FLVS Chemistry: Module 4 Notes (w/ Flashcards)
04.01 Conservation of Mass:
Chemical Equations:
A chemical reaction starts with a reactant (the initial substance before a chemical change) and ends with a product (the new substance created after a chemical change).
The phase of matter of each substance are sometimes shown in subscripts: (s) for solid, (l) for liquid, and (g) for gas.
Conservation of Mass:
Law of Conservation of Mass: the law that explains that mass cannot be created or destroyed within a closed system
H2 + O2 = H2O
In a chemical reaction, atoms cannot be created or destroyed, but they can be rearranged.
2H2 + O2 = 2H2O
The number 2 in front of the joined hydrogen atoms on the reactant side of this equation shows how many of these joint diatomic molecules are necessary for the chemical reaction to take place.
According to the law of conservation of mass, matter cannot be created or destroyed in a chemical reaction, the mass of all the reactants will always add up to the total mass of all the products.
Using Coefficients:
When you examine a chemical reaction, make sure the number of atoms of each element on the reactant side is equal to the number of atoms of each element on the product side.
2H2(g) + O2(g) → 2H2O(l)
Reactants:
| Products:
|
You can only change the coefficients in a chemical reaction when balancing a chemical equation.
You cannot change the subscripts in any of the compound's formula because that would change the identity of the compound.
Balancing Equations:
Balance the following reaction: Na + Cl2 → NaCl
Count the elements | |
No coefficient means a 1 is there. Start by counting the number of atoms of each element on the reactant (left) and product (right) sides. On the left: On the right: This is not balanced due to the chlorine atoms. | 1 Na + 1 Cl2 → 1 NaCl |
Balance one of the elements | |
The only way to balance the chlorine atoms is by changing the coefficient on the NaCl to a 2. Let's count the number of atoms again. On the left: On the right: Chlorine is now balanced! However, now sodium is not balanced. | 1 Na + 1 Cl2 → 2 NaCl |
Continue with balancing the other element | |
Do not undo the 2 on the NaCl because it balances the chlorine atoms. To balance the sodium atoms, change the coefficient on the Na atom on the left side. Count the number of atoms again. On the left: On the right: Sodium is now balanced, too! The chemical equation is balanced. Two atoms of sodium react with one molecule of chlorine gas to form two formula units of sodium chloride. | 2 Na + 1 Cl2 → 2 NaCl |
04.02 Synthesis and Decomposition Reactions:
Building Up and Breaking Down:
Synthesis Reaction: a reaction in which two or more reactants combine to form one product
Decomposition Reaction: a reaction in which a single compound reacts to form more than one product
The reaction between hydrogen and oxygen to form water is an example of a synthesis reaction.
In a decomposition reaction, one reactant breaks apart and forms two or more products.
When electricity is passed through water, the water will break down and produce hydrogen and oxygen.
In A + B → AB, the letters A and B represent elements, compounds, or polyatomic ions,
and AB represents a compound made from A and B.In AB → A + B, AB represents a compound made from elements or polyatomic ions,
which separates into other compounds or individual elements A and B.
Electrolysis: the process in which an electric current is used to decompose a substance
Burning Compounds:
In a combustion reaction, a compound or element reacts with oxygen, releasing a large amount of energy in the form of light and heat. We use combustion reactions for cooking and for fuel.
In a combustion reaction, oxygen is always one of the reactants.
Any reaction that gives off heat is called exothermic.
For example, wood burning in a fireplace is an exothermic combustion reaction, giving off both heat and light.
Organic Compounds | Inorganic Compounds |
Organic compounds are compounds that contain carbon covalently bonded with other elements. Example equations: Combustion of propane:
Cellular Respiration:
| Not all combustion reactions involve organic compounds made of carbon and hydrogen, which means the products are not always carbon dioxide and water. Other substances, both compounds and elements, can burn in oxygen. Example equation: 4Fe + 3O2 → 2Fe2O3 |
04.03 Single and Double Replacement Reactions:
Trading Partners:
Double Replacement Reaction: a type of reaction in which the ions of two compounds exchange places in an aqueous solution to form two new compounds
Single Replacement Reaction: a type of reaction in which one element replaces a similar element within a compound
In a single displacement reaction, one element switches places with another element in a compound. The reactants in a single displacement reaction are always an element and a compound, and the products are a different element and a different compound.
