Chap 1 Intro to Drugs
adverse effects: drug effects, sometimes called side effects, that are not the desired therapeutic effects; may be unpleasant or even dangerous
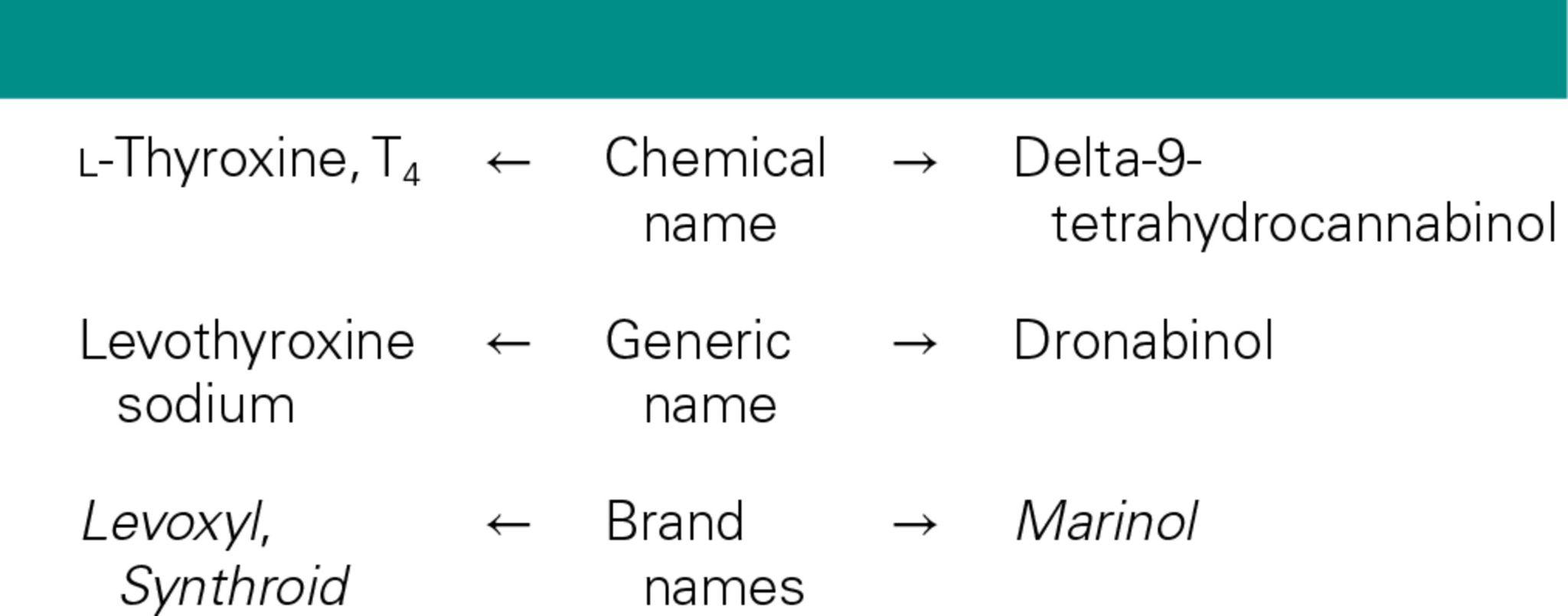
brand name: name given to a drug by the pharmaceutical company that developed it; also called a trade name or proprietary name
chemical name: name that reflects the chemical structure of a drug
drugs: chemicals that are introduced into the body to bring about change
Food and Drug Administration (FDA): federal agency responsible for the regulation and enforcement of drug evaluation and distribution policies
generic drugs: drugs sold by their generic name; not brand name or trade name product
generic name: the original designation that a drug is given when the drug company that developed it applies for the approval process
genetic engineering: process of altering DNA, usually of bacteria, to produce a chemical to be used as a drug
orphan drugs: drugs that have been discovered but would not be profitable for a drug company to develop; usually drugs that would treat only a small number of people; these orphans can be adopted by drug companies to develop
over-the-counter (OTC) drugs: drugs that are available without a prescription for self-treatment of a variety of complaints; deemed to be safe when used as directed; often formerly only available by prescription
pharmacology: the study of the biological effects of chemicals
pharmacotherapeutics: clinical pharmacology—the branch of pharmacology that deals with drugs; chemicals that are used in medicine for the treatment, prevention, and diagnosis of disease in humans
phase I study: a pilot study of a potential drug using a small number of selected, usually healthy human volunteers.
phase II study: a clinical study of a proposed drug by selected physicians using actual patients who have the disorder the drug is designed to treat; patients must provide informed consent.
phase III study: use of a proposed drug on a wide scale in the clinical setting with patients who have the disease the drug is thought to treat.
phase IV study: continuous evaluation of a drug after it has been released for marketing.
preclinical trials: initial trial of a chemical thought to have therapeutic potential; uses laboratory animals, not human subjects.
teratogenic: having adverse effects on the fetus.
