Lesson 10: Which Reagent Runs Out First?
Limiting and Excess Reagents
The Limiting Reagent is the reactant that is completely consumed in a chemical reaction. This is the reactant which determines how much product will be formed.
The Excess Reagent is the reactant that is still present after the reaction is complete.
Using a balanced chemical equation, determine the working molar ratio for all reactants. Identify the smallest WMR and use this to predict the mass of products produced. The smallest WMR is the limiting reagent and the other will be the excess reagent.
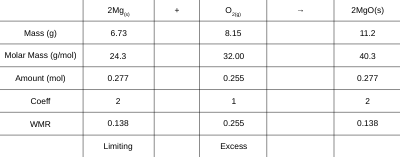
Example 1: In the combustion of magnesium metal in oxygen gas, 6.73 g of magnesium and 8.15 g of oxygen gas are available for reaction. Identify the limiting reagent in this reaction and determine what mass of magnesium oxide should be produced.
Calculating the Excess Reagent
When designing experiments it is important that the excess reagent is present in sufficient amounts that allow all of the reactant entities to react. The general rule is add 10% to whatever is the bare minimum is from pre-lab calculations. If you add more than 10% then it becomes costly and there is unneeded waste (environmentally irresponsible)
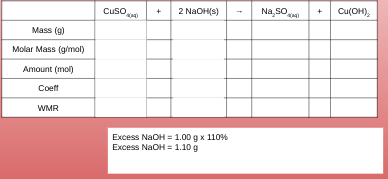
Example 1: In an experiment to test the stoichiometric method, 2.00 g of copper(II) sulfate solution is reacted with excess of sodium hydroxide in aqueous solution. What mass of solid sodium hydroxide is reasonable to use?