IB Biology Unit 2: Biochemistry
A: Water
A1.1.1 Water as the medium for life
Life needs chemical reactions to take place to gain energy, grow, and get rid of waste.
Water is a liquid medium that allows the chemistry of life to take place.
The first cells on the planet probably originated in water (hydrothermal vents)
A1.1.2 Hydrogen bonds as a consequence of the polar covalent bonds within water molecules.
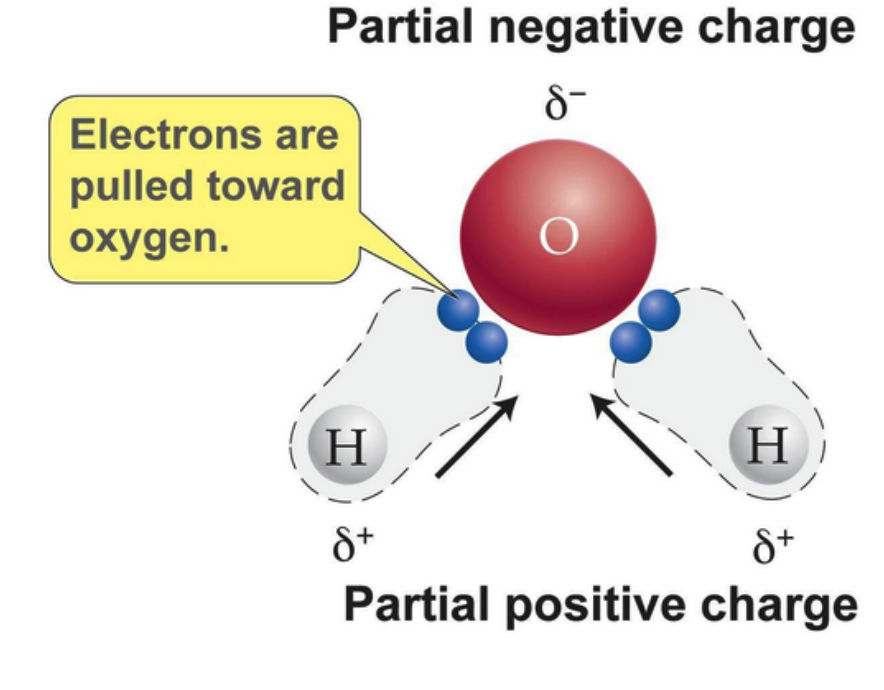
Water (H2O) is made up of two hydrogen atoms covalently bound to an oxygen atom
While this bonding involves the sharing of electrons, they are not shared equally
The number of protons in each atom is different; oxygen atoms have 8, whilst hydrogen atoms have just 1
Having more protons, the oxygen atoms attract the electrons more strongly
Thus, the oxygen atom becomes slightly negative and the hydrogen atoms become slightly positive (i.e., the oxygen has a higher electronegativity)
Covalently bonded molecules that have a slight potential charge are said to be polar
A1.1.3 Cohesion of water molecules due to hydrogen bonding and consequences for organisms.
Cohesion:
This property occurs as a result of the polarity of a water molecule and its ability to form hydrogen bonds
Although hydrogen bonds are weak, the large number of bonds present (each water molecule bonds to four others in a tetrahedral arrangement) gives cohesive forces great strength
Water molecules are strongly cohesive (they tend to stick to one another)
Water droplets form because the cohesive forces are trying to pull the water into the smallest possible volume, a sphere
A1.1.4 Adhesion of water to materials that are polar or charged and impacts for organisms
Result of the polarity of a water molecule and its ability to form hydrogen bonds
Water molecules tend to stick to other water molecules that are charged or polar for similar reasons that they stick to each other
Single hydrogen bonds are weak but a large number of bonds give adhesive forces a lot of strength
Capillary action
combination of adhesive forces that cause water to bond to a surface
Helpful in the movement of water during transpiration and when you drink using a straw
A1.1.5 Solvent properties of water linked to its role as a medium for metabolism and for transport in plants and animals
Properties of water molecules
Solvent
Water can dissolve many organic and inorganic substances that have charged or polar regions
Water is often wrongly referred to as being the universal solvent, it is good for many substances though
Metabolic reactions
Happen most readily in solutions of water - water in cells dissolves the reactants/substrates
Cells are mostly water so diffusion into and out of them happens most easily if the substance is in solution
Soluble substances such as sucrose can easily be transported around the plant in the phloem. Once dissolved in the water of the phloem, the sucrose can be moved to where it is needed by mass flow
Hydrophilic
Substances that are chemically attracted to water
All substances that dissolve in water are hydrophilic, including polar molecules like glucose, and particles with positive or negative charges like sodium and chloride ions
Substances that water adheres to are also hydrophilic
Hydrophobic
Substances that are insoluble in water
Molecules are hydrophobic if they DON’T have negative or positive charges and are nonpolar
All lipids are hydrophobic
Hydrophobic molecules dissolve in other solvents like propanone
Transport of molecules in the blood
Blood plasma consists of mainly of water, plus dissolved substances which it transports
Glucose
Polar molecule=freely soluble
Carried by the blood plasma
Amino acids
Positive and negative charges = soluble in water
Carried by the blood plasma
Oxygen
Non-polar molecule
Barely soluble
Water becomes saturated with oxygen at relatively low concentrations
As temperature increases, the solubility decreases
Hemoglobin in red blood cells carry the majority of oxygen
Fats
Insoluble
Non-polar
Carried in blood inside lipoprotein complexes
Cholesterol
Insoluble
Lipoprotein complex
Fats also need to travel in the blood but they’re nonpolar
Sodium chloride
Freely soluble in water
carried in the blood plasma
A1.1.6 Physical properties of water and the consequences for animals in aquatic habitat
Thermal
Water has a high specific heat capacity
Water needs a lot of heat to warm up and cools down slowly because it can store a lot of energy.
