2.Nomenclature and Structure of Carbonyl Group
I. Aldehydes and ketones
Aldehydes and ketones are the simplest and most important carbonyl
compounds.
There are two systems of nomenclature of aldehydes and ketones.
(a) Common names
Aldehydes and ketones are often called by their common names instead of IUPAC names.
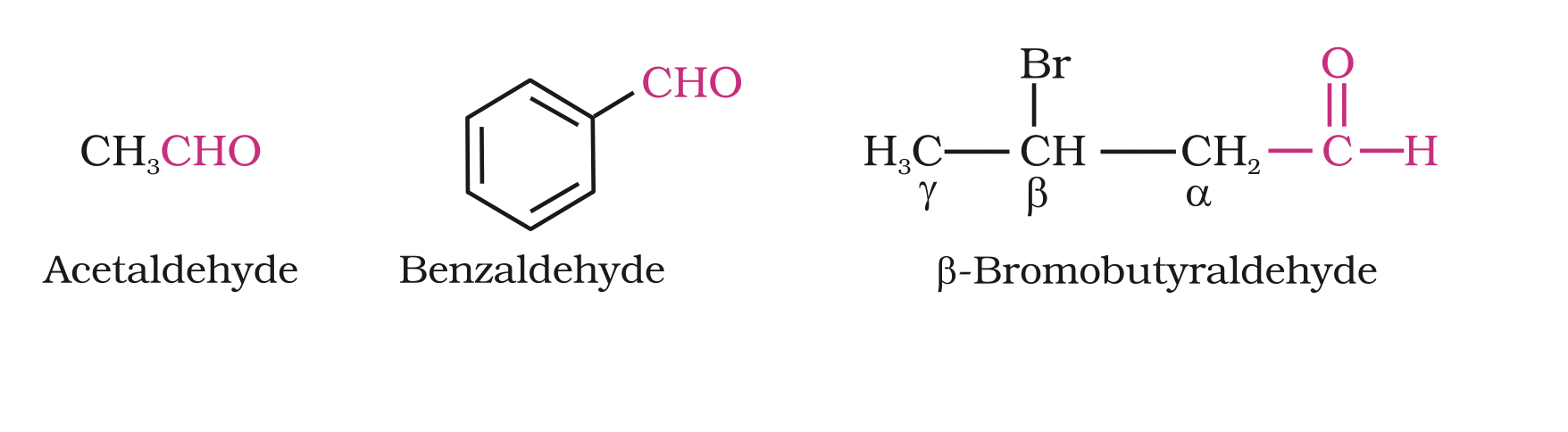
