Chapter 6 - Microscopic Examination of Urine
Preparation and Examination of the Urine Sediment
Specimen Preparation
- Specimens should be fresh or adequately preserved, and it should be thoroughly mixed prior to centrifuging (see Specimen Handling in Chapter 3 - Introduction to Urinalysis).
- A midstream clean-catch specimen is recommended (see Midstream Clean-Catch Specimen in Specimen Handling in Chapter 3 - Introduction to Urinalysis).
Specimen Volume
- 12 mL is usually needed (see Specimen Collection in Chapter 3 - Introduction to Urinalysis).
Centrifugation
- The speed of the centrifuge and the length of time the specimen is centrifuged should be consistent.
- Centrifugation for 5 minutes at a relative centrifugal force (RCF) of 400 produces an optimum amount of sediment with the least chance of damaging the elements.
- RCF is used rather than revolutions per minute (RPM) to correct for differences in the diameter of centrifuge heads.
- To convert, RCF = 1.118 x 10^(-5) x radius in cm x RPM^2
- Use of the braking mechanism to slow the centrifuge causes disruption of the sediment prior to decantation and should not be used.
- Centrifugation calibration should be routinely performed.
- All specimens must be centrifuged in capped tubes to prevent the spread of biohazardous aersols.
Sediment Preparation
- A uniform amount of urine and sediment should remain in the tube after decantation.
- The volume of urine centrifuged divided by the sediment volume equals the concentration factor.
- The sediment concentration factor relates to the probability of detecting elements present in low quantities and is used when quantitating the number of elements present per mL.
- Volumes of 0.5 and 1.0 mL are frequently used.
- To maintain a uniform sediment concentration factor, urine should be aspirated off rather than poured off, unless otherwise specified by the commercial system in use.
- The sediment must be thoroughly resuspended by gentle agitation using a commercial-system pipette or by repeatedly tapping the tip of the tube with the finger.
- Vigorous agitation should be avoided, as it may disrupt some cellular elements.
Volume of Sediment Examined
- When using the conventional glass-slide method, the recommended volume is 20 μL (0.02 mL) covered by a 22 x 22 mm glass cover slip.
- Allowing the specimen to flow outside of the cover slip may result in the loss of heavier elements such as casts as they have a tendency to locate near the edges of the cover slip.
- Commercial systems control the volume of sediment examined by providing slides with chambers capable of containing a specified volume.
- Care must be taken to ensure the chambers are completely filled.
- Product literature supplies the chamber volume, size of the viewing area, and approximate number of low-power and high-power viewing areas, based on the area of the field of view using a standard microscope.
Examination of the Sediment
- The manner by which the microscopic examination is performed should be consistent and should include observation of a minimum of 10 fields under both low (10x) and high (40x) power.
- The slide is first examined under low power to detect casts and to ascertain the general composition of the sediment.
- Once encountered, the setting is changed to high power.
- An epithelial cell will be present to provide a point of reference.
- Focusing on artifacts should be avoided, because they are often larger than the regular sediment elements and cause the microscopist to examine objects in the wrong plane.
- When the sediment is examined unstained, many sediment constituents have a refractive index similar to urine.
- for staining procedures, see Sediment Stains in Sediment Examination Techniques below.
- Staining also imparts identifying characteristics to cellular structures, such as the nuclei, cytoplasm, and inclusions.
Reporting the Microscopic Examination
Casts are reported as the average number per 10 low-power fields (lpfs).
RBCs and WBCs are reported as the average number per 10 high-power fields (hpfs).
Epithelial cells, crystals, and other elements are frequently reported in semiquantitative terms such as, rare, few, moderate, and many, or as 1+, 2+, 3+, and 4+.
Conversion of the average number of elements per lpf or hpf to the number per milliliter provide standardization among the various techniques in use.
Procedures should be completely documented and followed by all personnel.
Correlation of Results
- Microscopic results should be correlated with the physical and chemical findings to ensure the accuracy of the report, and results that do not correlate must be rechecked for both technical and clerical errors.
| Microscopic Elements | Physical | Chemical | Exceptions |
|---|---|---|---|
| RBCs | turbidity | + blood | number |
| red color | hemolysis | ||
| WBCs | turbidity | + protein | number |
| + nitrite | lysis | ||
| + leukocytes | |||
| Epithelial Cells | turbidity | number | |
| Casts | + protein | number | |
| Bacteria | turbidity | pH | number and type |
| + nitrite | |||
| + leukocytes | |||
| Crystals | turbidity | pH | number and type |
| color |
Sediment Examination Techniques
Sediment Stains
| Stain | Action | Function |
|---|---|---|
| Sternheimer-Malbin/Sedi-Stain/KOVA stain (crystal violet + safranin O) | delineates structure and contrasting colors of the nucleus and cytoplasm | identifies WBCs, epithelial cells, and casts |
| 0.5% toluidine blue | enhances nuclear detail | differentiates WBCs and renal tubular epithelial (RTE) cells |
| 2% acetic acid | lyses RBCs and enhances nuclei of WBCs | distinguishes RBCs from WBCs, yeast, oil droplets, and crystals; cannot be used for initial sediment analysis |
| oil red O, Sudan III | stains triglycerides and neutral fats | identifies free fat droplets and lipid-containing cells and casts |
| gram stain | differentiates gram-positive and gram-negative bacteria | identifies bacterial casts |
| Hansel stain (methylene blue + eosin Y), Wright’s stain | stains eosinophilic granules | identifies urinary eosinophils |
| Prussian blue stain | stains structures containing iron | identifies yellow-brown granules of hemosiderin in cells and casts |
| Elements in Urinary Sediment | Usual Distinguishing Color of Stained Elements | Comments |
|---|---|---|
| RBCc | pink to purple (neutral pH), purple (alkaline pH) | |
| WBCs | purple nucleus, purple granules in cytoplasm | |
| glitter cells (Sternheimer-Malbin positive cells) | colorless or light blue nucleus, pale blue or gray cytoplasm | some may exhibit Brownian motion or erratic random movement |
| renal tubular epithelial cells | dark blue-purple nucleus, light blue-purple cytoplasm | |
| bladder tubular epithelial cells | blue--purple nucleus, light purple cytoplasm | |
| squamous epithelial cells | dark orange-purple nucleus, light purple or blue cytoplasm | |
| triglycerides, neutral fats | orange-red | |
| cholesterol | not stained but polarized | |
| hyaline casts | pale pink or pale purple | very uniform color; slightly darker than mucous threads |
| coarse granular inclusion casts | dark purple granules in purple matrix | |
| finely granular inclusion casts | fine dark purple granules in pale pink or pale purple matrix | |
| waxy casts | pale pink or pale purple | darker than hyaline casts, but of a pale even color; distinct broken ends |
| fat-inclusion casts | fat globules unstained in a pink matrix | rare; presence is confirmed if examination under polarized light indicates double refraction |
| red cell inclusion casts | pink to orange-red | intact cells can be seen in matrix |
| blood (hemoglobin) casts | orange-red | no intact cells |
| bacteria | purple, but doesn’t stain if non-motile; blue for gram-positive, red for gram-negative | |
| Trichomonas vaginalis | light blue-green | |
| mucus | pale pink or pale blue | |
| background | pale pink or pale purple |
Cytodiagnostic Urine Testing
- The preparation of permanent slides using cytocentrifugation followed by staining with Papanicolaou stain provides an additional method for detecting and monitoring renal disease.
- Cytodiagnostic urine testing also provides more definitive information about renal tubular changes associated with transplant rejection; viral, fungal, and parasitic infections; cellular inclusions; pathologic casts; and inflammatory conditions.
- A voided first morning specimen is recommended for testing.
Microscopy
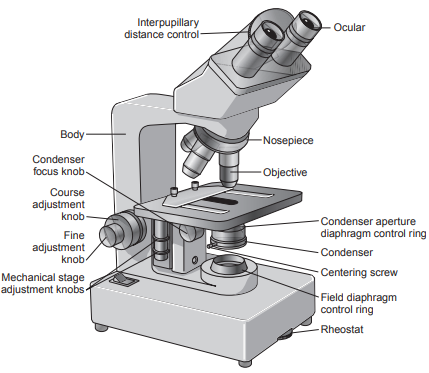
Essentially all types of microscopes contain a lens system, illumination system, and a body consisting of a base, body tube and nosepiece.
Primary components of the lens system are the oculars, the objectives, and the coarse and fine adjustment knobs.
Most microscopes are designed to be parfocal, indicating that they require only minimum adjustment when switching among objectives.
Objectives are adjusted to be near the specimen and perform the initial magnification of the object on the mechanical stage.
- Objectives are inscribed with information that describes their characteristics and includes the type of objective, magnification, numerical aperture, microscope tube length, and cover-slip thickness to be used.
- The numerical aperture number represents the refractive index of the material between the slide and the outer lens (air or oil) and the angle of the light passing through it.
- The higher the numerical aperture, the closer the lens is to the object and the better the light-gathering capability of the lens, yielding greater resolving power.
- The length of the objectives attached to the nosepiece varies with magnification (length increases from 10x to 100x magnification), thereby changing the distance between the lens and the slide when they are rotated.
- The objectives used for examination of urine sediment are 10x (low power, dry) and 40x (high power, dry).
- The final magnification of an object is the product of the objective magnification times the ocular magnification.
- Using a 10x ocular and a 10x objective provides a total magnification of 100x for lpf observations, and the 10x ocular and the 40x objective provide a magnification of 400x for hpf observations.
The image then passes to the oculars for further resolution.
- Resolution is the ability of the lens to distinguish two small objects that are a specific distance apart.
- It is best when the distance between the two objects is small.
- It is inversely proportional to the wavelength of light and the numerical aperture of the lens.