In a double displacement reaction, atoms from two different compounds switch places.
The reactants are always two compounds, and the products are always two different compounds.
Double Replacement:
In a double replacement reaction, the ions of two different compounds in an aqueous solution exchange places to form two new compounds. Because the reactants are dissolved in water, they can move and collide together to form new products.
One of the products formed is typically a solid, a gas, or water. The other product usually remains dissolved in the solution.
AB + CD → AD + CB
A, B, C, D in the reactants represent ions. A and C are positive ions and B and D are negative ions.
AD and CB represent ionic or molecular compounds that are formed.
Insoluble Product | Molecular Product |
Double replacement reactions form a product that is insoluble in water, but it bubbles out of the solution as a gas instead of forming a solid precipitate. | Some double replacement reactions can form a molecular product. |
04.04 Redox Reactions:
Losses and Gains:
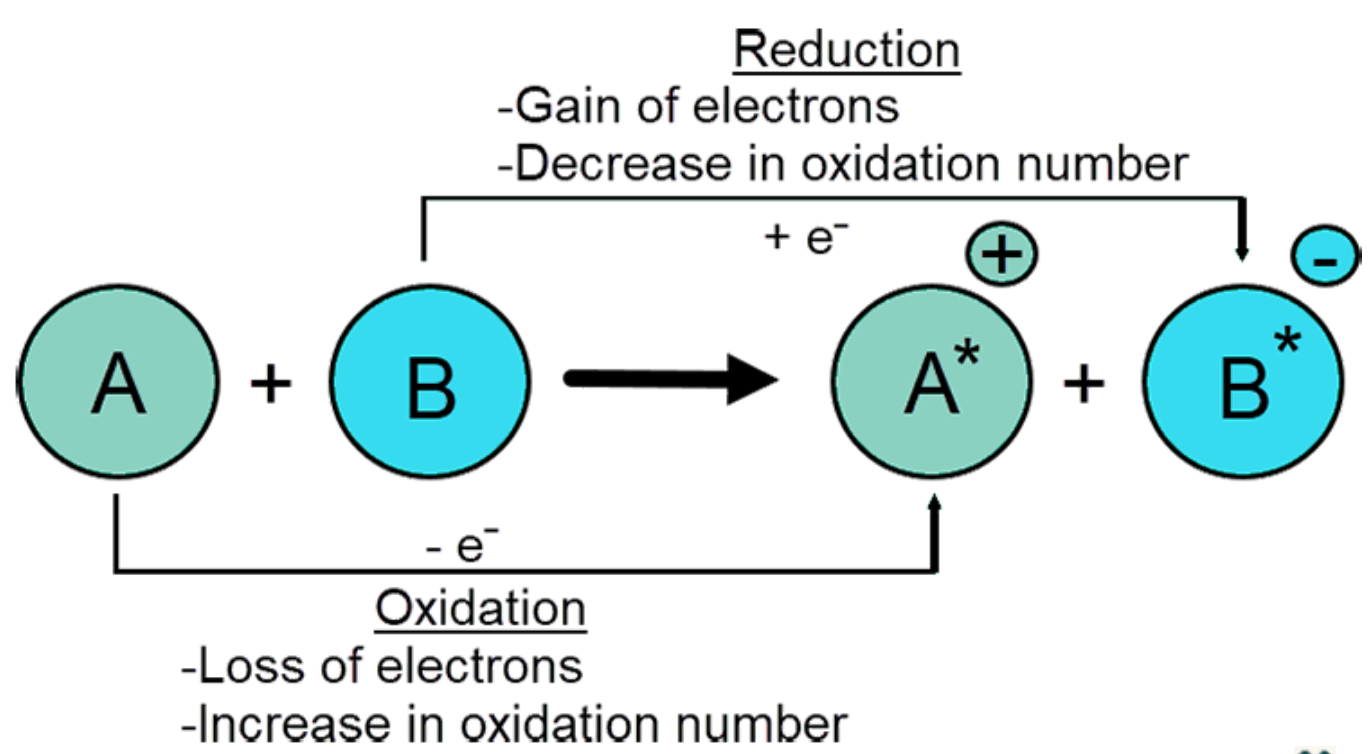
An oxidation-reduction reaction, also known as a redox reaction, involves the transfer of electrons where one species gains electrons and one loses electrons.
