2.2.3: Reaction Rates and Mechanisms
For a reaction to occur, two molecules with enough force, and in a proper alignment.
the steric factor is a factor in the Arrhenius equation that reflects the probability that molecules collide in the correct orientation for a reaction to occur (it is often combined with the frequency factor).
The number of successful collisions determines the reaction rate.
At a given temperature, reactant particles have a range of speeds. This distribution is shown by the maxwell-Boltzmann distribution.
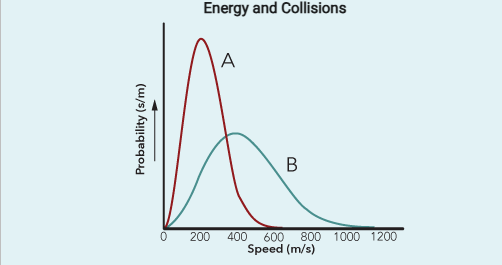
The rate of a reaction depends on the activation energy, which is the energy of the activated complex (transition state)
The Arrhenius equation: k = Ae^(-Ea / RT)
It relates the rate constant of a reaction to the activation energy and temperature.
The steric factor is a component in the Arrhenius equation that reflects the probability that molecules will collide in the correct orientation for a reaction to occur. It accounts for the spatial arrangement of the reacting molecules, which can affect whether a successful collision leads to a reaction. The steric factor is often combined with the frequency factor in the Arrhenius equation.
The rate law can be used to predict how the rate changes when the concentration of each reactant changes, which is determined by the reaction mechanism .
Reactions occur in elementary steps (Individual step in a reaction which describes a single molecular event. It can not be broken down into simpler steps).
The molecularity of a reaction is the number of reactant molecules involved in an elementary step, which can be classified as unimolecular, bimolecular, or termolecular depending on whether one, two, or three molecules collide and react simultaneously.
The step that has the highest activation energy has the smallest rate constant and is the slowest, thus it is the rate-determining step
The rate-determining step has the smallest rate constant.