- Laboratory microscopes normally contain oculars capable of increasing the magnification 10 times (10x) and are binocular to provide a more complete visualization.
- The oculars can be adjusted horizontally to adapt to differences in interpupillary distance between operators.
- A diopter adjustment knob on the oculars can be rotated to compensate for variations in vision between the operators’ eyes.
- The field of view is determined by the eyepiece and is the diameter of the circle of view when looking through the oculars.
- The field of view varies with the field number engraved on the eyepiece and the magnification of the objective.
- The higher the magnification, the smaller the field of view.
The distance between the slide and the objective is controlled by the coarse and fine focusing knobs located on the body tube.
- Initial focusing is performed using the coarse knob that moves the mechanical stage noticeably up and down until the object comes into view.
- This is followed by adjustment using the fine focusing knob to sharpen the image.
- When using a parfocal microscope, only the fine knob should be used for adjustment when changing magnifications.
The illumination system contains the light source, condenser, and field and iris diaphragms.
Illumination for the modern microscope is provided by a light source located in the base of the microscope.
The light source is equipped with a rheostat to regulate the intensity of the light.
Filters may also be placed on the light source to vary the illumination and wavelengths of the emitted light.
A field diaphragm contained in the light source controls the diameter of the light beam reaching the slide and is adjusted for optimal illumination.
A condenser located below the stage then focuses the light on the specimen and controls the light for uniform illumination.
The normal position of the condenser is almost completely up with the front lens of the condenser near the slide but not touching it.
The condenser adjustment (focus) knob moves the condenser up and down to focus light on the object.
Two adjustments to the condenser—centering and Köhler illumination—provide optimal viewing of the illuminated field.
- They should be performed whenever an objective is changed.
- Additional focusing of the object should be performed using the adjustment knobs and the rheostat on the light source.
An aperture diaphragm in the condenser controls the amount of light and the angle of light rays that pass to the specimen and lens, which affects resolution, contrast, and depth of the field of image.
- By adjusting the aperture diaphragm to 75% of the numerical aperture of the objective, maximum resolution is achieved.
- The aperture diaphragm should not be used to reduce light intensity because it decreases resolution.
- The microscope lamp rheostat is used for this adjustment.
To center the condenser and obtain Köhler illumination, the following steps should be taken:
Place a slide on the stage and focus the object using the low-power objective with the condenser raised.
Close the field diaphragm and lower the condenser until the edges of the field diaphragm are sharply focused.
Center the image of the field diaphragm with the condenser centering screws.
Open the field diaphragm until its image is at the edge of the field.
Remove an eyepiece and look down through the eyepiece tube.
Adjust the aperture diaphragm until approximately 75% of the field is visible.
Replace the eyepiece.
Objects to be examined are placed on a platform, referred to as the mechanical stage.
Routine preventive maintenance procedures on the microscope ensure good optical performance.
- The microscope should always be covered when not in use to protect it from dust.
- If any optical surface becomes coated with dust, it should be carefully removed with a camel-hair brush.
- Optical surfaces should be cleaned with lens paper immediately after contamination.
- An oil immersion lens must be wiped free of oil and cleaned after each use.
- Fingerprints and oil smears impair the sharpness of an image.
- An annual professional cleaning for the microscope is recommended.
- Light sources are replaced as necessary.
Types of Microscopy
Bright-Field Microscopy
- The compound bright-field microscope consists of a two-lens system combined with a light source.
- The first lens system is located in the objective and is adjusted to be near the specimen.
- The second lens system, the ocular lens, is located in the eyepiece.
- It is essential that sediments be examined under reduced light when using bright-field microscopy.
Phase-Contrast Microscopy
It makes use of phase-contrast objective lens and a matching condenser that create variations of contrast in the specimen image due to the various refractive indexes in the object.
The principle is that the scattered light from the substance being examined slows down in comparison to the rays passing through air (media), thereby decreasing the intensity of the light and producing contrast.
This is called phase difference and is affected by the thickness of the object, refractive index, and other light-absorbance properties.
The best contrast is obtained when the light that does not pass through the specimen is shifted one quarter of a wavelength and compared with the phase difference of the specimen.
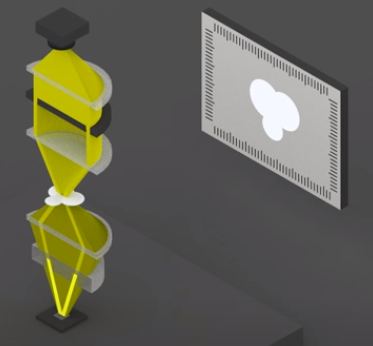
Two phase rings that appear as “targets” are placed in the condenser and the objective.
- One phase ring is placed in the condenser or below it, permitting light to only pass through the central clear circular area.
- A second phase-shifting ring with a central circular area that retards the light by one quarter wavelength is placed in the objective.
Phase-contrast microscopy is accomplished by adaptation of a bright-field microscope with a phase-contrast objective lens and a matching condenser..
Phase rings must match and be concentric, so it is important to check that the objective and condenser mode are the same.
- Focus the microscope in bright-field with a specimen slide.
- Select a low-power phase condenser ring and the corresponding ring objective.
- Remove an ocular, insert the adjustment telescope, and look through the telescope.
- Observe the dark and light rings (annuli).
- With the adjusting screw on the telescope, center the light annulus (condenser) over the dark annulus (objective).
- Replace the ocular.
The diameter of the rings varies with the magnification.
It is particularly advantageous for identifying low refractive hyaline casts or mixed cellular casts, mucous threads and Trichomonas without the need to stain.
The image has the best contrast when the background is darkest.
Polarizing Microscopy
- Polarizing microscopy is used in urinalysis to confirm the identification of fat droplets, oval fat bodies, and fatty casts that produce a characteristic Maltese cross pattern.
- Birefringent uric acid crystals can be distinguished from cystine crystals.
- Monohydrate calcium oxalate crystals can be distinguished from nonpolarizing RBCs.
- Calcium phosphate crystals can be distinguished from nonpolarizing bacteria.
- The halogen quartz lamp in the microscope produces light rays of many different waves, each with a distinct direction and a vibration perpendicular to its direction.
It passes through a filter which selects a wave with the selected polarization or orientation.
It then passes through the specimen to be examined, and can either have positive birefringence or negative birefringence.
- Positive birefringence means the object can refract light in two dimensions and rotate it’s plane 90 degrees to each other in a clockwise direction.
- In contrast, negative birefringence rotates the plane in a counterclockwise direction.
- If the substance has no refractive properties, light passes through unchanged.
The refracted light then passes through another filter rotated 90° to the first filter.
- If the two filters are perpendicular to each other, no light will be able to pass through.
- However, if the substance is able to refract it to the polarization of the second filter, light will pass through and the substance will be seen.
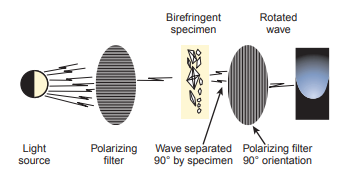
- Bright-field microscopes can be adapted for polarizing microscopy.
- The first filter, the polarizing filter, is placed in the condenser filter holder; the second filter, the analyzer, is placed in the head between the objectives and the ocular.
Interference-Contrast Microscopy
Interference-contrast microscopy provides a three-dimensional image showing very fine structural detail by splitting the light ray so that the beams pass through different areas of the specimen.
The light interference produced by the varied depths of the specimen is compared, and a three-dimensional image is visualized.
Two types of interference-contrast microscopy are available: modulation contrast (Hoffman) and differential interference contrast (Nomarski).
In the modulation-contrast microscope, a split aperture is placed below the condenser, a polarizer is placed below the split aperture, and an amplitude filter is placed in back of each objective.
- The modulator has three zones of light transmission: a dark zone that transmits 1% of light, a gray zone that transmits 15% of light, and a clear zone that transmits 100% of light.
- The polarized light rays pass through a split aperture to the various areas of the specimen and to the modulator where they are converted into the variations of light intensity to produce a three-dimensional image.