Clinical pharmacology addresses 2 key concerns:
The drug’s effects on the body
The body’s response to the drug.
The nurse is in a unique position regarding drug therapy because nursing responsibilities include the following:
Administering drugs
Assessing drug effects
Intervening to make the drug regimen more tolerable
Providing patient teaching about drugs and drug regimens
Monitoring the overall patient care plan to prevent medication errors
Therapeutic actions on the label of a drug is the action of drug on the body.
Indications: Uses of the drug; Evaluation points-- resolution or stabilization of those conditions.
Contraindication and Cautions: Conditions limiting the use of drug.
Assessment points; History or physical assessment indicating these conditions.
Black box label: Many of adverse reactions; could be fatal.
Sources of Drugs:
Drugs come in varies sources both natural and synthetic.
Natural sources include: plants, animals and inorganic compounds.
Natural Source: Chemicals that could be helpful for medicines can come from many different places, like plants, animals, or inorganic materials. To become a drug, a chemical needs to be proven to be helpful and not too dangerous or harmful.
Plants: Have a long history since prehistoric time; they are important sources of chemicals that are developed today.
Example: Digitalis used for cardiac disorder and various opiates used for sedation were originally derived from plants.
Digitalis Purpurea: Leaves, Dried leaves, digitalis leaf.
Papaver somniferous: Unripe capsule, juice, opium (paregoric), morphine (MS Contin), Codeine, Papaverine.
Example of Synthetic version of active chemical found in a plant.
Dronabinol (Marinol); contains the active ingredient (delta-9-terahydrocannbinol) found in Marijuana. Drug helps prevent nausea/vomiting in cancer patients w/o adverse effects of smoking marijuana leaf (controlled substance w/ high abuse potential). Synthetic version of active ingredient allows for accepted form to achieve desired effect in cancer patients. Legal for medical use in some states, not recreational use in most.
Eating a plant-based food can sometimes cause a drug-like effect.
For example: Ingestion of a plant-derived food can sometimes lead to a drug effect. The body converts natural licorice to a false aldosterone—a hormone found in the body—resulting in fluid retention and hypokalemia or low serum potassium levels if large amounts of licorice are consumed.
Animals Products
insulin for treating diabetes was obtained exclusively from the pancreas of cows and pigs.
Genetic engineering: the process of altering DNA—permits scientists to produce human insulin by altering Escherichia coli bacteria, making insulin a better product without some of the impurities that come with animal products.
Thyroid drugs and growth hormone preparations also may be obtained from animal thyroid but synthetic preparations are considered to be purer and safer than preparations derived from animals.
Inorganic Compounds: Salts of various elements, like aluminum, fluoride, iron, and gold, have therapeutic effects. These were discovered when a cause–effect relationship was observed.
Aluminum:
Antacid to decrease gastric acidity
management of hyperphosphatemia
prevention of the formation of phosphate urinary stones.
Fluoride:
Prevention of dental cavities; prevention of osteoporosis
Gold:
Treatment of rheumatoid arthritis
Iron:
Treatment of iron deficiency anemia.