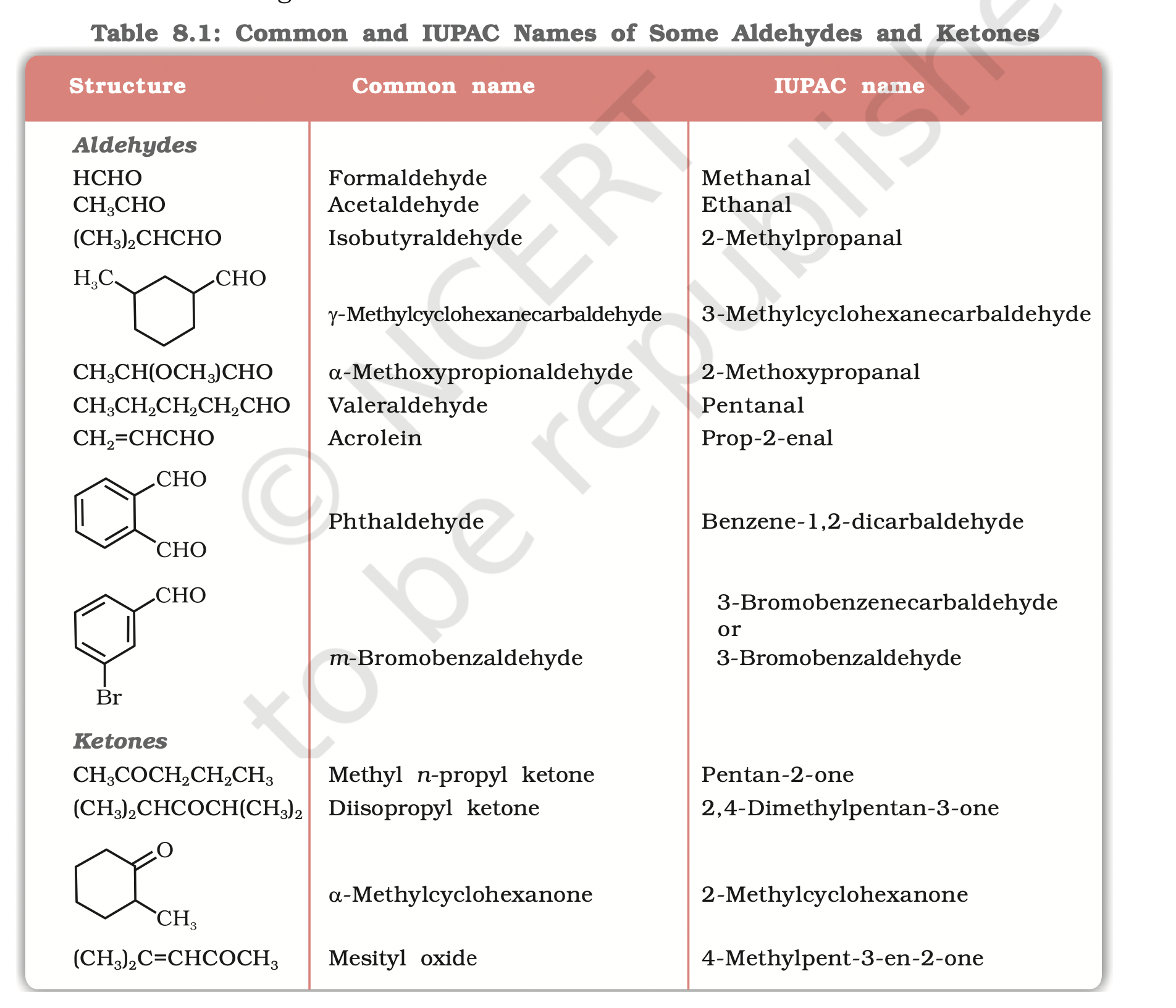
The common names of most aldehydes are derived from the common names of the corresponding carboxylic acids by replacing the ending –ic of acid with aldehyde.
At the same time, the names reflect the Latin or Greek term for the original source of the acid or aldehyde.
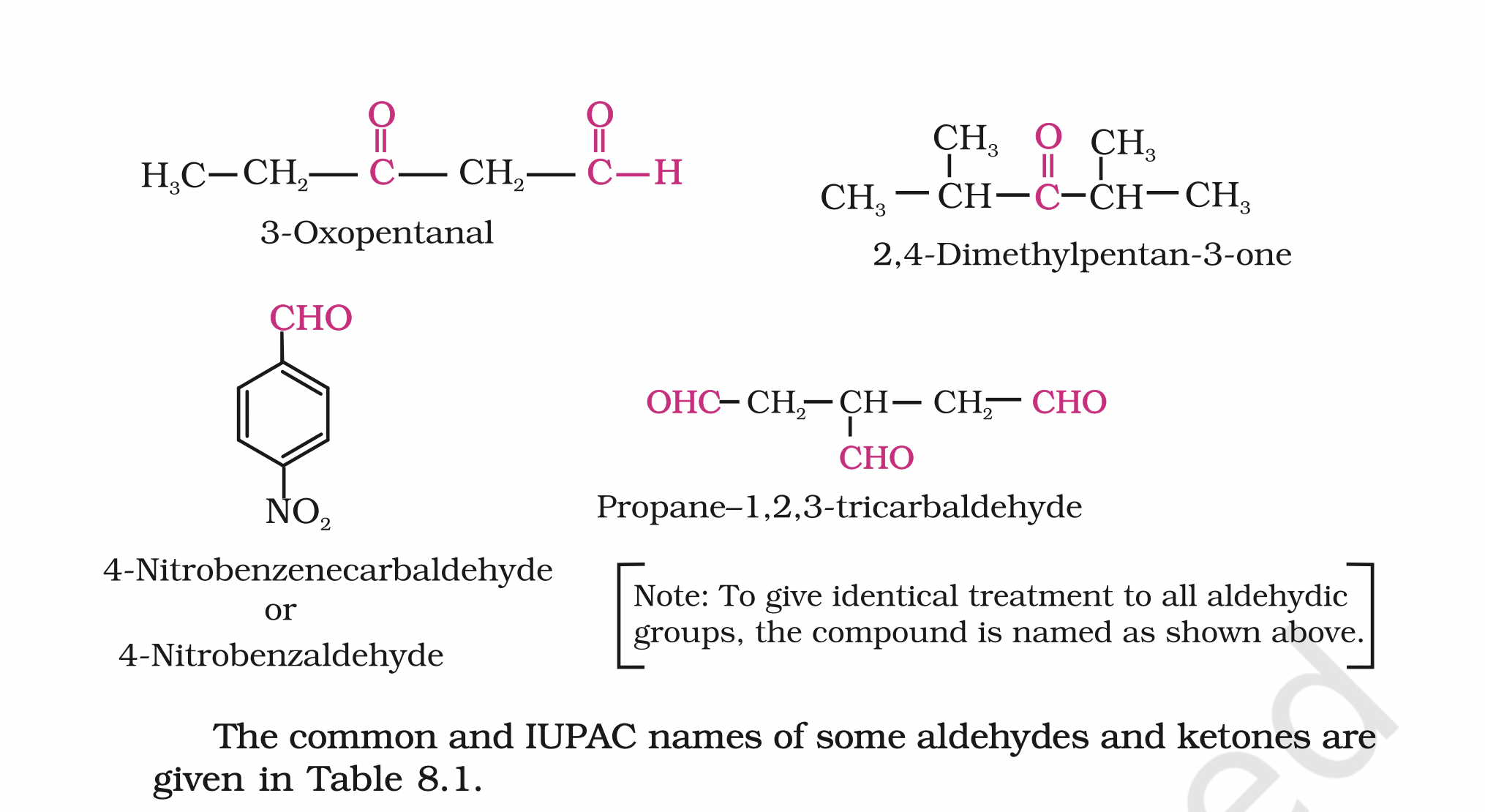