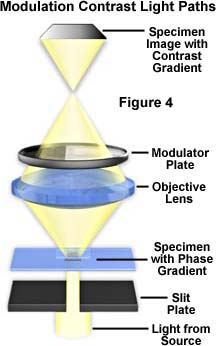
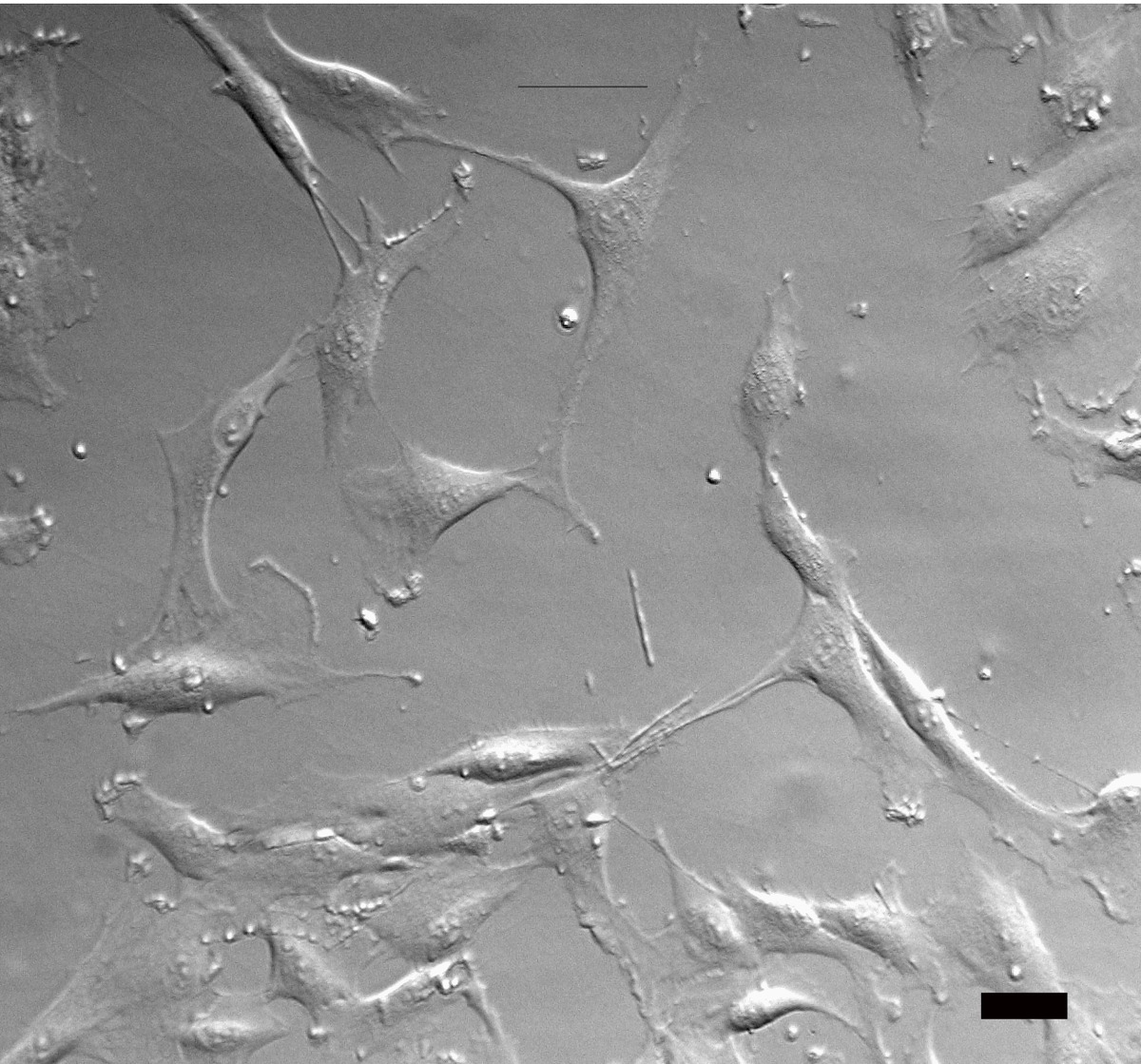
The differential interference-contrast microscope uses prisms.
- A polarizing filter to output plane-polarized light is placed between the light source and the condenser.
- A two-layered Nomarski-modified Wollaston prism that separates individual rays of light into ray pairs is required.
- The lower Wollaston prism is built into the condenser of the microscope.
- The upper prism is placed between the objective and the eyepiece and recombines the rays.
- Above the top Wollaston prism, another polarizing filter is placed that causes wave interference to occur and produce the three-dimensional image.
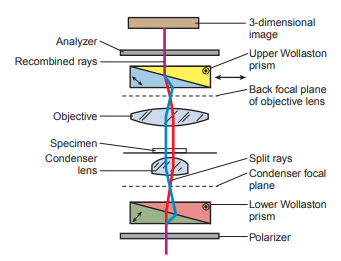
The advantage of interference-contrast microscopy is that an object appears bright against a dark background but without the diffraction halo associated with phase-contrast microscopy
These two types of microscopy provide layer-by-layer imaging of a specimen and enhanced detail for specimens with either a low or high refractive index.
However, it requires more extensive modifications to the bright-field microscope to perform this technique.
Dark-Field Microscopy
It makes use of an opaque disk that blocks the light from directly entering the objective, making the field of view black; however, as the light rays pass through the specimen, the light scatters, diffracts or reflects off the specimen and is captured by the objective lens.
The specimen appears light against the black background or dark-field.
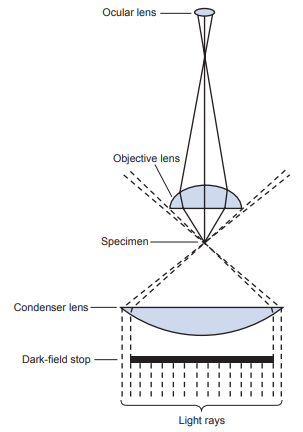
It is used to enhance visualization of specimens that cannot be seen easily viewed with a bright-field microscope without staining.
In particular, it is used to identify the spirochete Treponema pallidum.
Fluorescence Microscopy
In this method, the specimen is illuminated with a light of a specific wavelength in the color spectrum that excites it’s fluorophores, which then emits a light of another wavelength that is is visualized with the use of special filters, called the excitation filter and the emission filter.
Powerful light sources are required and are usually either mercury or xenon arc lamps.
The excitation filter selects the excitation wavelength of light from a light source.
The emission filter selects a specific wavelength of emitted light from the specimen to become visible.
The filters are chosen to match the excitation and emission wavelengths of the fluorophore used to label the specimen.
A dichroic mirror reflects the excitation light to the specimen and transmits the emitted light to the emission filter, which is collected with the objective and imaged by the detector.
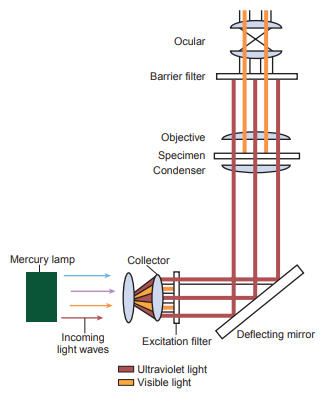
The fluorescent substance can be observed in the fluorescent microscope as a bright object against a dark background with high contrast when ultraviolet light source is used.
It is used to detect bacteria and viruses within cells and tissues through a technique called immunofluorescence.
Sediment Constituents
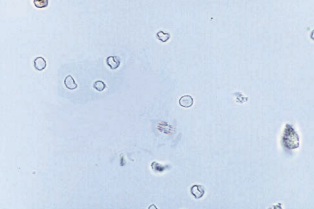
Red Blood Cells (RBCs)
They must be identified using high-power (40x) objective (x400 magnification) and reported as the average number per 10 high-power fields (hpfs)..
- RBCs appear as smooth, non-nucleated, biconcave disks measuring approximately 7 mm in diameter.
- In concentrated (hypersthenuric) urine, the cells shrink due to loss of water and may appear crenated or irregularly shaped.
- In dilute (hyposthenuria) urine, the cells absorb water, swell, and lyse rapidly, releasing their hemoglobin and leaving only the cell membrane.
RBCs are the most difficult for students to recognize.
- The reasons for this include RBCs’ lack of characteristic structures, variations in size and close resemblance to other sediment constituents.
- RBCs are frequently confused with yeast cells, oil droplets and air bubbles.
- Yeast cells usually exhibit budding.
- Oil droplets and air bubbles are highly refractile when the fine adjustment is focused up and down, and they may also appear in a different plane than other sediment constituents.
- The rough appearance of crenated RBCs may resemble the granules seen in WBCs; however, they are much smaller than WBCs.
- Adding acetic acid to a portion of the sediment will lyse the RBCs, leaving the yeast, oil droplets, and WBCs intact.
- Ghost cells, or RBCs that have lysed in hyposthenuric urine can be easily missed if specimens are not examined under reduced light.
- Supravital staining may also be helpful (for staining procedures, see Sediment Stains in Sediment Examination Techniques above).
The morphology of RBCs can aid in determining the site of renal bleeding.
- RBCs that vary in size, have cellular protrusions, or are fragmented are termed dysmorphic and have been associated primarily with glomerular bleeding.
- Small numbers of dysmorphic cells are found with non-glomerular hematuria.
- Dysmorphic RBCs also have been demonstrated after strenuous exercise.
- The dysmorphic cell most closely associated with glomerular bleeding appears to be the acanthocyte with multiple protrusions.
- It is difficult to observe under bright-field microscopy, so staining is recommended.
The number of cells present is indicative of the extent of damage to the glomerular membrane or vascular injury within the genitourinary tract.
- When macroscopic hematuria is present, the urine appears cloudy with a red to brown color.
- Microscopic analysis may be reported in terms of greater than 100 per hpf or as specified by laboratory protocol.
- Macroscopic hematuria is frequently associated with advanced glomerular damage, but it is also seen with damage to the vascular integrity of the urinary tract caused by trauma, acute infection or inflammation, and coagulation disorders.
- The urine appears cloudy with a red to brown color.
- Microscopic hematuria is recognized by the presence of hyaline, granular, and RBC casts.
- However, it may be from non-pathological causes such as after strenous exercise or from menstrual contamination.
- The urine may appear clear and unassuming, so correlation with color or blood test may not be accurate.
- The observation of microscopic hematuria can be critical to the early diagnosis of glomerular disorders and malignancy of the urinary tract and to confirm the presence of renal calculi.
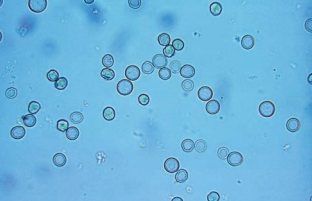
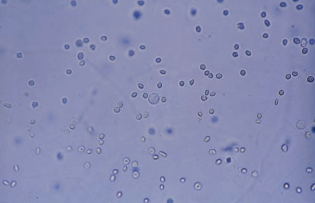
Leukocytes/White Blood Cells (WBCs)
WBCs are larger than RBCs, measuring an average of about 12 mm in diameter.
Characteristics and clinical implications differ depending on the type present.
- The predominant WBC found in the urine sediment is the neutrophil, and they contain granules and multilobed nuclei.
- They are reported as the average number seen in 10 hpfs.