Water has a high boiling point and latent heat of vaporization
It takes a lot of heat to boil and turn into steam
Latent heat is the extra energy needed to change from a liquid to a gas without changing its temperature
Water has a high heat of fusion
It takes a lot of heat to melt ice into water
Water as a coolant
High temperatures damage tissues and denature proteins
enzymes don’t work
It takes a lot of energy for water to change temperature
Heats and cools more slowly than air or land
Useful for animals in hot climates who can use water to cool off
When water evaporates it removes a lot of energy from the system
Cooling sensation
Helps aquatic animals remain at fairly constant temperatures in hot weather
Physical states of water
Water is less dense as a solid because of hydrogen bonding
Seals
Buoyancy helps them stay afloat without using too much energy
Water has a greater thermal conductivity than air so the seal needs to insulate itself with blubber
B: Carbohydrates and Lipids
B1.1.1 Carbon atoms can form four covalent bonds allowing a diversity or stable compounds to exist
Carbon forms the backbone of every organic molecule
Carbon atoms form covalent bonds
Strongest type of bond between atoms
Stable molecules can be formed
Carbon atoms have 4 electrons in their outer shell
Allows them to form 4 covalent bonds with 4 other different atoms
Carbohydrates
Have carbon, hydrogen and oxygen
They have a monomer (little molecules) and a polymer (a bunch of monomers chain up together that form a polymer)
Monomers are commonly ring shaped molecules
Lipids
Made up of fatty acids
Common lipids
Triglycerides