Drug Evaluation
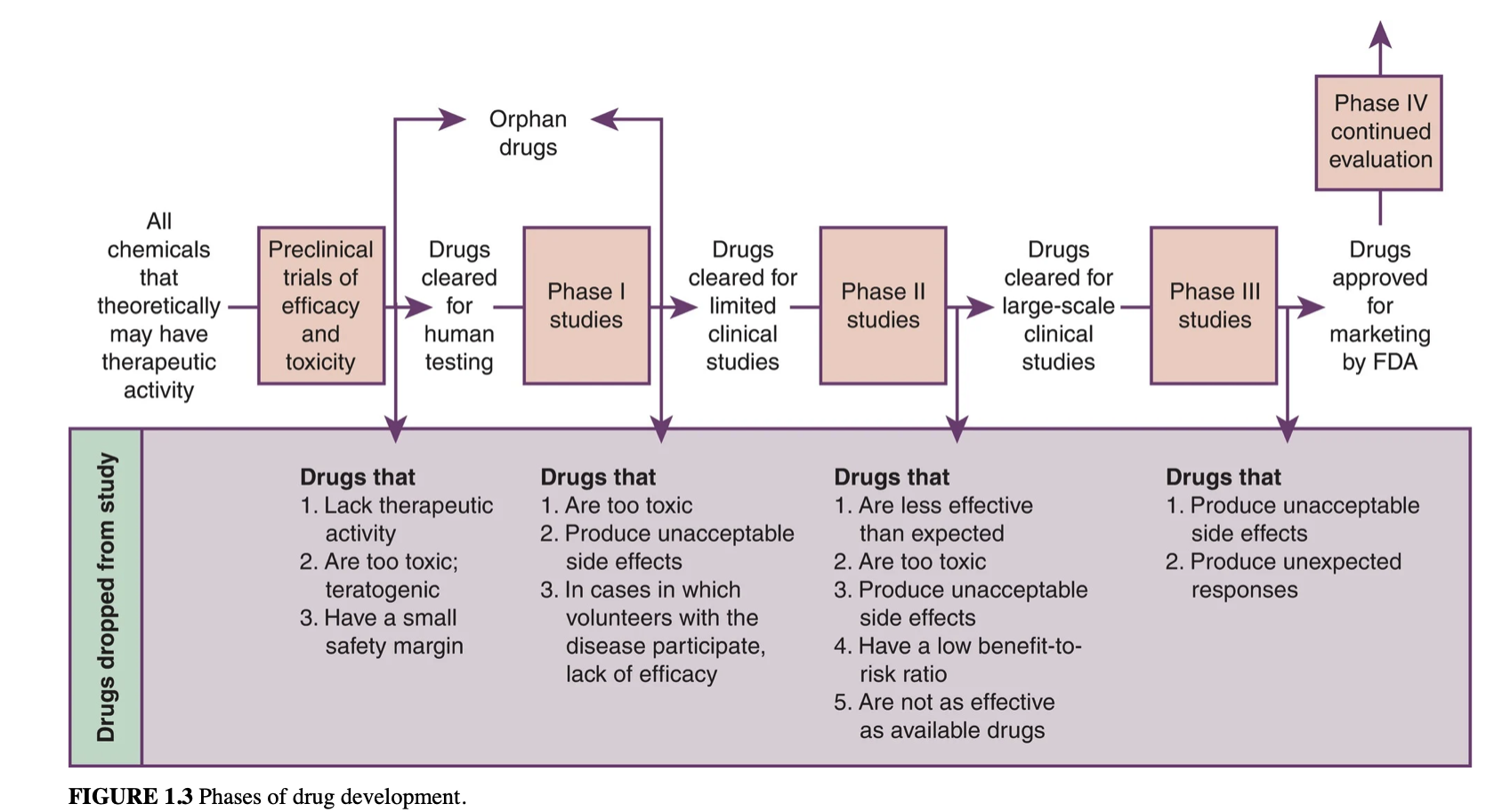
Before receiving final FDA approval to be marketed to the public, drugs must pass through several stages of development. These include:
preclinical trials and phase I, II, and III studies.
The drugs listed in this book have been through rigorous testing and are approved for sale to the public, either with or without a prescription from a healthcare provider.
Preclinical Trials
Chemicals may have therapeutic values tested on lab animals for two main purposes:
To determine whether they have the presumed effects in living tissue.
To evaluate any adverse effects.
At the end of the preclinical trials, some chemicals are discarded for the following reasons:
The chemical lacks therapeutic activity when used with living animals.
The chemical is too toxic to living animals to be worth the risk of developing into a drug.
The chemical is highly teratogenic (causing adverse effects to a fetus).
The safety margins are so small that the chemical would not be useful in the clinical setting.
Phase I Study:
A phase I study uses human volunteers to test drugs. Volunteers are fully informed of risks and may be paid. Usually, they are healthy young men/women, with women of childbearing potential sometimes excluded. Volunteers must sign a consent form outlining possible effects.
Chemicals may be effective in animals but not humans. In phase I studies, investigators assess effects in humans and adverse reactions. Many chemicals are dropped due to lack of effectiveness or toxicity.
Examples:
They cause unacceptable adverse effects.
They are highly teratogenic.
They are too toxic.
They lack evidence of potential therapeutic effect in humans.
Some chemicals go to the next step of testing, even though they have bad effects. For example, minoxidil is a drug that lowers high blood pressure, but it can make hair grow in strange places. Even though it was not safe, it was still more effective than any other medicine for high blood pressure. So, it moved on to the next testing stage (Phase II study), and is now used in special treatments to help people grow their hair back e.g. Rogaine.