- Characteristics change depending on the solution.
- In dilute alkaline urine, neutrophils lyse rapidly and begin to lose nuclear detail.
- Neutrophils exposed to hypotonic urine absorb water and swell, and the Brownian movement or erratic movement of the granules within these larger cells produces a sparkling appearance, hence the name “glitter cells”.
- They are of no pathologic significance.
- Eosinophils are not normally seen in the urine; therefore, the finding of more than 1% eosinophils in 100-500 cells is considered significant.
- Evaluation of a concentrated, stained urine sediment is required for performing a urinary eosinophil test.
- Sediment may be concentrated by routine centrifugation alone or with cytocentrifugation.
- For stains, see Sediment Stains in Sediment Examination Techniques above.
- The presence of urinary eosinophils is primarily associated with drug-induced interstitial nephritis; however, small numbers of eosinophils may be seen with urinary tract infection (UTI) and renal transplant rejection.
- Mononuclear WBCs such as lymphocytes, monocytes, macrophages and histiocytes may be present in small numbers and are usually not identified in the wet preparation urine microscopic analysis.
- Because lymphocytes are the smallest WBCs, they may resemble RBCs.
- They may be seen in increased numbers in the early stages of renal transplant rejection.
- Monocytes, macrophages, and histiocytes are large cells and may appear vacuolated or contain inclusions.
- Specimens containing an increased amount of mononuclear cells that cannot be identified as epithelial cells should be referred for cytodiagnostic urine testing.
- Epithelial cells, specifically renal tubular epithelial (RTE) cells, are usually larger than WBCs and more polyhedral in shape, with an eccentrically located nucleus. WBCs have a more irregular shape.
- Supravital staining or the addition of acetic acid can be used to enhance nuclear detail.
Usually, fewer than five leukocytes per hpf are found in normal urine; however, higher numbers may be present in urine from females.
- They enter the urine through glomerular or capillary trauma, or from ameboid migration through the tissues to sites of infection or inflammation in the genitourinary system.
- Pyuria or increased WBCs in the urine is caused by bacterial infections (e.g. pyelonephritis, cystitis, prostatitis and urethritis) or nonbacterial disorders (e.g. glomerulonephritis, lupus erythematosus, interstitial nephritis and tumors).
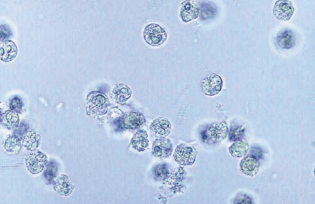
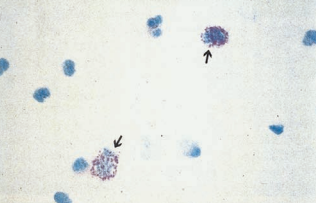
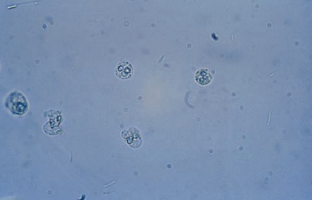
Epithelial Cells
They represent normal sloughing of old cells from linings of the genitourinary system.
Three types of epithelial cells are seen in urine: squamous, transitional (urothelial), and renal tubular.
Squamous epithelial cells originate from the linings of the vagina and female urethra and the lower portion of the male urethra.
Squamous cells are the largest cells found in the urine sediment.
They may occasionally appear folded, and they contain abundant irregular cytoplasm and a prominent nucleus about the size of an RBC.
They are often the first structures observed when the sediment is examined under low-power magnification and can serve as a good reference for focusing of the microscope.
They are commonly reported in terms of rare, few, moderate, or many and in terms of low-power or high-power magnification based on laboratory protocol.
Difficulties with these cells include confusion with casts, disintegration in urine that’s not fresh and obstruction of RBCs and WBCs in examination.
They represent normal cellular sloughing and have no pathologic significance.
They are more frequently seen in urine from female patients.
Specimens collected using the midstream clean-catch technique contain less squamous cell contamination.
A variation of the squamous epithelial cell is the clue cell, which does have pathologic significance indicating vaginal infection by the bacterium Gardnerella vaginalis.
It is normally part of the vagina’s normal flora and small numbers of clue cells may be present in the urinary sediment, so routine testing is needed to monitor it’s number.
- Routine testing for clue cells is performed by examining a vaginal wet preparation for the presence of the characteristic cells.
To be considered a clue cell, the bacteria should cover most of the cell surface and extend beyond the edges of the cell, giving a granular, irregular appearance.
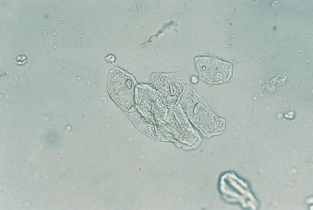
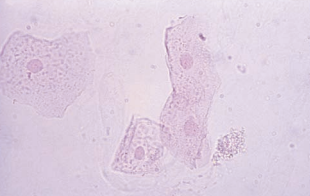
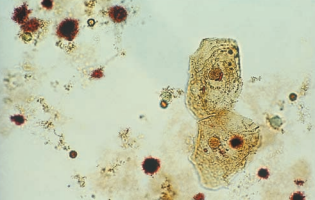
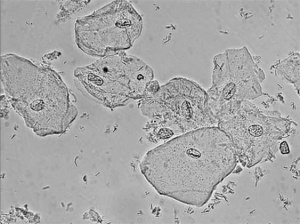
Renal tubular epithelial (RTE) cells come from the renal tubules and have different characteristics depending on their area of origin.
RTE cells must be identified and enumerated using high-power magnification and reported as rare, few, moderate or many, or as the actual number per high-power field.
- Classification of RTE cells as to site of origin is not considered a part of the routine sediment analysis and often requires special staining techniques.
The presence of more than two RTE cells per high-power field indicates tubular injury, necrosis or glomerular disorder, and such specimens should be referred for cytologic urine testing.
- Conditions producing tubular necrosis include exposure to heavy metals, drug-induced toxicity, hemoglobin and myoglobin toxicity, viral infections (hepatitis B), pyelonephritis, allergic reactions, malignant infiltrations, salicylate poisoning and acute allogenic transplant rejection.
It can absorb other substances as they pass through during reabsorption.
In cases of liver damage, they absorb bilirubin and appear a deep yellow color.
After episodes of hemoglobinuria (transfusion reactions, paroxysmal nocturnal hemoglobinuria, etc.), RTE cells absorb hemoglobin and convert it to yellow-brown hemosiderin granules.
The granules may also be seen free-floating in the sediment.
Confirmation of the presence of hemosiderin is performed by staining the sediment with Prussian blue.
RTE cells absorb lipids that are present in the glomerular filtrate and become oval fat bodies.
They appear highly refractile and difficult to observe, so staining and polarizing microscopy is used.
- Examination of the sediment using polarized light results in the appearance of characteristic Maltese cross formations in droplets containing cholesterol.
They are reported as the average number per hpf.
The droplets are composed of triglycerides, neutral fats, and cholesterol.
They are usually seen in conjunction with free-floating fat droplets.
- Free-floating fat droplets also stain or polarize depending on their composition.
- They may be observed floating on the top of the specimen.
- Care should be taken not to confuse the droplets with starch and crystal particles that also polarize.
- Specimen contamination by vaginal preparations and lubricants used in specimen collection must be considered when only free-floating fat droplets are present.
They may be confused with large fat-laden histiocytes and “bubble cells”.
- They can be differentiated from oval fat bodies by their large size.
- Large fat-laden histiocytes are indicative of lipid-storage diseases.
- Bubble cells are RTE cells containing large nonlipid-filled vacuoles, and they represent injured cells in which the endoplasmic reticulum has dilated prior to cell death and are indicative of acute tubular necrosis.
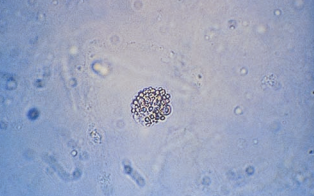
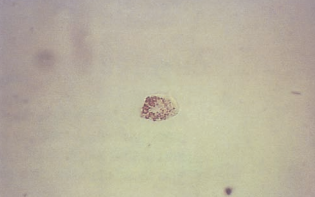
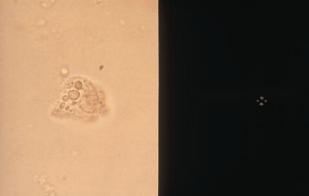
The cells from the proximal convoluted tubule (PCT) are larger, have a rectangular convoluted shape resembling casts, and a coarsely granular cytoplasm.
- To differentiate from casts, a nucleus should be present.
The cells from the distal convoluted tubule (DCT) are round or oval and have an eccentrically placed round nucleus.
- To differentiate from WBCs and spherical transitional epithelial cells, observe the nucleus.
The cells from the collecting duct are cuboidal with at least one straight edge and never round, and they have an eccentrically placed nucleus that is not easily visible.
- To differentiate from spherical and polyhedral transitional cells, observe the nucleus and edges.
- They frequently appears as large sheets of cells in groups of three or more called renal fragments.
- They may indicate severe tubular injury with basement membrane disruption.
- If they are single, it may indicate salicylate poisoning.