Glycerol + 3 fatty acids
Phospholipids
Phosphate + glycerol + 2 fatty acids
Steroids
4 fused hydrocarbon rings
Proteins
Contain carbon, hydrogen, oxygen, and nitrogen
Large organic compounds made of amino acids
Arranged into one or more linear chains
Structural or part of the plasma membrane
Nucleic Acids
Contain carbon, hydrogen, oxygen, nitrogen, and phosphorus
Chains of subunits called nucleotides
Base, sugar, and phosphate groups covalently bonded together
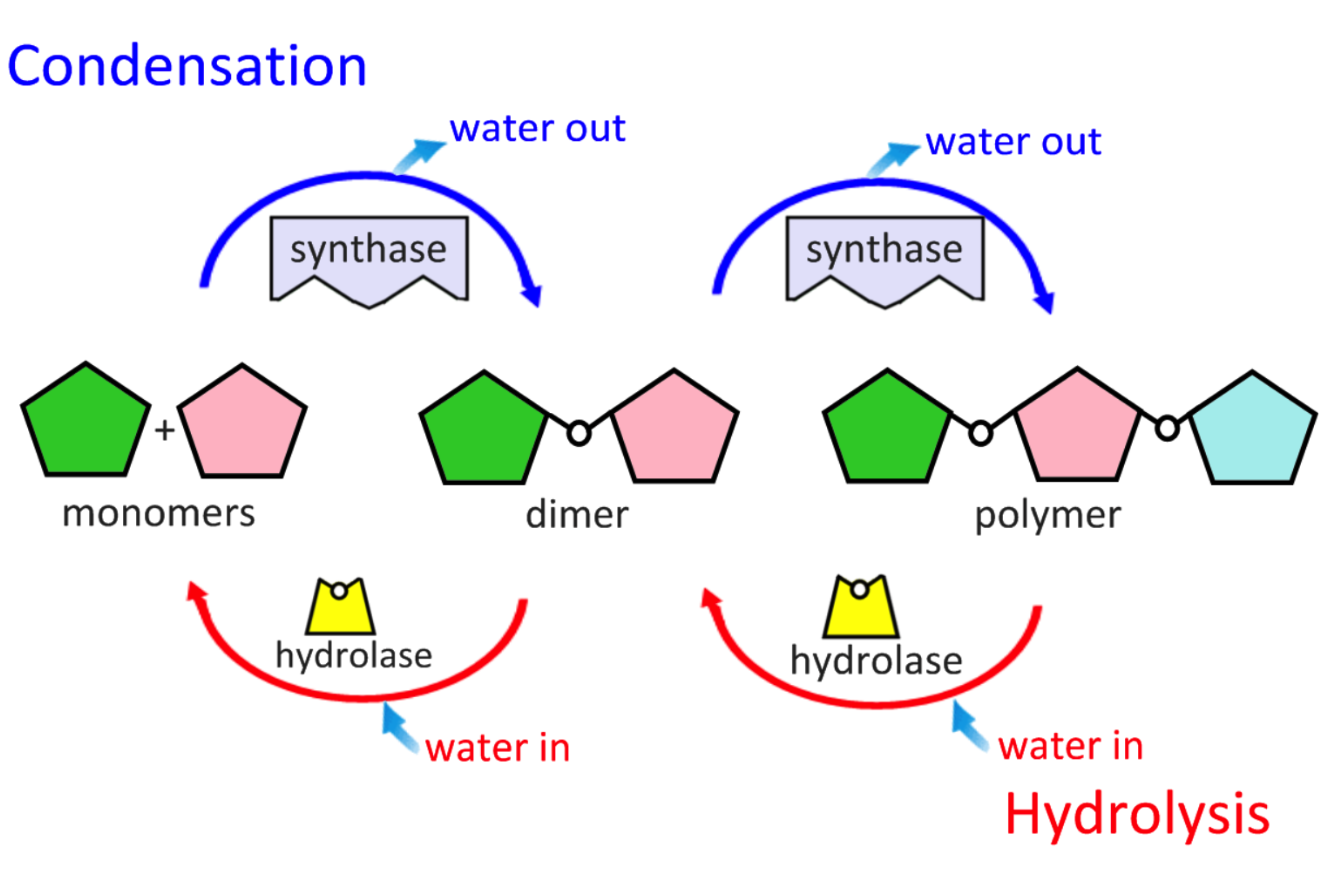
B1.1.2 Carbon production of macromolecules by condensation reactions that link monomers to form a polymer
Condensation makes bonds
water releasing
Anabolic reactions are those which build molecules
Hydrolysis breaks bonds
water splitting
Catabolic reactions are those which break down molecules
Monosaccharides (sugars) are the monomers of polysaccharides (carbs)
B1.1.3 Digestion of polymers into monomers by hydrolysis reactions
B1.1.4 Form and functions of monosaccharides
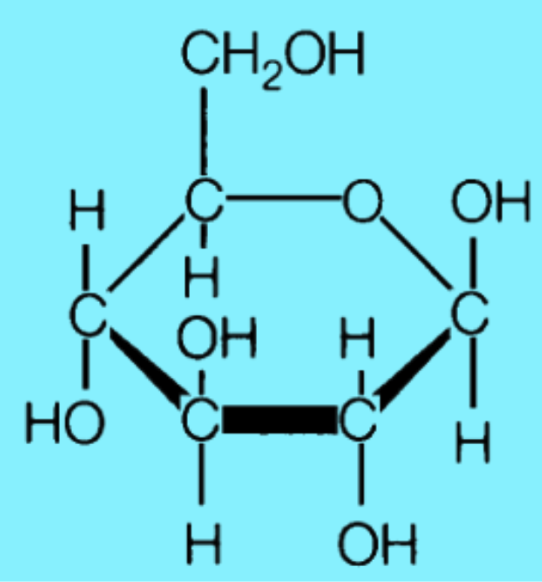
Monosaccharide 1: Glucose
Forms a hexagonal ring
Form of sugar that fuels respiration
Forms base unit for many polymers
Highly soluble in water
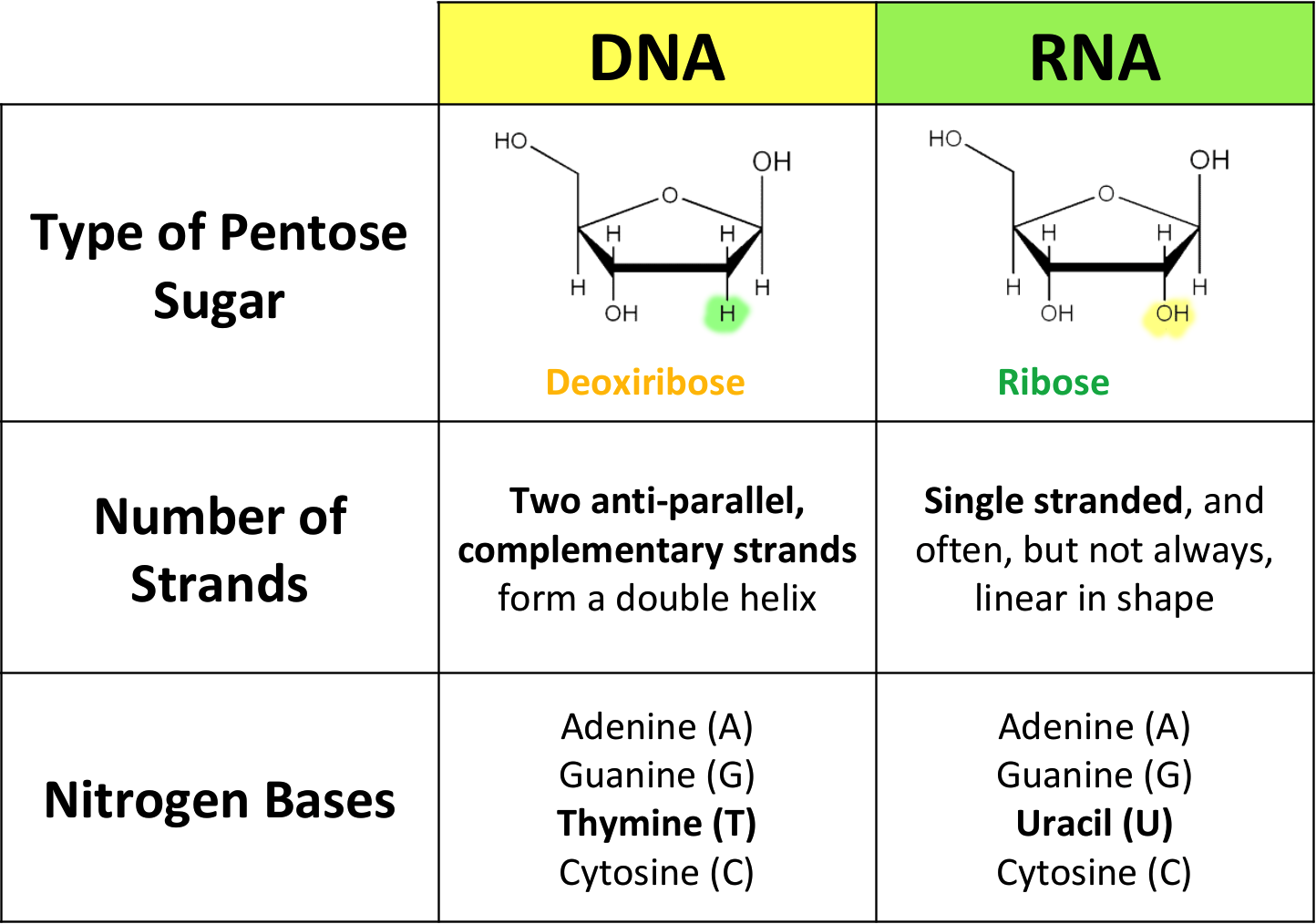
Monosaccharide 2: Ribose
Forms a pentagonal ring
Backbone of RNA
Deoxyribose differs as shown in the diagram and forms backbone of DNA
B1.1.5 polysaccharides as energy storage compounds
Polysaccharides
Polymers with more than two molecules
Often long and may be branched
Cellulose
The tensile strength of cellulose (the basis of cell walls) prevents plant cells from bursting, even under very high (water) pressure.