The location of the substituent in the carbon chain is indicated by Greek letters a, b, g, d, etc.
The a-carbon being the one directly linked to the aldehyde group, b- carbon the next, and so on. For example
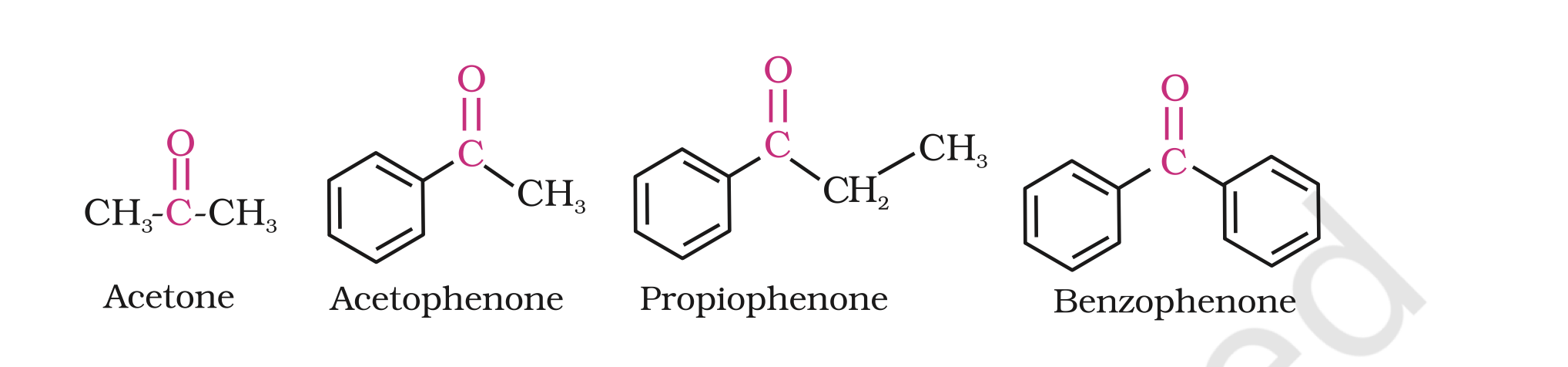
the common names of ketones are derived by naming two alkyl or aryl groups bonded to the carbonyl group.
