Practical: Experiment to Investigate Osmosis
Involves putting potato cylinders into different concentrations of sucrose solution to see what effect different water concentrations have on them
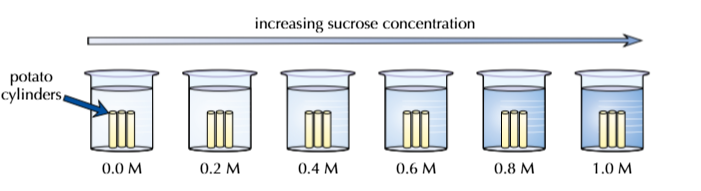
Prepare sucrose solutions of different concentrations, ranging from pure water to a very concentrated sucrose solution
Use a cork borer to cut a potato into the same sized pieces (the pieces should be about 1cm in diameter and preferably from the same potato)
Divide the cylinders into groups of three and use a mass balance to measure the mass of each group
Place one group in each solution
‘M’ is a unit of concentration (also written as mol dm-3). The solution with a concentration of 0.0M is pure water
Leave the cylinders in the solution for at least 40 minutes (making sure that they all get the same amount of time)
Remove the cylinders and pat dry gently with a paper towel. This removes excess water from the surface of the cylinders, so you get a more accurate measurement of their final masses
Weigh each group again and record your results
The independent variable is the concentration of sucrose solution, and the control variables is the volume of solution, the size of the potato cylinders, the type of potatoes used and the amount of drying. Otherwise the results won’t be valid
Interpreting the Results
You need to calculate the percentage change in their mass for each group of cylinders before and after their time in the sucrose
percentage change = final mass - initial mass / initial mass x 100
Then you can plot a graph and analyse your results
Above the x-axis = the water concentration of the sucrose solutions is higher than in the cylinders - the cylinders gain mass as water is drawn in by osmosis
No change in mass = the fluid inside the cylinders and the sucrose solution are isotonic - they have the same water concentration
Below the x-axis = the water concentration of the sucrose solutions is lower than in the cylinders - this causes the cylinders to lose water so their mass decreases