Starch (amylopectin)
Contains hundreds of glucose molecules
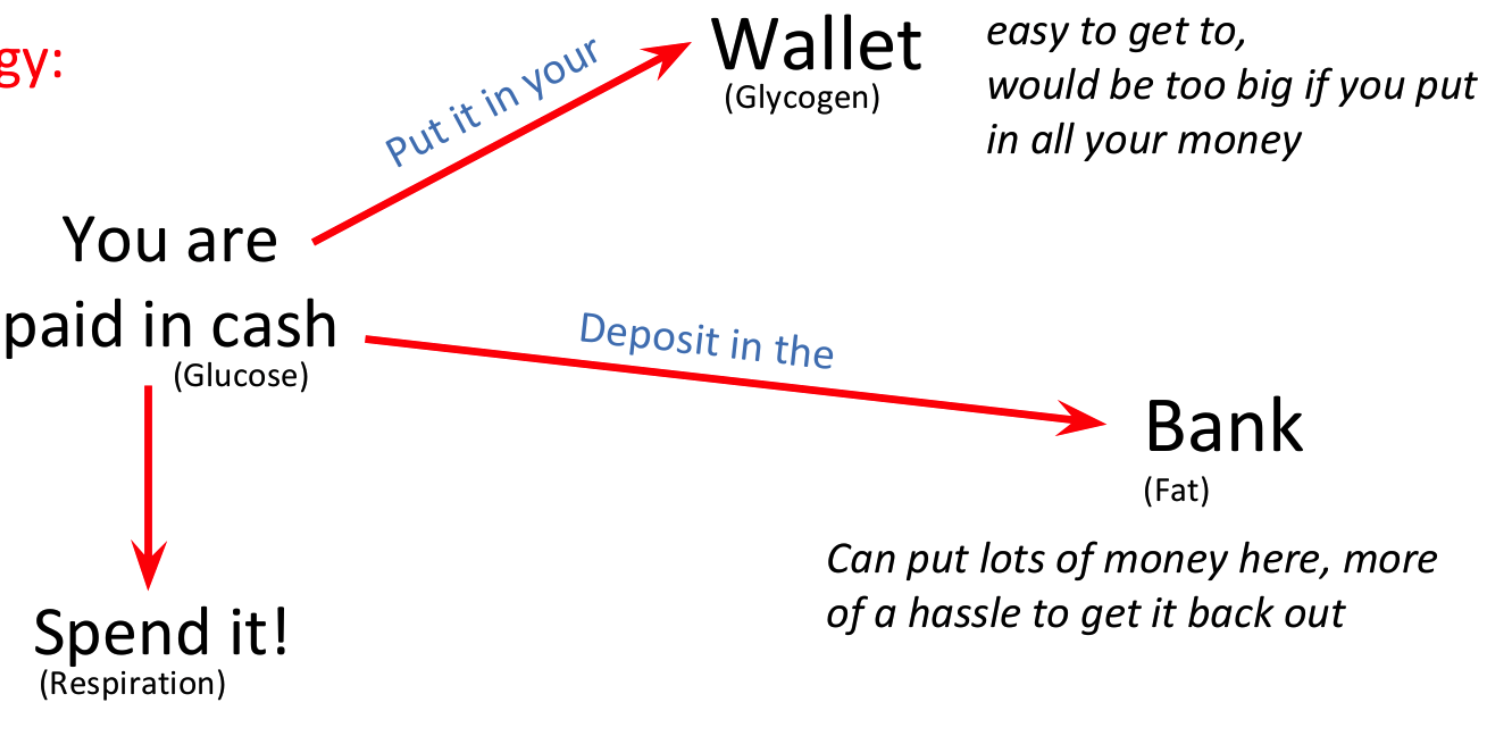
Glycogen
Found in animals and some fungi
Stored in the liver and some muscles in humans
Short-term energy storage
Made up of repeating glucose subunits
Excess glucose is converted into glycogen
Doesn’t affect the osmotic balance of cells
The way the water moves in a cell
Energy storage by lipids and carbohydrates
Why is glycogen needed at all?
Fats in adipose tissue cannot be mobilized as rapidly
Easily transported by the blood
Adipose: fat storage tissue in mammals
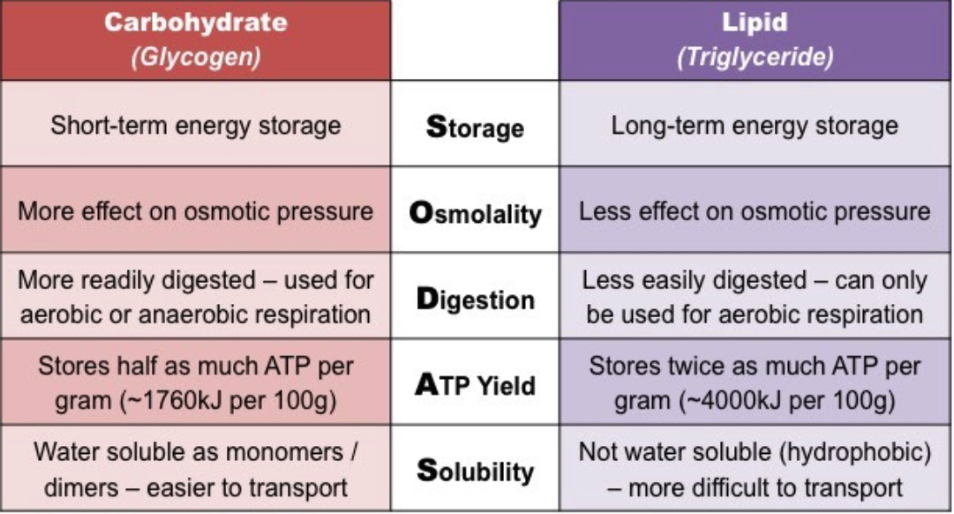
B1.1.6 Structure of cellulose related to its function as a structural polysaccharide in plants
Polysaccharide 1: Cellulose
Hydrogen bonds link the molecules together
Straight chain - not curved
Basis of cell walls
Very strong to keep plant cells from bursting even under very high water pressure
Polysaccharide 2: Starch
Amylose and amylopectin
Forms of starch made from repeating glucose units
Curved molecule
Only made by plant cells
Hydrophilic but too large to be soluble in water
Easy to add or remove extra glucose molecules
Short term energy storage
Polysaccharide 3: Glycogen
Polymer made from repeating glucose subunits
Made by animals and some fungi
Stored in the liver and some muscles in humans
Good for energy storage
B1.1.7 Role of glycoproteins in cell–cell recognition
Glycoprotein
Enable cells to recognize another cell as familiar or foreign
Cell-cell recognition (labeling)
Naming of the cell
Liver cells, skin cells, etc.