Oxidation is (the loss of electrons) when a substance loses one or more electrons during a reaction, therefore attaining a higher, more positive oxidation number.
Reduction is (the gain of electrons) when a substance gains one or more electrons during a reaction, therefore attaining a lower, more negative oxidation number.
Oxidation Numbers: a number assigned to an atom of an element that represents the number of electrons lost or gained
Transferring the Electrons:
Na(s) + Cl2(g) → 2NaCl(s)
Oxidation: Oxidation is defined as the loss of electrons in a chemical reaction. Many metals lose electrons when they react with oxygen to make an ionic metal oxide compound.
In the reaction of sodium and chlorine, neutral sodium was oxidized when it lost an electron to chlorine. This increased the initial charge of zero to +1 to make a positive Na+ ion.
Na(s) + | Cl2(g) → | 2NaCl(s) |
Charge: 0 | Charge: 0 | Charge of Na: +1 |
Reduction: Reduction is defined as the gain of electrons in a chemical reaction. When an atom or particle gains a negative electron, its overall charge is lowered, or "reduced."
In the reaction of sodium and chlorine, neutral chlorine got reduced when it gained electrons from the sodium. This decreased the initial charge of zero to -1 to make a negative Cl- ion.
Na(s) + | Cl2(g) → | 2NaCl(s) |
Charge: 0 | Charge: 0 | Charge of Cl: -1 |
Elements that are covalently bonded can be "oxidized" if they have less control over their electrons than before their "oxidation." The same goes for reduction, except they have more control of their electrons after "reduction."
The Redox Pattern:
Redox in single replacement | Redox absent in double replacement |
A single replacement reaction is almost always a redox reaction because it involves a neutral element that changes its electrostatic charge to combine with a new compound. | In a double replacement reaction, ions do not change their charges. They do not follow the pattern of redox reactions. |
OIL RIG = Oxidation Is Loss (of electrons), Reduction Is Gain (of electrons)
LEO the lion says GER = Losing Electrons is Oxidation, Gaining Electrons is Reduction
Oxidizing and Reducing Agents:
Reducing Agent: A reactant that causes another substance to be reduced (not being reduced itself). The reducing agent is the reactant that is oxidized.
Oxidizing Agent: A reactant that causes another substance to be oxidized (not being oxidized itself). The oxidizing agent is the reactant that is reduced.
An oxidizing agent causes another substance to be oxidized.
The only way to cause oxidation is to remove electrons from that other substance (because oxidation is loss of electrons).
Therefore, the oxidizing agent is reduced as it gains electrons from the other substance.
04.05 Reactions:
Fission vs. Fusion:
In chemical reactions, the transfer of electrons does not change an element's identity. In nuclear reactions, a nucleus is split apart (fission) or two nuclei are combined (fusion).