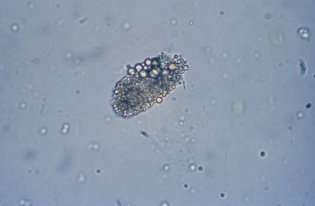
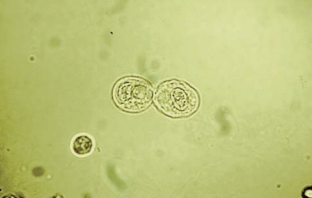
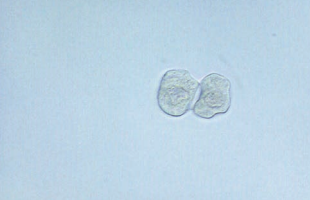
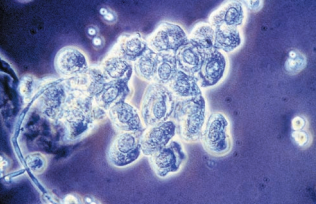
Transitional epithelial cells originate from the lining of the renal pelvis, calyces, ureters, and bladder, and from the upper portion of the male urethra.
Transitional epithelial cells are smaller than squamous cells, have distinct centrally-located nuclei, and appear in several forms, including spherical, polyhedral and caudate.
- Cells in direct contact with the urine absorb water, becoming spherical in form and much larger than the polyhedral and caudate cells.
- Spherical forms of transitional epithelial cells are sometimes difficult to distinguish from RTE cells.
- The presence of a centrally located rather than eccentrically placed nucleus and supravital staining can aid in the differentiation.
Transitional cells are identified and enumerated using high-power magnification.
They are usually reported as rare, few, moderate, or many following laboratory protocol.
They are usually present in small numbers in normal urine, representing normal cellular sloughing.
Increased numbers of transitional cells seen singly, in pairs, or in clumps (syncytia) are present following invasive urologic procedures such as catheterization and are of no clinical significance.
- However, an increase in transitional cells exhibiting abnormal morphology such as vacuoles and irregular nuclei may be indicative of malignancy or viral infection and should be referred for cytologic examination.
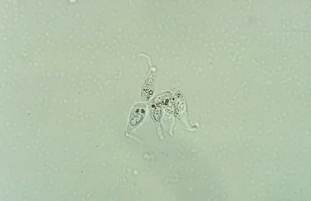
Bacteria
Bacteria may be present in the form of cocci (spherical) or bacilli (rods).
They must be observed using high-power magnification and reported as few, moderate, or many per high-power field.
- Use of phase microscopy aids in the visualization of bacteria.
To be considered significant for UTI, bacteria should be accompanied by WBCs.
The presence of bacteria can be indicative of either lower or upper UTI and are routinely followed up with a specimen for quantitative urine culture.
- The bacteria most frequently associated with UTI are the Enterobacteriaceae (referred to as gram-negative rods); however, the cocci-shaped Staphylococcus and Enterococcus are also capable of causing UTI.
- The actual bacteria producing an UTI cannot be identified with the microscopic examination.
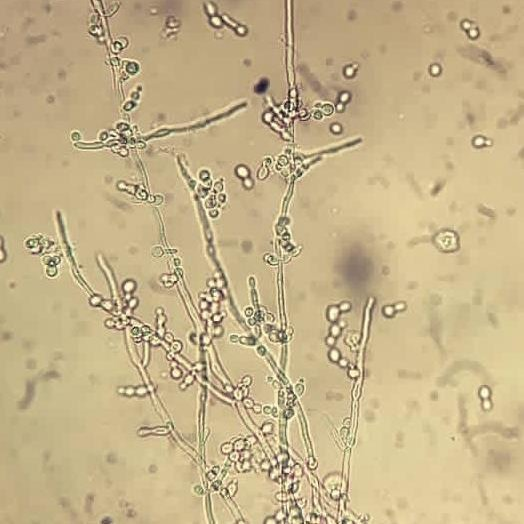
Yeast
Yeast cells are reported as rare, few, moderate, or many per hpf.
- Yeast cells appear as small, refractile oval structures that may or may not contain a bud.
- They may appear as branched, mycelial forms in severe infections.
Yeast cells, primarily Candida albicans, are seen in the urine of diabetic immunocompromised patients and women with vaginal moniliasis.
- The acidic, glucose-containing urine of patients with diabetes provides an ideal medium for the growth of yeast.
- A true yeast infection should be accompanied by the presence of WBCs.
Parasites
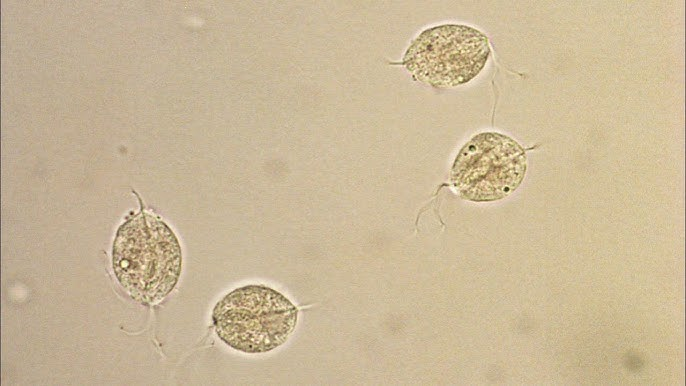
The most frequent parasite encountered in the urine is Trichomonas vaginalis.
- It is a sexually transmitted pathogen associated primarily with vaginal inflammation.
- Infection of the male urethra and prostate is asymptomatic.
- It is a pear-shaped flagellate with an undulating membrane.
- It is easily identified in wet preparations of the urine sediment by its rapid darting movement in the microscopic field, but it may be confused with a WBC or a transitional or RTE epithelial cell if not moving.
- Phase microscopy may enhance visualization of the flagella or undulating membrane.
- It is usually reported as rare, few, moderate, or many per hpf.
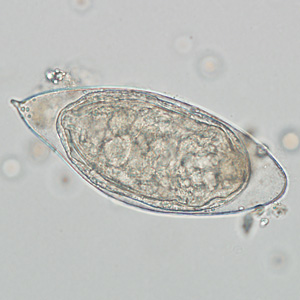
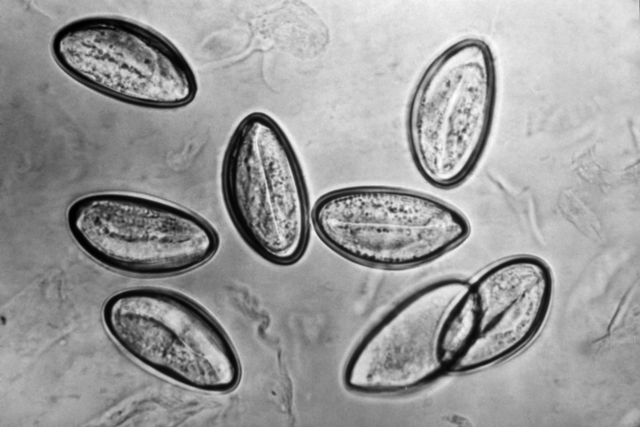
The ova of the bladder parasite, Schistosoma haematobium, will appear in the urine.
- It is seldom seen in the United States.
The presence of the ova from the pinworm Enterobius vermicularis indicates fecal contamination.
- It is normally found in the intestinal flora.
Spermatozoa
They are characterized by oval slightly tapered heads, long flagella-like tails and no motility.
- Urine is toxic to spermatozoa.
Spermatozoa are occasionally found in the urine of both men and women following sexual intercourse, masturbation, or nocturnal emission.
They are rarely of clinical significance except in cases of male infertility or retrograde ejaculation in which sperm is expelled into the bladder instead of the urethra.
A positive reagent strip test for protein may be seen when increased amounts of semen are present.
Reporting protocols vary due to varied degrees of clinical significance and possible legal consequences.
Mucus
Mucus is a protein material produced by the glands and epithelial cells of the lower genitourinary tract and the RTE cells.
It is characterized by thread-like structures with a low refractive index.
- Subdued light is required when using bright-field microscopy.
- Care must be taken not to confuse clumps of mucus with hyaline casts.
- The differentiation can usually be made by observing the irregular appearance of the mucous threads.
Mucous threads are reported as rare, few, moderate, or many per lpf.
Mucus is more frequently present in female urine specimens.
It has no clinical significance when present in either female or male urine.
Casts
They are formed within the lumens of the distal convoluted tubules and collecting ducts and are unique to the kidney.
The major constituent of casts is Tamm-Horsfall protein, a glycoprotein excreted by the RTE cells of the distal convoluted tubules and upper collecting ducts.
Under normal conditions, Tamm-Horsfall protein is excreted at a relatively constant rate but can increase from stress, exercise, dehydration and heat exposure.
- Tamm-Horsfall protein is aggreated into individual protein fibrils attached to the RTE cells.
- They are interweaved to form a loose fibrillar network, and urinary constituents may become enmeshed in the network.
- The process continues until it forms a solid structure.
- The protein fibrils detach from the epithelial cells and is excreted.
As the cast forms, urinary flow within the tubule decreases as the lumen becomes blocked.
The width of the cast depends on the size of the tubule in which it is formed.
- Broad casts are formed from tubular distention or originate from the collecting ducts.
- Cylindroids are formed at the junction of the ascending loop of Henle and the distal convoluted tubule, producing structures with a tapered end.
The appearance of a cast is also influenced by the materials present in the filtrate at the time of its formation and the length of time it remains in the tubule.
Hyaline casts consist almost entirely of Tamm-Horsfall protein.
The morphology of hyaline casts is varied, consisting of normal parallel sides and rounded ends, cylindroid forms and wrinkled or convoluted shapes, occasional with an adhering cell or granules.
Hyaline casts appear colorless in unstained sediments and have a refractive index similar to that of urine.
- The Sternheimer-Malbin stain produces a pink color in hyaline casts.
- Increased visualization can be obtained by phase microscopy.
They are the most common type, and the presence of zero to two hyaline casts per lpf is considered normal with the exception of increases from stress, exercise, dehydration and heat exposure.
Hyaline casts are increased in acute glomerulonephritis, pyelonephritis, chronic renal disease and congestive heart failure.