Carbohydrate tails
Ex. Blood antigens
A, B, O, AB
B.1.1.8 Hydrophobic properties of water
º1Substances that are insoluble in water
Molecules are hydrophobic if they DON’T have negative or positive charges and are NONPOLAR
All lipids are hydrophobic, including fats and oils
Hydrophobic molecules dissolve in other solvents such as propanone (acetone)
B1.1.9 Formation of triglycerides and phospholipids by condensation reaction
Triglycerides formation
Condensation reaction between glycerol and fatty acids
Glycerol + 3 fatty acids = triglyceride
Example of condensation
smaller molecules linking up to create a larger molecule
Lipids are glycerol combined with 1, 2, or 3 fatty acids
Triglycerides are lipids
B.1.1.10 Difference between saturated, monounsaturated and polyunsaturated fatty acids
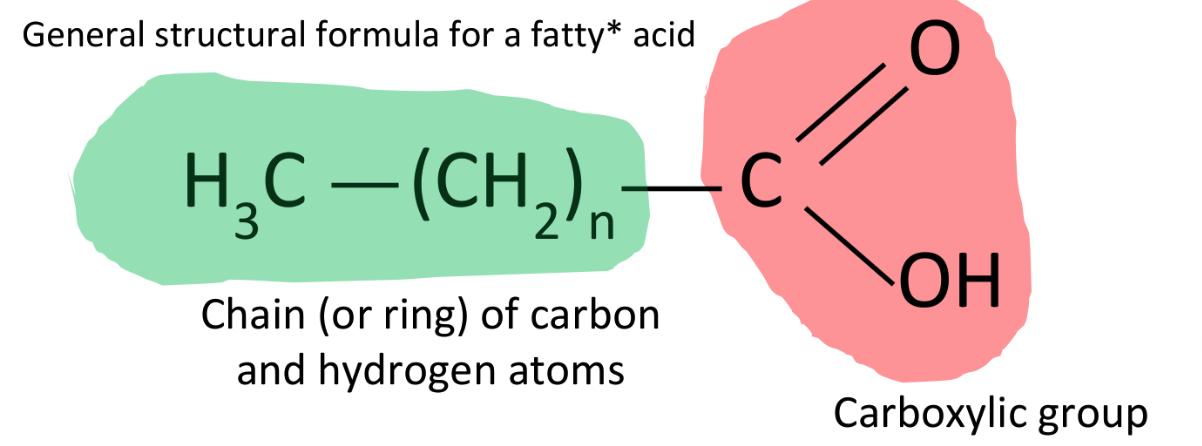

Cis-isomers
Natural
Tend to be curved because the hydrogen atoms are on the same side of the two carbon atoms
loosely packed
Liquid at room temperature
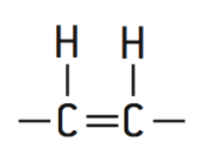
Trans-isomers
Artificial
Tightly packed
Solid at room temp
Increased chance of heart disease
Straight
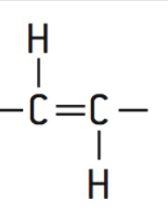
B.1.1.11 Triglycerides in adipose tissues for energy storage and thermal insulation
Functions of lipids
Structure: Phospholipids are a main component of cell membranes
Hormonal signaling: Chemical messengers, steroids are involved in hormonal signaling (estrogen, testosterone, etc)
Insulation: Fats in animals can serve as heat insulators while sphingolipids in the myelin sheath can serve as electrical insulators. Fat keeps them warm
Protection: Triglycerides form a tissue layer around many key internal organs and provide protection against physical injury. Cushioning. (The heart)
Storage of energy: Triglycerides can be used as a long-term energy storage source
B.1.1.12 Formation of phospholipid bilayers as a consequence of the hydrophobic and hydrophilic region
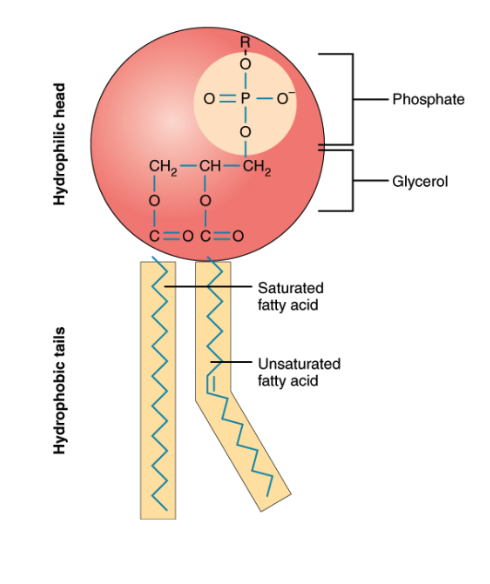
Cell membrane is made up of phospholipids
Phospholipids are amphipathic
Have a hydrophilic head
Have a hydrophobic tail
Phospholipid bilayer is very stable, but also flexible
They form double layers (cell membrane)
B.1.1.13 Ability of non-polar steroids to pass through the phospholipid bilayer
Hormones are chemical messengers that produce a response in the target cells of an organism
Lipid based hormones are called steroids
Steroids are nonpolar so they can pass freely through the cell membrane
Ex: Testosterone
C: Proteins
A
A.1.2.1 DNA as the genetic material of all living organisms
Universal code
Every living thing uses DNA as the way of storing information
Some viruses use RNA or DNA but never both as their genetic material but they are not considered to be living
Acellular
Can’t do any of the life stuff without invading a cell
A.1.2.2 Components of a nucleotide
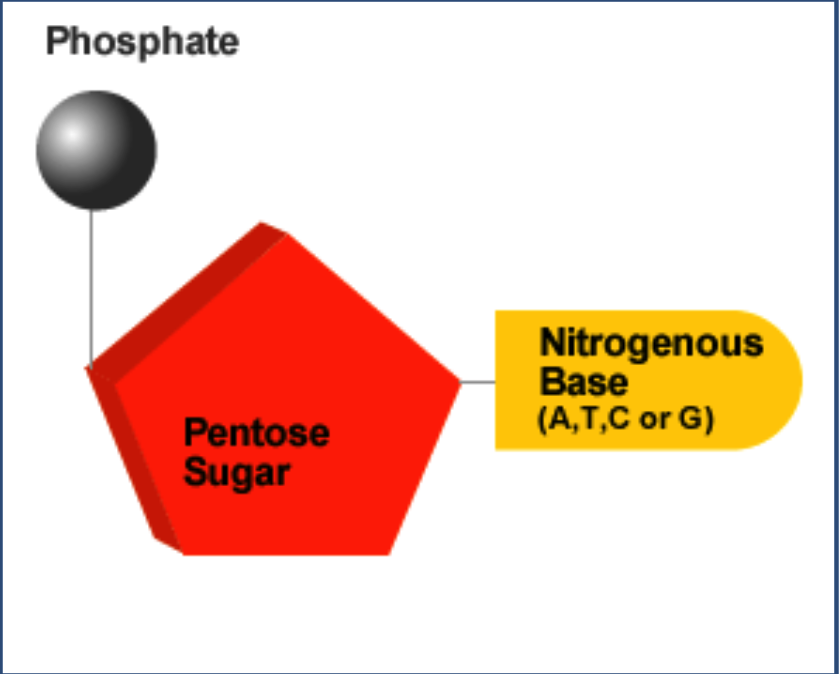
A.1.2.3 Sugar- phosphate bonding and the sugar phosphate “backbone” of DNA and RNA
DNA nucleotides are linked together by covalent bonds formed in condensation reactions into a single strand
A.1.2.4 Bases in each nucleic acid that forms the basis of a code
State the names of the four bases in DNA
Purines
Adenine
Always bonds with the T
Guanine
Pyrimidines
Thymine
Cytosine
Always bonds with G
Purines need to go with pyrimidines because they need to balance out the lengths
A.1.2.5 RNA as a polymer formed by condensation of nucleotide monomers
Has Uracil instead of Thymine
Function of RNA
Protein synthesis
Making a gene into a trait
It takes a copy of DNA out of the nucleus because DNA is too big to leave the nuclear pores and functions as a working copy of DNA
Working copy of the DNA
Made through transcription
RNA can go to a ribosome and create a protein
A.1.2.6 DNA as a double helix made of two antiparallel strands of nucleotides with two strands linked by hydrogen bonding between complementary base pairs
Secondary structure of DNA is the double helix
Two strands of DNA
How is the double helix structure maintained?