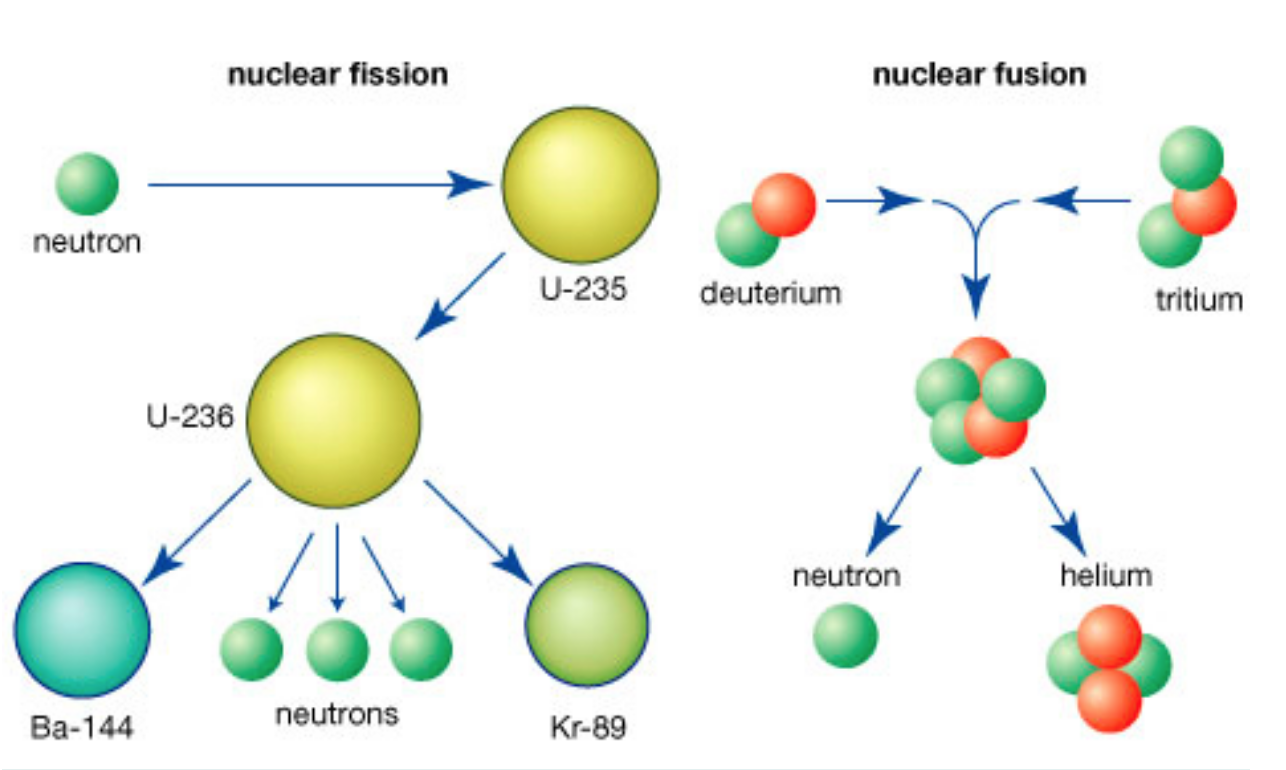
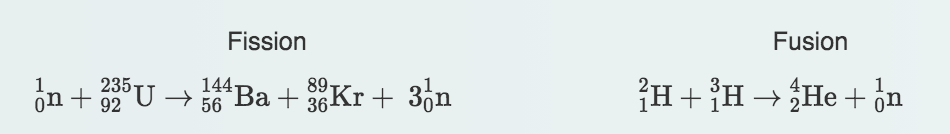
Fission:
Uranium-235 becomes an unstable isotope of becomes an unstable isotope of uranium, uranium-236.
This isotope splits into barium, krypton, and three neutrons. With the smaller particles, there is a release of energy after the split.
Fusion:
Two isotopes of hydrogen (deuterium has one neutron and tritium has two neutrons) collide and fuse.
This fusion reaction produces a helium nucleus (two protons and two neutrons) and a neutron particle. What is also released is a vast amount of energy.
From Mass to Energy:
When it comes to matter in a chemical reaction, the atoms in the reactants are the same as in the product, just rearranged to form new compounds.
Both chemical and nuclear reactions release energy.
As an energy source, nuclear fusion has several advantages over fission. The small isotopes of hydrogen are plentiful on Earth and easy to obtain.
However, the major disadvantage of fusion is that it is difficult to initiate and control.