Granular casts represent the disintegration of cellular casts and tubule cells or protein aggregates filtered by the glomerulus.
They are easily visualized under low-power microscopy; however, final identification should be performed using high power to determine the presence of a cast matrix.
- They may contain an occasional recognizable cell.
- Artifacts, such as clumps of small crystals and fecal debris, may occur in shapes resembling casts and must be differentiated.
- Columnar RTE cells may also resemble granular casts, and staining for nuclear detail may be required.
- Granular casts seen in conjunction with WBC casts contain WBC granules of varying sizes.
- When granular casts remain in the tubules for extended periods, the granules further disintegrate, and the cast matrix develops a waxy appearance, the structure becomes more rigid, the ends of the casts may appear jagged or broken, and the diameter becomes broader.
The origin of the granules in nonpathologic conditions appears to be from the lysosomes excreted by RTE cells during normal metabolism.
- It is not unusual to see hyaline casts containing one or two of these granules.
- Increased cellular metabolism occurring during periods of strenuous exercise accounts for the transient increase of granular casts that accompany the increased hyaline casts.
Waxy casts have a brittle, highly refractive cast matrix caused by degeneration of the hyaline cast matrix and any cellular elements or granules contained in the matrix.
Waxy casts are more easily visualized than hyaline casts because of their higher refractive index.
- As a result of the brittle consistency of the cast matrix, they often appear fragmented with jagged ends and have notches in their sides.
Waxy casts are representative of extreme urine stasis, indicating chronic renal failure.
- They are usually seen in conjunction with other types of casts associated with the condition that has caused the renal failure.
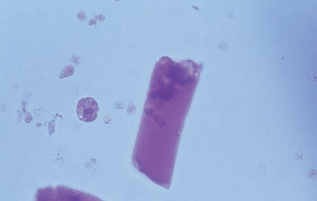
Broad casts, also known as renal failure casts, are a a mold of the distal convoluted tubules or collecting duct.
They represent extreme urine stasis and indicate the destruction (widening) of the tubular walls or the compromise of the collecting duct.
Bile-stained broad, waxy casts are seen as the result of the tubular necrosis caused by viral hepatitis.
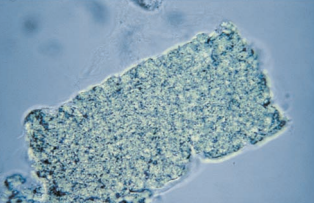
RBC casts are more fragile than other casts and may exist as fragments or have a more irregular shape as the result of tightly packed cells adhering to the protein matrix.
RBC casts are easily detected under low power by their orange-red color, but high-power magnification is used to observe the presence of a cast matrix, thereby differentiating the structure from a clump of RBCs.
- As an RBC cast ages, cell lysis begins and the cast develops a more homogenous appearance but retains its color.
- In the presence of massive hemoglobinuria or myoglobinuria, homogenous orange-red or red-brown casts and granular dirty brown casts may also be present.
- They are reported as the number of RBC casts per lpf.
It is highly improbable that RBC casts will be present in the absence of free-standing RBCs and a positive reagent strip test for blood.
They indicate bleeding within the nephron, specifically damage to the glomerulus (glomerulonephritis) and nephron capillary structure.
- They are usually associated with proteinuria and dysmorphic erythrocytes.
- Homogenous brownish casts indicate severe hemoglobinuria or myoglobinuria where toxic degradation products are present, leading to acute tubular necrosis and renal failure.
- They must be present in conjunction with other pathologic findings such as RTE cells and a positive reagent strip test for blood.
- RBC casts have also been observed in healthy individuals following participation in strenuous contact sports.
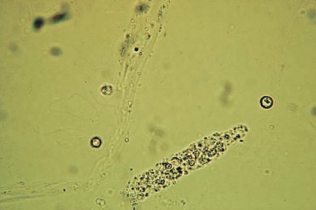
WBC casts are composed of neutrophils and may appear granular with multilobed nuclei and irregular borders.
WBC casts are visible under low-power magnification but must be positively identified using high power, along with supravital staining.
- Staining may be necessary to demonstrate the characteristic nuclei to differentiate it from RTE casts.
- They may be confused with WBC clumps, so a matrix must be observed.
The appearance of WBC casts in the urine signifies infection or inflammation within the nephron such as pyelonephritis (upper UTI) or glomerulonephritis, or nonbacterial inflammations such as acute interstitial nephritis.
- Bacteria are present in cases of pyelonephritis and glomerulonephritis, but are not present with acute interstitial nephritis.
- RBC casts accompany EBC casts in cases or glomerulonephritis.
- Eosinophil casts may be present in appropriately stained specimens.
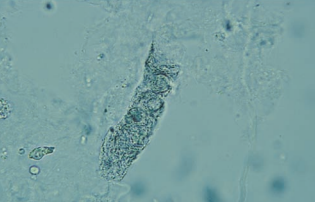
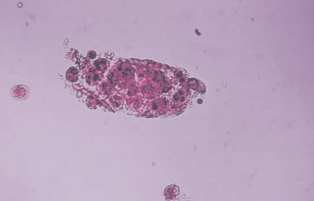
Bacterial casts resemble granular casts containing bacilli both within and bound to the protein matrix, and sometimes with WBCs.
Their presence should be considered when WBC casts and many free WBCs and bacteria are seen in the sediment, and it is confirmed by performing a Gram stain on the dried or cytocentrifuged sediment.
It is usually indicative of pyelonephritis.
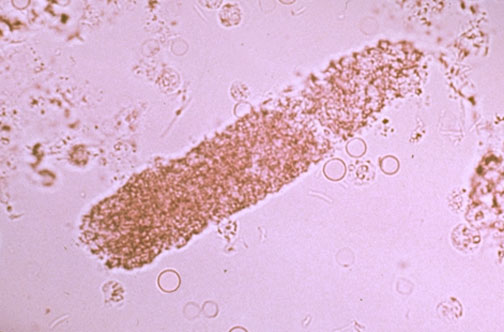
Epithelial cell casts are similar to hyaline casts but have attached RTE cells that produce them.
Casts containing RTE cells represent the presence of advanced tubular destruction, producing urinary stasis along with disruption of the tubular linings.
- They are associated with heavy metal and chemical or drug-induced toxicity, viral infections, and allograft rejection.
- When tubular damage is present, some cells may be incorporated into the cast matrix, but the majority will be very noticeably attached to the cast surface.
- The cells visible on the cast matrix are the smaller round and oval cells as they are formed in the distal convoluted tubule.
- To differentiate from WBCs, they are stained and observed through phase microscopy to enhance nuclear detail.
- Fragments of epithelial tissue may also be attached to the cast matrix.
- Bilirubin-stained RTE cells are seen in cases of hepatitis.
- They also accompany WBC casts in cases of pyelonephritis.
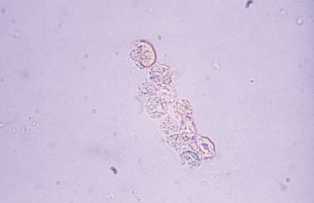
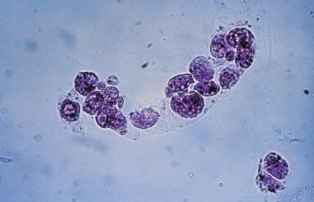
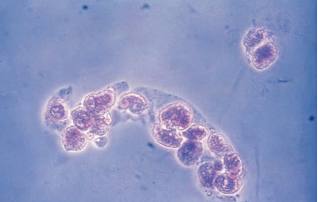
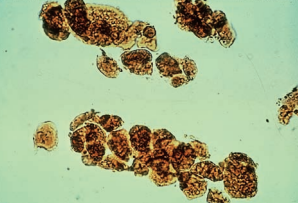
Fatty casts are highly refractile with a cast matrix containing few or many fat droplets and intact oval fat bodies attached to the matrix.
Fatty casts are confirmed using polarized microscopy and Sudan III or Oil Red O fat stains.
Fatty casts are seen in conjunction with oval fat bodies and free fat droplets in disorders causing lipiduria.
They are most frequently associated with the nephrotic syndrome, but are also seen in toxic tubular necrosis, diabetes mellitus and crush injuries.
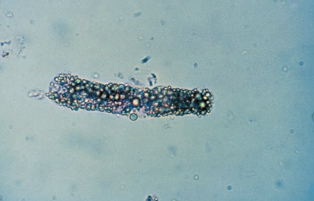
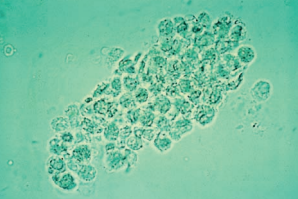
Mixed cellular casts containing multiple cell types are not uncommon.
Mixed cellular casts most frequently encountered include RBC and WBC casts in glomerulonephritis and WBC and RTE cell casts, or WBC and bacterial casts in pyelonephritis.
When mixed casts are present, there should also be homogenous casts of at least one of the cell types, and they will be the primary diagnostic marker.
- For example, in glomerulonephritis, the predominant casts will be RBC, and in pyelonephritis, the predominant casts will be WBC. Bacteria are often incorporated into WBC casts and provide little additional diagnostic significance.
The protein gels more readily under conditions of urine-flow stasis, acidity and the presence of sodium and calcium.
Tamm-Horsfall protein is found in both normal and abnormal urine but is not detected by reagent strip protein methods.
Other proteins present in the urinary filtrate, such as albumin and immunoglobulins, are also incorporated into the cast matrix.
Their shape is representative of the tubular lumen with parallel sides and somewhat rounded ends, a high refractive index and additional elements present in the filtrate.
It should be examined immediately after collection as possible under reduced light and from low-power to high-power magnification.
- The cast matrix dissolves quickly in dilute alkaline urine.