Hydrogen bonds hold sections together
Hydrogen bonds hold complementary base pairs together
Complementary base pairing ensures that mistakes are not made when copying or transcribing DNA
Covalent bonds
Sugar to phosphate
Sugar to nitrogenous base
Hydrogen bonds
Base pair to base pair
Each line to each line
Sections of the backbone to each other that makes it twirly
Nucleotides to nucleotides
DNA double helix is formed using complementary base pairing and hydrogen bonds
C and G have 3 hydrogen bonds
A and T have 2 hydrogen bonds
Sequence of bases on DNA make up genes
Genes are heritable factors that control specific characteristics
Nuclear DNA contains single-copy genes and regions of highly repetitive sequences
Coding DNA and non-coding DNA
A.1.2.7 Differences between DNA and RNA
A.1.2.9 Diversity of possible DNA base sequences and the limitless capacity of DNA for storing information
The human genome project which has decoded the case sequence for the whole 6 feet of the human genome requires a data warehouse to store the information electronically
Entirety of all the genes inside a living organism
Divided up the mapping of the human information code between many different universities
About 500,000 dvds worth of data in 1 gram of DNA
A.1.2.10 Conservation of the genetic code across all life forms as evidence of universal common ancestry
Strongest evidence in the theory of evolution is in the sharing of DNA across all life forms