The locations of substituents are indicated by Greek letters, a a¢, b b¢ and so on beginning with the carbon atoms next to the carbonyl group, indicated as aa¢.
Some ketones have historical common names, the simplest dimethyl ketone is called acetone.
Alkyl phenyl ketones are usually named by adding the name of acyl group as prefix to the word phenone. For example
 (b) IUPAC names
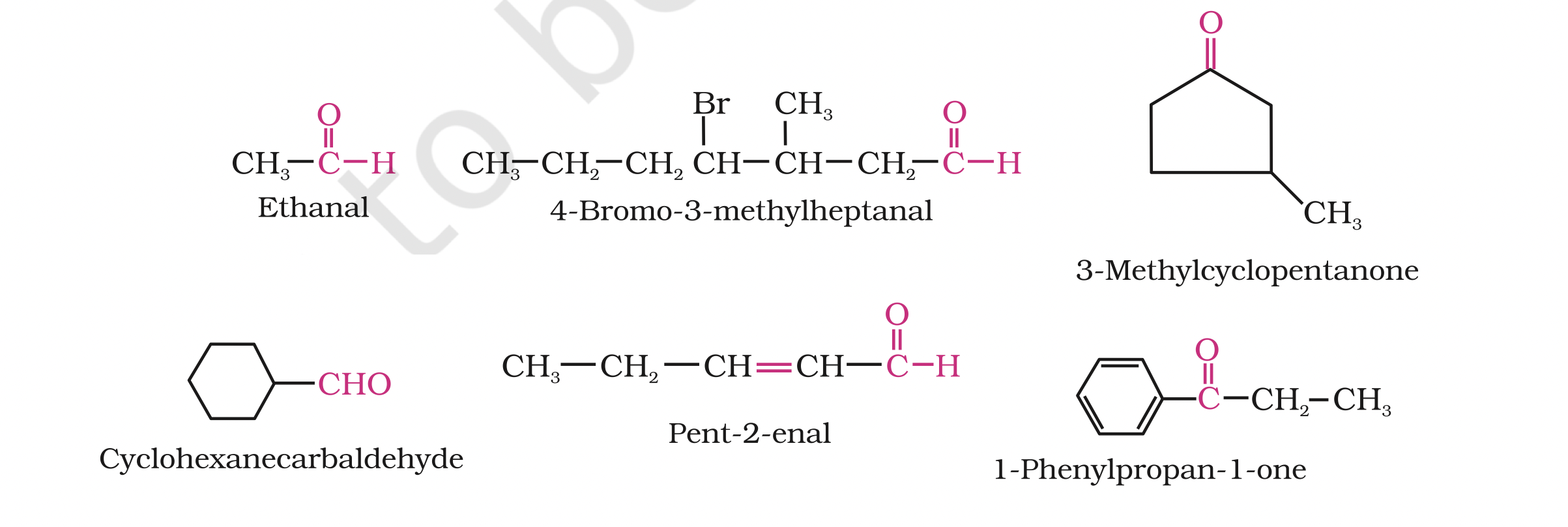
(b) IUPAC namesThe IUPAC names of open chain aliphatic aldehydes and ketones are derived from the names of the corresponding alkanes by replacing the ending –e with –al and –one respectively.
In case of aldehydes the longest carbon chain is numbered starting from the carbon of the aldehyde group
in case of ketones the numbering begins from the end nearer to the carbonyl group.
The substituents are prefixed in alphabetical order along with numerals indicating their positions in the carbon chain.
The same applies to cyclic ketones, where the carbonyl carbon is numbered one.
When the aldehyde group is attached to a ring, the suffix carbaldehyde is added after the full name of the cycloalkane.
