | The rocket pushes on the gas | The gas pushes on the rocket |
A sprinter pushing off from the start blocks. | Foot pushes off start block | Start block pushes on foot |
The Moon in orbit around the Earth. | Earth is pulling on moon | Moon is pulling on earth |
Weight and mass
Mass - measure of how much matter there is in an object (Kg).
Weight - force oh how much gravity pulling on an object (N).
Formulae:
w = m*g
m = w/g
g = w/m

Examples:
Question: | Answer: |
What is the weight of a small car if it's mass is 20,000g? | W = 20kg * 9.8
= 196 N |
What is the mass of an item if it's weight is 65 N on Earth? | M = w/g M = 65N/9.8
M = 6.63kg |
If a person weighs 78N on Earth, what is their mass? | M = 78/9.8
M = 7.95kg |
What is Jupiter's gravitational pull if the same person from the scenario above weighs 197.3N on Jupiter? | G = w/m G = 197.3/7.95 G = 24.79m/s |
Mercury has 0.37 g's of Earth. What is Mercury's gravitational pull? | G=9.8*0.37 = 3.63 ms-2 |
What is the weight of a person with a mass of 45kg on the moon, if the moon's gravitational pull is 1.6 m/s2? | W=mg
45*1.6 = 72 N |
Work and energy
Energy - the ability to do work (J)/(kJ).
Main types of energy:
kinetic - energy associated with movement.
potential - energy associated with position (stored energy).
Law of conservation of energy:
energy cannot be created nor destroyed.
the total energy of a system remains constant.
Example:
Consider two identical, large boxes placed on the floor. Both of them are heavy and are difficult for you to move.
You are challenged to EITHER move both boxes 1 m each, OR to move just one box 10 m.
Intuitively, which challenge would you accept? | Two boxes x2 |
Explain your choice. (You don't need to use physics principals yet). | 2m is less than 10m so you need less energy |
Which of the two challenges would make you the most 'tired'? In other words, which challenge would require you to expend the most energy? | The second one |
Moving the box requires you to expend energy? Where does that energy go? | Kinetic energy Thermal energy Sound energy |
What do you need to apply to the boxes to actually get them to move at all? (This is a physics term). | Force |
Work - measure of the amount of energy transferred into moving or rearranging an object.
The relationship between force and work - they are directly proportionate to one another, and as distance increases, work also increases.
The relationship between distance and work - they are directly proportionate to one another, and as distance increases, work also increases.

Examples:
How much work is done if a force of 200 N moves an object 6 m? | W=fd 200*6 = 1200J |
How far does an object move is 500 J of work is done on it by a constant force of 75 N? | D=w/f 500*75 = 6.67m |
How much work is done by a student who pushes a backpack with a force of 20 N for 5 m along the hallway? | W = f*d W = 20*5 = 100 j |
A soccer player kicks a ball with a force of 100 N for 0.1 m. How much work does the player do on the ball? | W = fd W = 100*0.1 = 100 j |
A cyclist travels 20 m and does 3000 J of work on their bike. What force did the cyclist apply to the bike pedals? | F = w/d F= 3000/20 = 150 N |
Gravitational potential energy
Formulae:
G = 9.8
M = GPE/(g*h)
GPE = m*g*h
H = GPE/(m*g)
G = GPE/(m*h)
Examples:
Consider a person at the gym lifting weights from the floor.
Mass (kg) | Exercise | Mass lifted to height of (m) |
30 |
| 0.75 |
30 |
| 2 |
60 |
| 2 |
Exercise | Force (N) | Work (J) |
1 | 294 | 220.5 |
2 | 294 | 588 |
3 | 588 | 1176 |
A person with a mass of 60 kg climbs up a ladder that is 5 m high. How much work does the person do on their body? | M= 60 kg H = 5 m G= 9.8 m/s/s GPE = m. g. h 60x9.8x5 = 2940 J 2.94 kJ |
A roller coaster with a mass of 500 kg starts at a height of 10 m. As it is winched up the start ramp, 196 kJ of work is done on it by the motor. What height does the rollercoaster reach? | M = 500 kg W = 196 000 J H =?
GPE/ m x g = h 196 000/ 500 x 9.8 = 40 m
H= 40 -10 m = 30 m |
Kinetic energy
What type of energy does a barbell have at the top of the lift? | Gravitational potential |
What type of energy does a barbell have just before it hits the ground? | Kinetic energy |
Qualitatively describe the change in the barbells energy up until it hits the ground. | The gravitational potential energy is transforming into kinetic energy |
Can you use this to relate kinetic and gravitational potential energy. | Total energy= GPE + KE At the top total energy = GPE ( KE =0) At the bottom total energy = KE (GPE = 0) In the middle total energy = KE + GPE |
Define the formula for Kinetic Energy. | KE = 1/2 mv2 |
A 100 kg cyclist on a bike is travelling at 15 m/s. Calculate the total kinetic energy of the cyclist and bike.
| KE = 1/2 mv2 KE = 1/2 x 100 x 15 x 15 = 11,250 J |
A force of 900 N acts on a car with a mass of 1 tonne over 0.5 km.
|
V = square root of 2 KE/m = square root of 2 x 450000/1000 = 30 m/s |
Power stations: thermal energy
Steps involved in the generation of electricity in a power station:
burn coal/gas to release heat.
heat turns water in boiler into steam.
steam turns turbine to generate electricity.
steam condensed back into water, and fed back into boiler

Temperature:
a measure of the average kinetic of the particles in a substance.
Heat:
heat is an energy type that transfers from an object of higher temperature to an object of lower temperature.
Which has more energy, an ice-burg or a cup of coffee?
ice-burg has higher energy because it has more particles.
Which has a higher temperature?
the cup of coffee has a higher temperature because the particles are moving faster.
Specific heat capacity:
Consider two saucepans of water sitting on a stove. One is large and contains 5 L of water, the other small and contains only 1 L. The stove applies heat to the saucepans at the same rate.
Which saucepan would take longer to start boiling? | The larger one. |
Why is this? | The larger one has more particles so more total energy is required to increase by the same amount. |
What does this mean about the relationship between heat transfer and mass? | As mass increases, the heat required also increases. |
Formula:
Q = mcΔT
Units used:
J/kgoC
Example:
Water is fed into the boiler of a thermal power station at a temperature of 14 oC. A particular boiler has a volume of 9000 L of water. Calculate the energy required to start boiling all of the water in the boiler. The specific heat capacity of water is 4180 J/kg oC | Q = mcΔT
m = 9000 L = 9000 kg c = 4180 J/kg oC ΔT = 100 - 14 = 86 oC
Q = 9000 x 4180 x 86 = 3,235,320,000 J |
1155 J of energy is required to heat 50 g of copper metal from 20 oC to 80 oC. Calculate the specific heat capacity of copper. | Q = mcΔT
Q = 1155 J m = 50 g = 0.05 kg ΔT = 80 - 20 = 60 oC
c = Q / (mΔT) = 1155 / (0.05 x 60) = 385 J/kg oC |