CHEMISTRY: BEHAVIOR OF GASES
Kinetic Molecular Theory
The kinetic molecular theory (KMT) is a model or theory that is used to explain the behavior of gasses. This Theory describes the relationships among pressure, temperature, velocity, frequency, and force of collisions.
The kinetic molecular theory is summarized in the below statements:
- Gasses contain particles that are in constant, random, straight-line motion.
- Gas particles collide with each other and with the walls of the container. These collisions may result in a transfer of energy among the particles, but there is no net loss of energy as the result of these collisions. The collisions are said to be perfectly elastic.
- Gas particles are separated by relatively great distances. Because of this the volume occupied by the particles themselves is negligible.
- Gas particles do not attract each other
The Phase of Gas
Gas is spread out indefinitely unless they are confined in a container. In a closed container, the gaseous particles always expand to fill the volume of the container.
Relationship of Pressure and Numbers of Gas Particles
- Gas molecules collide with the walls of their container. These collisions with the container wall exert a force over the surface area of a wall - the particles exert pressure on the wall.
- As the number of air particles increases ↑, pressure increases ↑ as well.
- Pressure and the number of gas molecules are directly proportional.
Relationship of Pressure and Volume of a gas
- The volume and pressure are indirectly related.
- If one of the variables (volume or pressure) increases ↑, the other must decrease ↓.
Relationship of Temperature and Pressure of a Gas
- Temperature of a substance is defined as a measure of the average kinetic energy of its particles.
- The kinetic energy Formula:
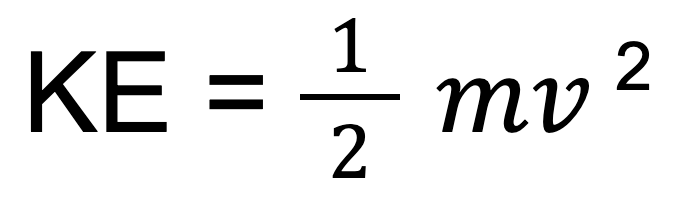
- As temperature rises the kinetic energy increases, due to an increase in velocity.
- As the temperature rises, the velocity of the particles increases, causing them to hit walls of the container more often and with greater force. Thus an increase in temperature causes the pressure to increase.
- Pressure and temperature are directly proportional.
Relationship of Temperature and Volume of Gas
- As temperatures increase, so does the volume of gas.
- When pressure is constant, temperature and the volume of gas are directly proportional.
Relationship of Temperature and Velocity
- The higher the temperature the greater the average velocity of the particles.
Combined Gas Law Equation
The relationships among pressure temperature and volume can be mathematically presented by an equation known as the combined gas law
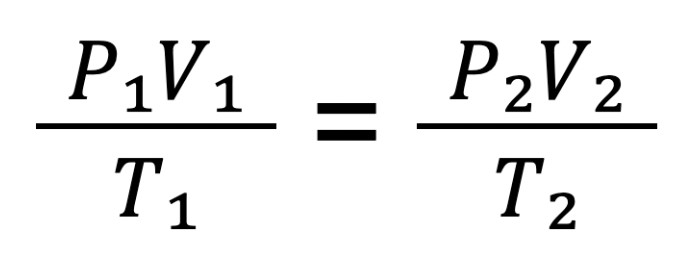
This model can be used to solve problems of gas properties including, temperature, volume, and pressure, whenever two more of these properties are involved.