- They are reported as the average number per 10 lpfs.
Crystals
Crystals are formed by the precipitation of urine solutes, including inorganic salts, organic compounds and medications (iatrogenic compounds).
- Solutes precipitate more readily at low temperatures, and it takes place in specimens that have remained at room temperature or been refrigerated prior to testing.
- As the concentration of urinary solutes increases, their ability to remain in solution decreases, resulting in crystal formation.
- The presence of crystals in freshly voided urine is most frequently associated with concentrated (high specific gravity) specimens.
- In general, organic and iatrogenic compounds crystallize more easily in an acidic pH, whereas inorganic salts are less soluble in neutral and alkaline solutions.
- An exception is calcium oxalate, which precipitates in both acidic and neutral urine.
- All abnormal crystals are found in acidic urine.
- The reversal of these changes can cause crystals to dissolve.
- When solubility characteristics are needed for identification, the sediment should be aliquoted to prevent destruction of other elements.
Crystals are usually reported as rare, few, moderate or many per hpf while abnormal crystals may be averaged and reported per lpf.
- Additional aids in crystal identification include the use of polarized microscopy and solubility characteristics of the crystals.
- The geometric shape of a crystal determines its birefringence regardless of size.
- Although the size of a particular crystal may vary (slower crystallization produces larger crystals), the basic structure remains the same.
The most common crystals seen in acidic urine are urates, consisting of amorphous urates, uric acid, acid urates and sodium urates.
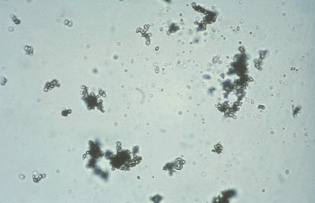
Amorphous urates occur in clumps resembling granular casts and appear microscopically as yellow-brown granules.
They are frequently encountered in specimens that have been refrigerated.
They are found in acidic urine with a pH greater than 5.5.
Uric acid crystals occur in a variety of shaped and appear yellow-brown or colorless.
They may be rhombic, four-sided and flat (whetstones), wedged, six-sided and in rosettes.
- Six-sided crystals are usually colorless and resemble cystine crystals.
- They are distinguished by their high birefringent under polarized light.
Increased amounts of uric acid crystals, particularly in fresh urine, are associated with increased levels of purines and nucleic acids and are seen in patients with leukemia who are receiving chemotherapy, in patients with Lesch-Nyhan syndrome and, sometimes, in patients with gout.
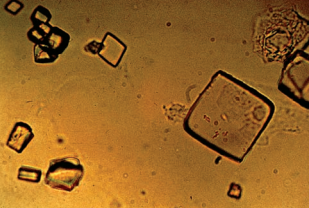
Acid urates appear as larger granules and may have spicules similar to the ammonium biurate crystals seen in alkaline urine.
They are rarely encountered and are frequently seen in conjunction with amorphous urates.
They are seen in less acidic urine.
They have little clinical signifance.

Sodium urate crystals are needle-shaped and are seen in synovial fluid during episodes of gout but appear in the urine.
They are rarely encountered and are frequently seen in conjunction with amorphous urates.
They are seen in less acidic urine.
They have little clinical signifance.

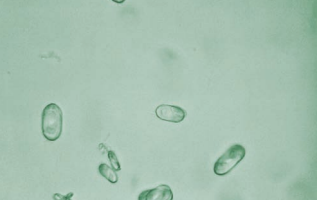
Calcium oxalate crystals appear either in dihydrate or monohydrate form.
The most common form of calcium oxalate crystals is the dihydrate that is easily recognized as a colorless octahedral envelope or as two pyramids joined at their bases.
- They are birefringent under polarized light.
Less characteristic and less frequently seen is the monohydrate form that are oval or dumbbell shaped.
- They may be confused with RBCs, so it is distingued by it’s birefringence upon examination under polarized light.
- Massive amounts of crystals are frequently produced in cases of ethylene glycol (antifreeze) poisoning.
Both types are frequently seen in acidic urine, but they can be found in neutral urine and even rarely in alkaline urine.
They are sometimes seen in clumps attached to mucous strands and may resemble casts.
- The finding of clumps of calcium oxalate crystals in fresh urine may be related to the formation of renal calculi.
- The majority of renal calculi are composed of calcium oxalate.
- They are also associated with foods high in oxalic acid, such as tomatoes and asparagus and ascorbic acid.
- Oxalic acid is an end product of ascorbic acid metabolism.
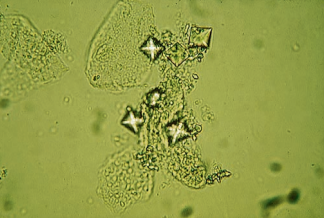
Phosphates represent the majority of the crystals seen in alkaline urine and include amorphous phosphate, triple phosphate, calcium phosphate, calcium carbonate and ammonium biurate.
Amorphous phosphates are granular in appearance, similar to amorphous urates.
When present in large quantities following specimen refrigeration, they cause a white precipitate that does not dissolve on warming.
They can be differentiated from amorphous urates by the color of the sediment and the urine pH.
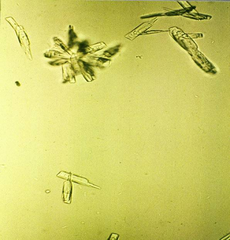
Triple phosphate (ammonium magnesium phosphate) crystals have a prism shape that frequently resembles a “coffin lid”.
As they disintegrate, the crystals may develop a feathery appearance.
Triple phosphate crystals are birefringent under polarized light.
They have no clinical significance; however, they are often seen in highly alkaline urine associated with the presence of urea-splitting bacteria.

Calcium phosphate crystals are not frequently encountered and may appear as colorless flat rectangular plates or thin prisms often in rosette formations.
The rosette forms may be confused with sulfonamide crystals when the urine pH is in the neutral range.
- Calcium phosphate crystals dissolve in dilute acetic acid and sulfonamides do not.
They have no clinical significance, though calcium phosphate is a common constituent of renal calculi.
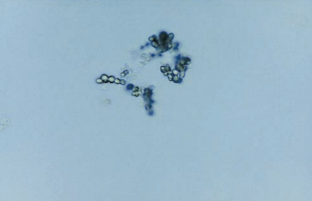
Calcium carbonate crystals are small and colorless with dumbbell or spherical shapes.
They may occur in clumps that resemble amorphous material, but they can be distinguished by the formation of gas after the addition of acetic acid.
They are also birefringent, which differentiates them from bacteria.
Calcium carbonate crystals have no clinical significance.
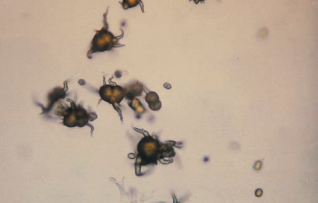
Ammonium biurate crystals exhibit the characteristic yellow-brown color of the urate crystals seen in acidic urine.
They are frequently described as “thorny apples” because of their appearance as spicule-covered spheres.
Ammonium biurate crystals resemble other urates in that they dissolve at 60°C and convert to uric acid crystals when glacial acetic acid is added.
Ammonium biurate crystals are almost always encountered in old specimens and may be associated with the presence of the ammonia produced by urea-splitting bacteria.
Abnormal urine crystals are found in acidic urine or rarely in neutral urine and can be caused by a variety of compounds, particularly when they are administered in high concentrations.
Cystine crystals appear as thick or thin colorless hexagonal plates, and they may disintegrate in the presence of ammonia.
They may be difficult to differentiate from colorless uric acid crystals.
- Uric acid crystals are very birefringent under polarized microscopy, whereas only thick cystine crystals have polarizing capability.
- Positive confirmation of cystine crystals is made using the cyanide-nitroprusside test.
Cystine crystals are found in the urine of persons who inherit a metabolic disorder that prevents reabsorption of cystine by the renal tubules (cystinuria).
- Persons with cystinuria have a tendency to form renal calculi, particularly at an early age.
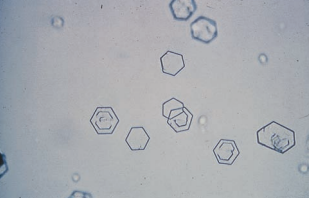
Cholesterol crystals resembling a rectangular plate with a notch in one or more corners, but they are rarely seen unless refrigerated and more commonly found in droplet form.
Cholesterol crystals are highly birefringent with polarized light.
They are associated with disorders producing lipiduria, such as the nephrotic syndrome, and are seen in conjunction with fatty casts and oval fat bodies.
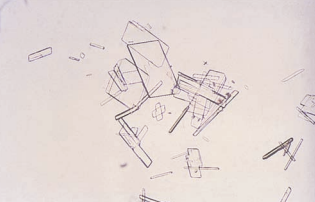
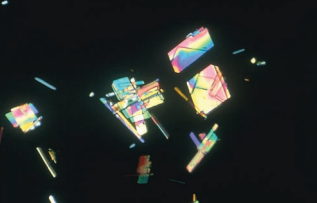
Crystals of radiographic contrast media have a very similar appearance to cholesterol crystals and also are highly birefringent.
Differentiation is best made by comparison of the other urinalysis results, examining the patient history, and observing for accompanying substances.
- Cholesterol crystals should be accompanied by other lipid elements and heavy proteinuria.
- The specific gravity of a specimen containing radiographic contrast media is markedly elevated when measured by refractometer.

Tyrosine crystals appear as fine colorless to yellow needles that frequently form clumps or rosettes.
They are usually seen in conjunction with leucine crystals in specimens with positive chemical test results for bilirubin.
Tyrosine crystals may also be encountered in inherited disorders of amino-acid metabolism.