All life shares descent from a Last Universal Common Ancestor (LUCA)
First origin of life
Evidence that life has a common origin
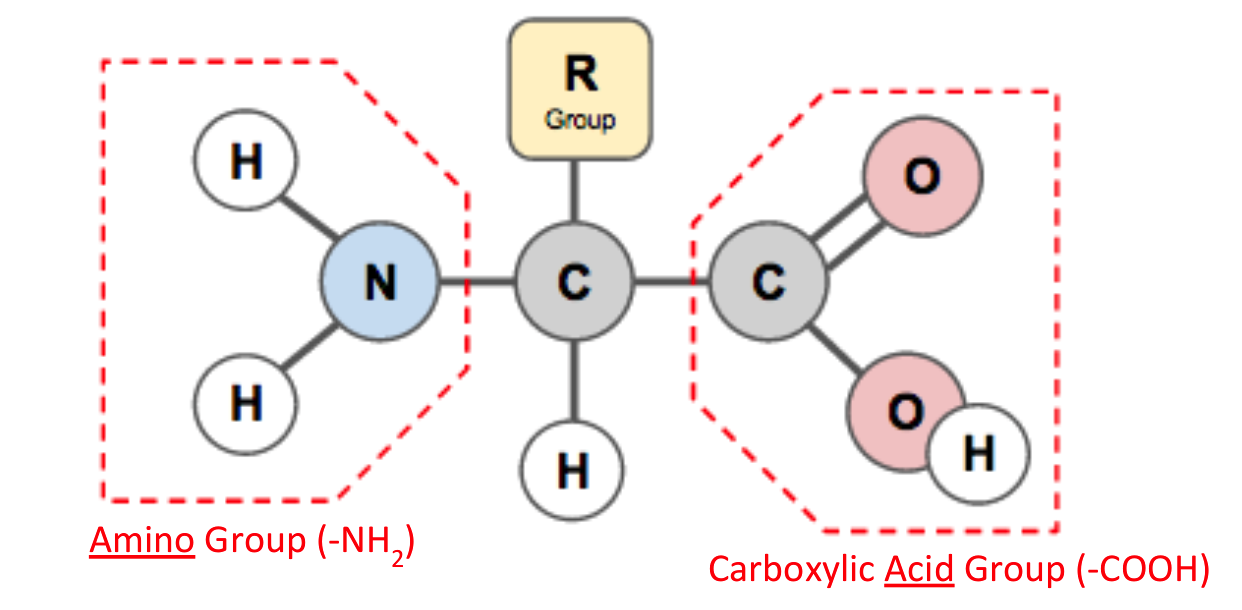
B.1.2.1 Generalized structure of an amino acid
The amino group is one of the reasons why nitrogen is an important element in living things
The carboxylic acid group contains an oxygen double-bonded to the carbon and a hydroxyl group that can be lost to form new bonds
In proteins there are 20 amino acids that build up proteins in different ways
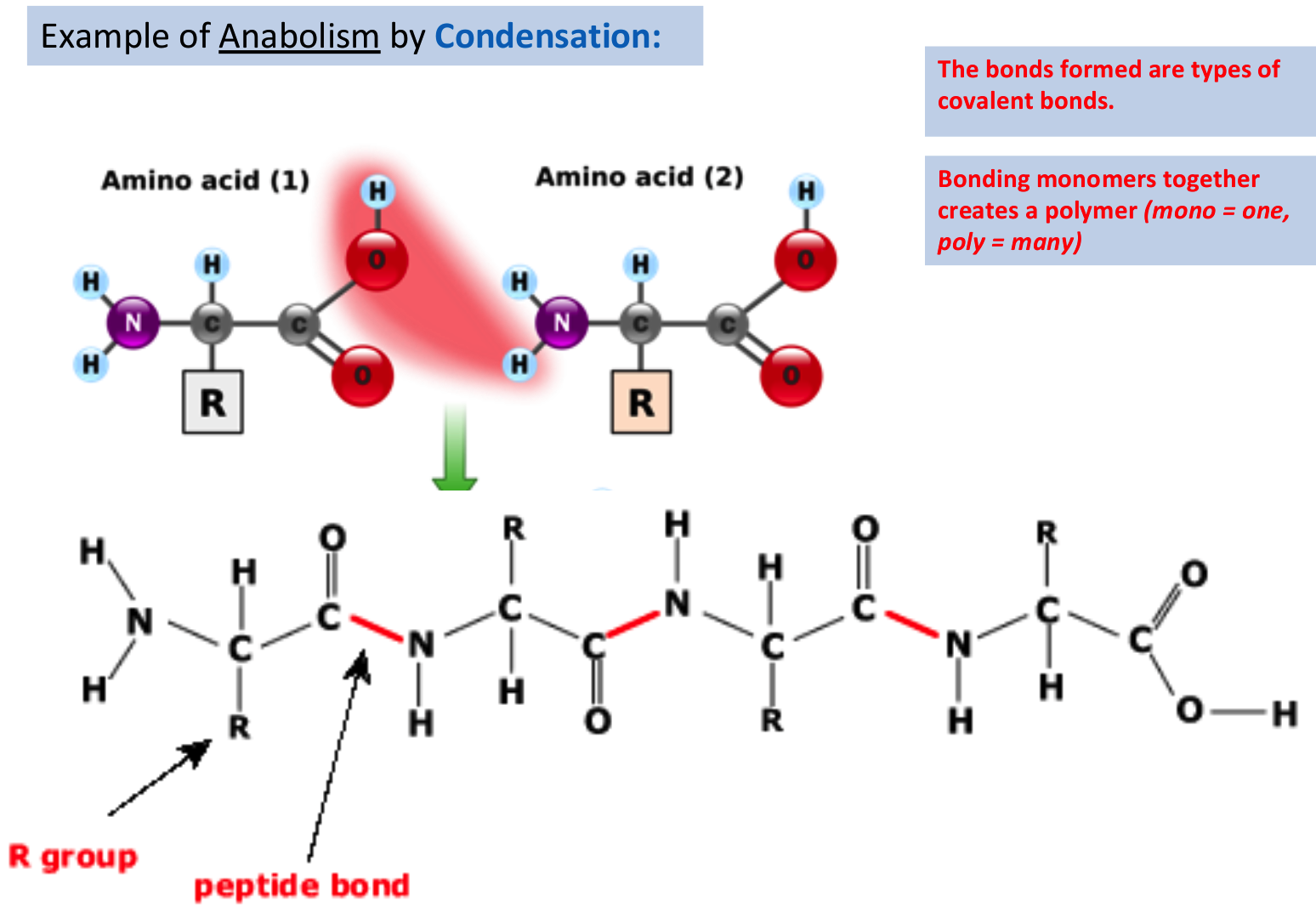
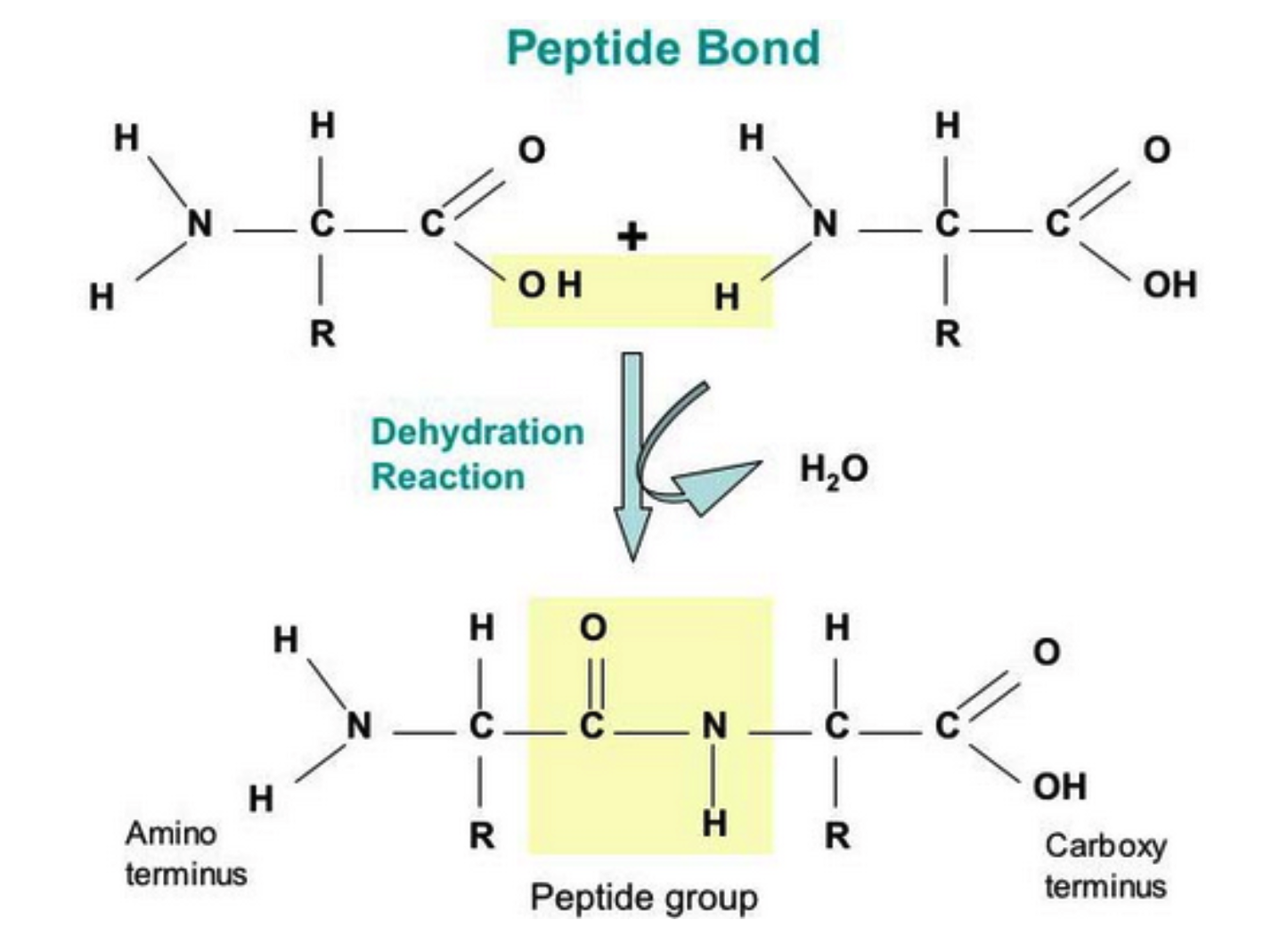
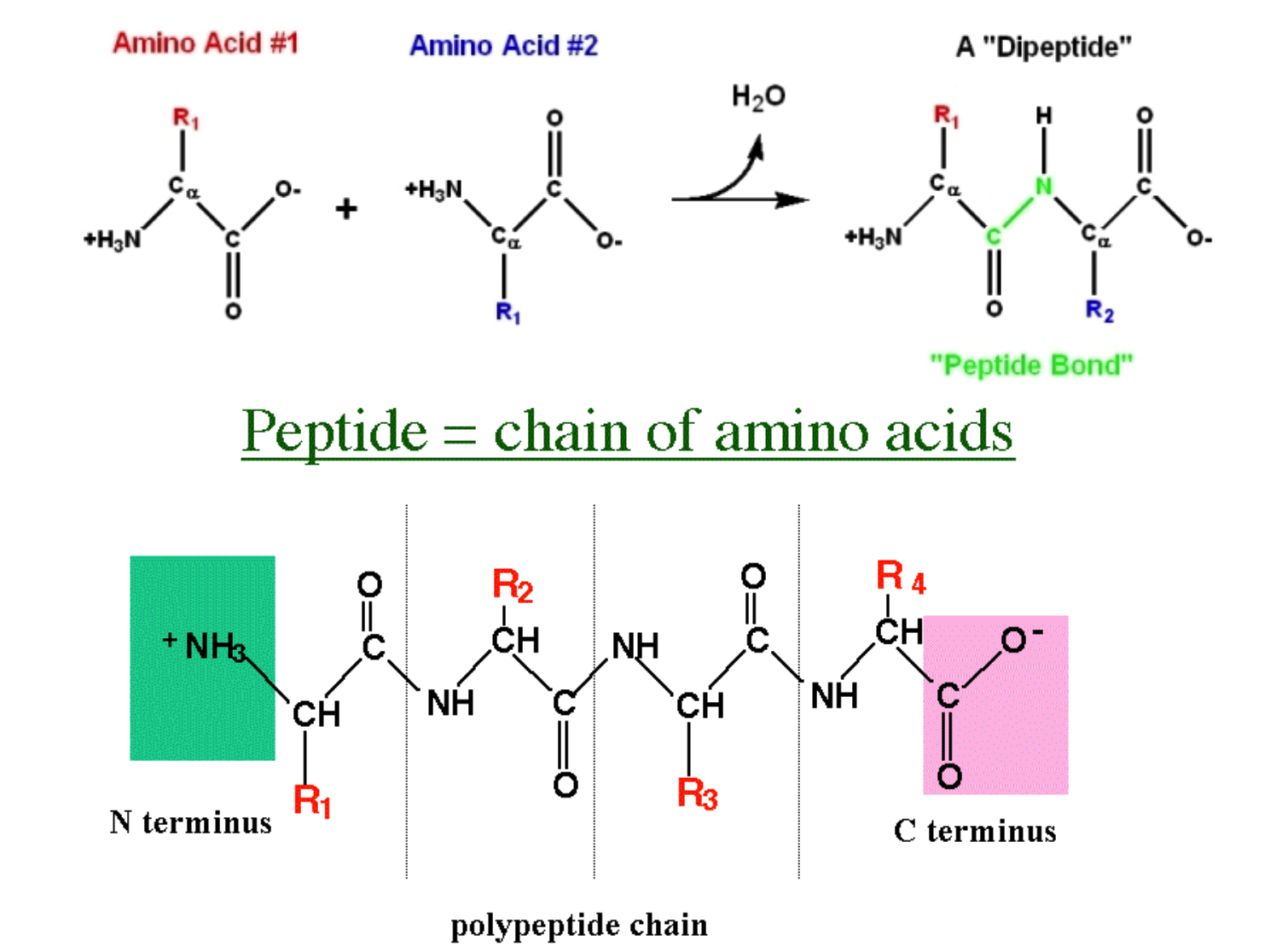
B.1.2.2 Condensation reactions forming dipeptides and longer chains of amino acids
B.1.2.3. Dietary requirements for amino acids
Obtained from nutrition
Basic things to make amino acids come from food
Synthesized by the body
Essential amino acids
The ones you can’t make
Need to get them directly from food
Valine
Non essential amino acids
Once you break down food, your body can make them
Serine
B.1.2.4 Infinite variety of peptide chains
Polypeptides are chains of amino acids joined by peptide bonds
Proteins are versatile
There are 20 different amino acids
Can be combined in any order
Each amino acid has unique properties
Some a polar (hydrophilic)
Some are non-polar (Hydrophobic)
Some are positively or negatively charged
Some contain sulphur
Properties determine how a polypeptide folds up into a protein