The numbering of the ring carbon atoms start from the carbon atom attached to the aldehyde group.
The name of the simplest aromatic aldehyde carrying the aldehyde group on a benzene ring is benzenecarbaldehyde.
However, the common name benzaldehyde is also accepted by IUPAC. Other aromatic aldehydes are hence named as substituted benzaldehydes.
Structure of the Carbonyl Group
The carbonyl carbon atom is sp2-hybridised and forms three sigma (s) bonds.
The fourth valence electron of carbon remains in its p-orbital and forms a p-bond with oxygen by overlap with p-orbital of an oxygen.
In addition, the oxygen atom also has two non bonding electron pairs.
Thus, the carbonyl carbon and the three atoms attached to it lie in the same plane and the p-electron cloud is above and below this plane.
The bond angles are approximately 120° as expected of a trigonal coplanar structure (Figure 8.1).
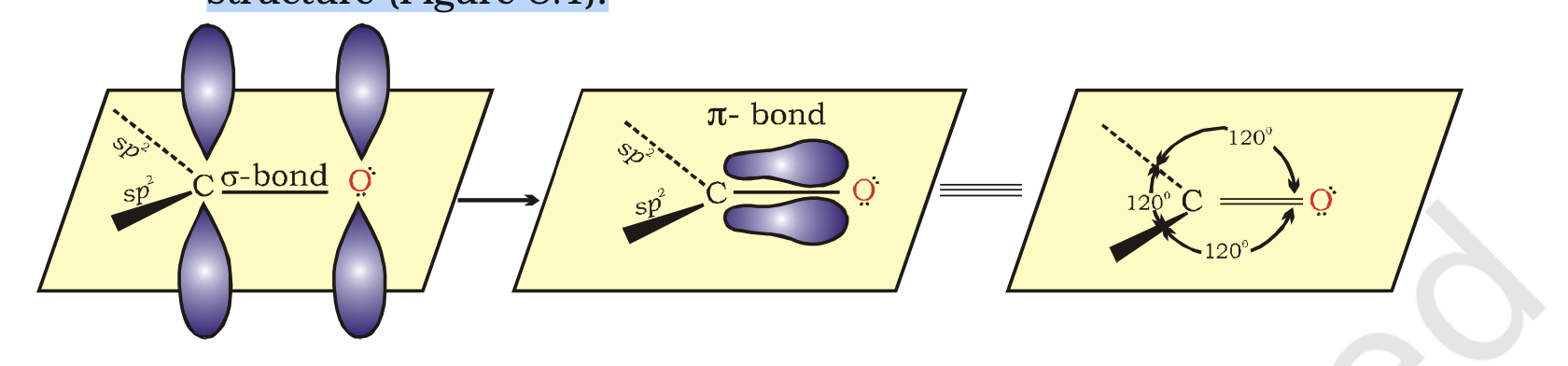
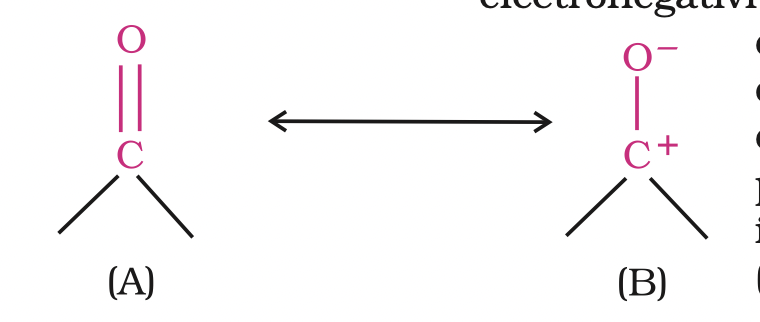
The carbon-oxygen double bond is polarised due to higher electronegativity of oxygen relative to carbon.
Hence, the carbonyl carbon is an electrophilic (Lewis acid), and carbonyl oxygen, a nucleophilic (Lewis base) centre.
Carbonyl compounds have substantial dipole moments and are polar than ethers. The high polarity of the carbonyl group is explained on the basis of resonance involving a neutral
(A) and a dipolar (B) structures as shown.