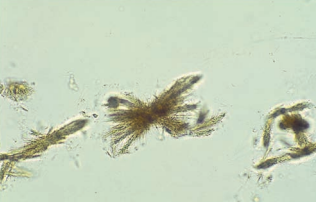
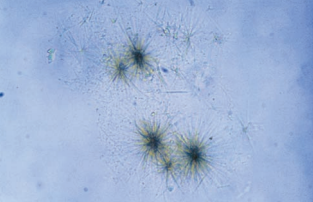
Leucine crystals are yellow-brown spheres that demonstrate concentric circles and radial striations.
They are seen less frequently than tyrosine crystals and, when present, should be accompanied by tyrosine crystals.
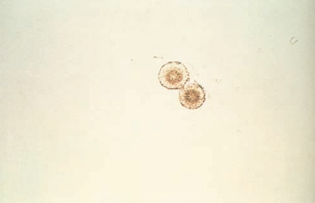
Bilirubin crystals appear as clumped needles or granules with the characteristic yellow color of bilirubin.
Bilirubin crystals are present in hepatic disorders producing large amounts of bilirubin in the urine.
- A positive chemical test result for bilirubin would be expected.
In disorders that produce renal tubular damage, such as viral hepatitis, bilirubin crystals may be found incorporated into the matrix of casts.
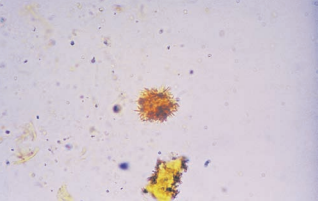
Sulfonamide crystals appear in a variety of crystal shapes and colors.
Shapes most frequently encountered include needles, rhombics, whetstones, sheaves of wheat and rosettes with colors ranging from colorless to yellow-brown.
- There are a variety of sulfonamide medications with different formulations.
- A check of the patient’s medication history aids in the identification confirmation.
- If necessary, a diazo reaction can be performed for further confirmation.
This is commonly found in patients treated for UTIs.
Inadequate patient hydration was and still is the primary cause of sulfonamide crystallization.
The appearance of sulfonamide crystals in fresh urine can suggest the possibility of tubular damage if crystals are forming in the nephron.
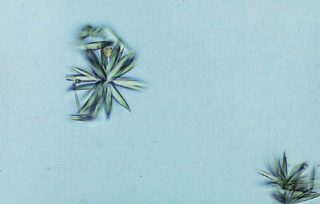
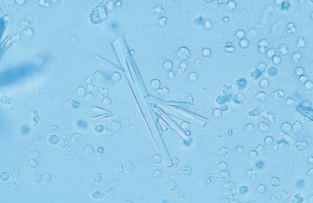
Ampicillin crystals appear as colorless needles that tend to form bundles following refrigeration.
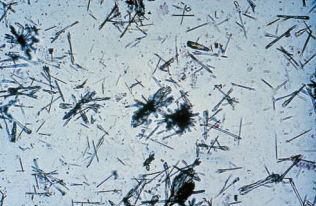
Precipitation of antibiotics is not frequently encountered except for the rare observation of ampicillin crystals following massive doses of this penicillin compound without adequate hydration.

Contaminants of all types can be found in urine, particularly in specimens collected under improper conditions or in dirty containers.
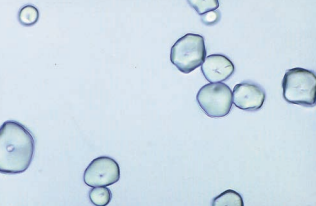
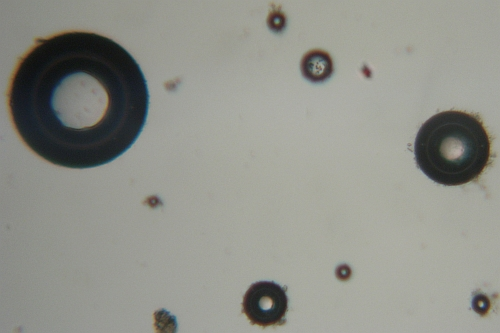
Starch granules are highly refractile spheres, usually with a dimpled center.
They resemble fat droplets when polarized, producing a Maltese cross formation, and may also occasionally be confused with RBCs.
- Differentiation between starch and pathologic elements can be made by considering other urinalysis results, including chemical tests for blood or protein and the presence of oval fat bodies or fatty casts.
Starch granule contamination may occur when cornstarch is the powder used in powdered gloves.
Oil droplets and air bubbles also are highly refractile.
They may resemble RBCs to inexperienced laboratory personnel.
Oil droplets may result from contamination by immersion oil or lotions and creams.
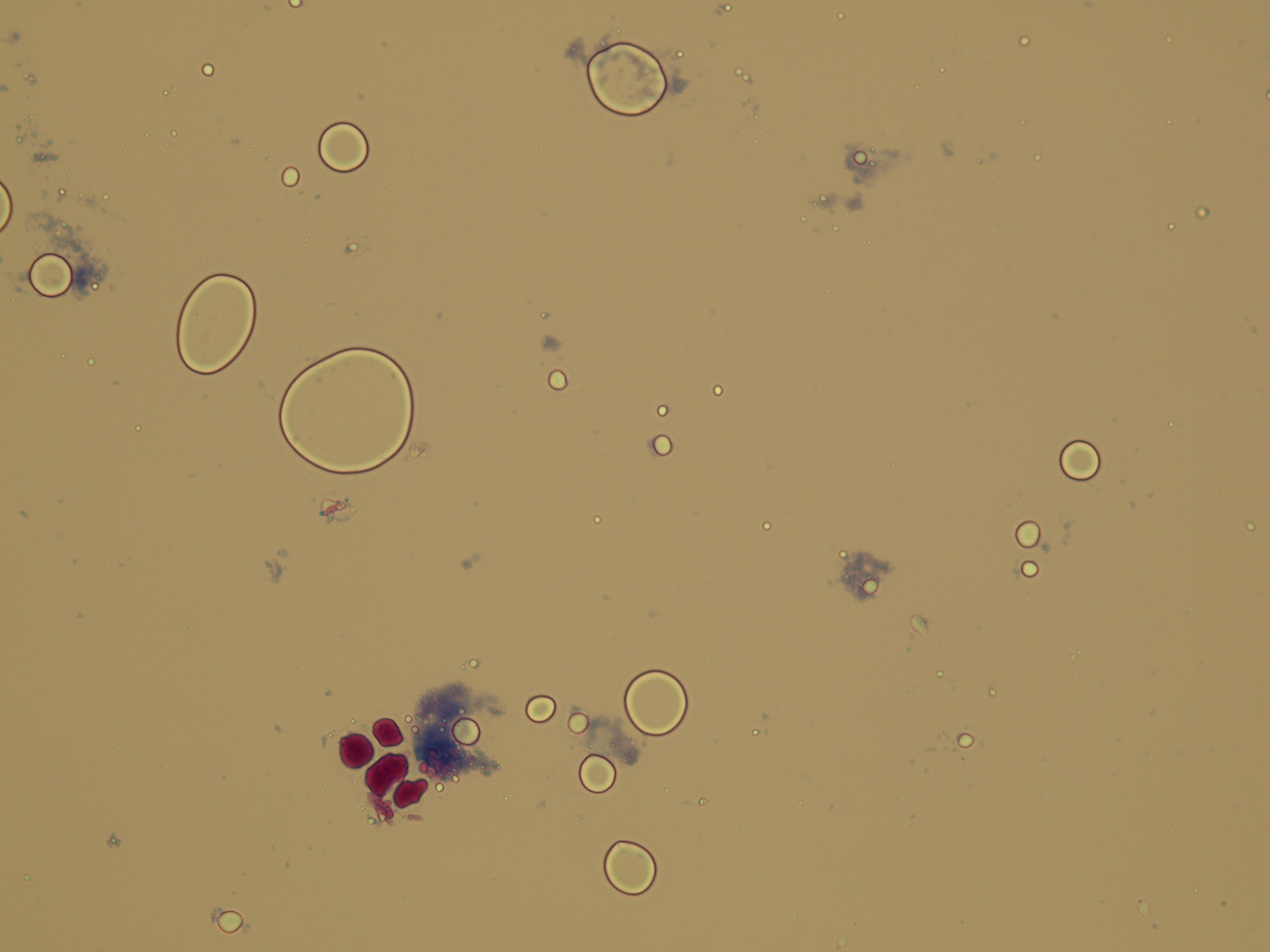
Air bubbles occur when the specimen is placed under a cover slip.
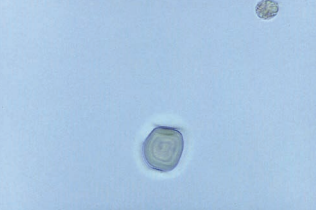
Pollen grains appear as spheres with a cell wall and occasional concentric circles.
Their large size may cause them to be out of focus with true sediment constituents.
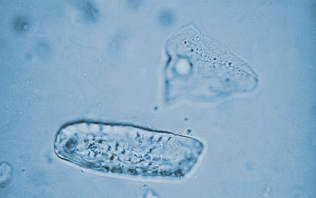
Hair and fibers from clothing and diapers are long and refractile.
They may be mistaken for casts, so they are differentiated by being birefringent under polarized light.
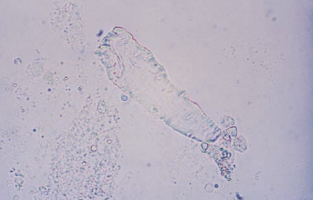
Fecal artifacts may appear as plant and meat fibers or as brown amorphous material in a variety of sizes and shapes.
Improperly collected specimens or rarely the presence of a fistula between the intestinal and urinary tracts may produce fecal specimen contamination.
\