Why are there infinite variety of polypeptides?
Because there are 20 amino acids
many possibilities in how they are built
Many different lengths we can make proteins
If a polypeptide has 7 amino acids there can be 20^7 possible polypeptides generate
Proteins have different levels of structure
Once a chain is made, it can link with other polypeptide chains
Proteins can fold and that gives them versatility
Fibrous proteins tend to be structures in nature which means its building material
Keratin (hair), collagen
Insoluble
Doesn’t want to build things that would melt in water
Globular proteins are functional in nature
Transport, have functions
Haemoglobin, insulin
Soluble
Functions of proteins
Digestion
Keep us healthy
immunoglobulins
Muscles
Involved in DNA stuff
Support to the body
Coordination for bodily function
Move essential molecules around the body
Immunoglobulins
Globular protein
Keep us healthy
Fight off viruses and bacteria
Antibodies
Spider silk
Structural protein
Very strong
B.1.2.5 Effect of pH and temperature on protein structure
Denaturation
What happens to a protein when subjected to extreme conditions of heat or pH
If you burn a protein or drop it in acid it will denature
Breaks down
Loses shape
Loses function
Sometimes in high salt or heavy metals can denature proteins

Genes are codes for making polypeptides
DNA is stored in the nucleus
Polypeptide made in the cytoplasm
m.