04.06 Radioactive Decay (HONORS):
Radioactivity Around Us:
Radioactivity: the emission of radiation caused by the spontaneous disintegration of atomic nuclei
Although radiation itself occurs naturally, our society's use of radioactive material does increase our chances of exposure to radioactivity. Medical devices and procedures, and the byproducts produced by medical facilities and nuclear power plants, can also add to our exposure to radioactivity.
Fundamental Forces:
Strong Nuclear | Electromagnetic |
A strong nuclear force is an attractive force that holds the nucleus of an atom together. It is associated with holding the protons and other subatomic particles together inside the nucleus. Protons in an atom's nucleus all have a positive charge, so they naturally repel each other due to electromagnetic forces. The strong nuclear force overrides the electromagnetic repulsion and holds the positive protons and neutral neutrons packed tightly together in an atom's nucleus. | Electromagnetic forces are the second strongest of the four fundamental forces. They are the only forces that can both attract and repel each other, depending on the charges. The electromagnetic force also has a very long range. Electromagnetic force attracts negative electrons to the positive nucleus of an atom and underlies the interactions between atoms and molecules. |
Weak Nuclear | Gravitational |
Weak nuclear force is a very powerful force; it is given its name in comparison to strong nuclear force that is even stronger. The weak nuclear force is responsible for different types of particle decay and radioactivity. Radioactivity occurs when the nucleus of an unstable atom breaks down, | Gravity is the weakest of the four fundamental forces. It has a large range and can be experienced over a long distance. Gravity is the force of attraction that pulls objects toward the center of Earth and that holds the moon in orbit around Earth. Greater masses attract with more gravitational force, but gravitational force weakens as objects get farther apart. |
Radioactive Decay:
Atoms with unstable nuclei are radioactive; their nuclei eventually break down to form a different substance and release radiation in the process. We refer to this process as radioactive decay.
alpha (α) | beta (β) | gamma (γ) |
Alpha radiation is made up of a stream of alpha particles. Charge: Its atomic number: 2 Its mass number: 4 Alpha particles have a high amount of kinetic energy and can cause damage to surface materials such as skin and living tissue. They cannot normally penetrate lightweight materials such as paper or fabric. | Beta radiation is made up of a stream of beta particles. Beta particles are fast-moving electrons released from a nucleus when a neutron breaks apart into one proton and one electron. When the negative beta particle is released from the nucleus of an atom, the atom ends up with one more proton and one less neutron. Beta particles are more difficult to protect against than alpha particles; they can penetrate cloth and paper. Beta particles can penetrate deeply into skin and potentially harm or kill living cells. They cannot penetrate thin layers of denser materials such as aluminum and other metals. | Gamma radiation can be given off during different types of nuclear decay. Gamma rays are a form of electromagnetic waves with a very high frequency and greater energy than ultraviolet light or X-rays. They can penetrate through most materials. Gamma rays can cause much more damage to living cells than alpha or beta particles. They can be stopped by very dense materials, such as thick layers of lead. |
Transmutation:
When a radioactive element's nucleus decays, the number of protons and neutrons inside the nucleus changes. When this happens, the atom becomes another element. The changing of one element into another by radioactive decay is called transmutation.
Carbon-14 is a radioactive isotope of carbon that goes through beta decay to produce nitrogen-14. As a plant or animal lives, the ratio of carbon-14 to carbon-12 in its molecules is the same as the ratio of these two isotopes in the atmosphere. However, once the organism dies, the amount of carbon-14 begins to decrease as it undergoes radioactive decay. By determining how much carbon-14 has already decayed in the artifact, chemists can estimate the age of the item.
Half-Life:
Radioactive isotopes decay at different rates, but the rates are all measured in terms of the substance's half-life.
The half-life is the time needed for half of the radioactive atoms in a sample to decay.
The half-life of a given isotope is constant and is independent of external conditions or the number of atoms in the sample.
They measure the rate of the isotope's decay using a radiation detector.
The time it takes for half of a specific isotope to decay will be the same whether you have one million atoms or ten atoms
The amount of time it takes for only half of the original sample to remain is equal to the amount it takes for the radioactive sample to decrease from one-half to one-quarter of the original amount.