Phase II studies:
Allow clinical investigators to test a drug's effectiveness in treating a specific disease that the patient may have. Patients are made aware of potential benefits and risks and monitored closely, often free of charge. Studies are conducted across the nation in hospitals, clinics and doctors' offices, monitored by a pharmaceutical company. If a drug does not meet certain criteria, it may be removed from further investigation.
Some examples of removal:
It is less effective than anticipated.
It is too toxic when used with patients.
It produces unacceptable adverse effects.
It has a low benefit-to-risk ratio, meaning that the therapeutic benefit it provides does not outweigh the risk of potential adverse effects that it causes.
It is no more effective than other drugs already on the market, making the cost of continued research and production less attractive to the drug company.
Phase III studies:
A phase III study involves use of a drug in a clinical market. Prescribers monitor patients for adverse effects and may ask them to keep journals. Drug company collects this info and shares with FDA. Unacceptable adverse effects or unforeseen reactions can lead to drug being removed from study/market.
Approval of a drug = Brand name (Trade name)
Generic name: Original designation that the drug was given when the drug company applied for the approval process.
Chemical names: Names that reflect the chemical structure of a drug .
Some drugs known as 3 names
The generic and chemical names always appear in straight print, and the brand name is always capitalized and italicized (e.g., minoxidil [Rogaine])
Drug development and approval can take 5-6 years, resulting in "drug lag" in the U.S. FDA prioritizes public safety, but can accelerate the process in certain cases, e.g. drugs with great promise and no alternative in phase III studies. These drugs may be "fast tracked" or given "breakthrough" status in phase II studies. Such drugs may not have long-term effects known, as indicated in the literature.
Phase IV Studies:
After a drug is approved for marketing, it enters a phase of continual evaluation.
Healthcare professionals must report adverse effects to the FDA, even if not related to the drug. Also, drug companies must manage risk of side effects with labeling and warnings. Unexpected effects may be seen with wider distribution, as seen with Symmetrel, which had fewer influenza cases, revealing its antiviral properties. example, patients taking the antiparkinsonism drug amantadine (Symmetrel) were found to have fewer cases of influenza than other patients, leading to the discovery that amantadine is an effective antiviral agent.
Legal Regulation of Drugs
The FDA regulates drug development and sale, while local laws further regulate distribution and administration. Nurses should be familiar with the area's rules and regulations, which can vary by state. The FDA's power has increased in response to drug disasters, such as the 1930s “elixir of sulfanilamide” incident, resulting in the 1938 Federal Food, Drug and Cosmetic Act, giving the FDA power to test drug toxicity and monitor labeling.
the Federal Food, Drug and Cosmetic Act of 1938, which gave the FDA power to enforce standards for testing drug toxicity and monitoring labeling.
In the 1960s, thalidomide (Thalomid) was used as a sleeping aid by pregnant women, causing birth defects. The Kefauver-Harris Act of 1962 gave the FDA control over drug testing and efficacy/safety standards. Other laws have given the FDA control over potentially addictive drugs and non-prescription drugs.
Safety during Pregnancy:
FDA required each new drug be assigned a pregnancy category. Research into fetal development led many to recommend no drugs be used in pregnancy. In 2014, new guidelines established categories related to presence in breast milk. In 2015, FDA stopped using categories, replaced with risk levels for fertility, pregnancy, and lactation. High risk for pregnancy indicates fetal toxicity, high risk for lactation indicates drug enters breast milk, low risk indicates no problems. Evidence-based data provides a safer, clearer guide to drug use in these populations.
Food and Drug Administration Pregnancy Categories
Category A: Adequate studies in pregnant women have not demonstrated a risk to the fetus in the first trimester of pregnancy, and there is no evidence of risk in later trimesters.
Category B: Animal studies show no risk to fetus; no adequate studies in pregnant women. Risk to fetus during first trimester unknown; no evidence of risk in later trimesters.
Category C: Animal studies show adverse effects on fetus; benefits from drug use in pregnant women may be acceptable despite potential risks, or no animal/human reproduction studies.
Category D: There is evidence of human fetal risk, but the potential benefits from the use of the drug in pregnant women may be acceptable despite its potential risks.
Category X: Studies in animals/humans show fetal abnormalities/adverse reactions; reports suggest fetal risk. Risk of use during pregnancy exceeds any potential benefit.
Controlled Substances
The Controlled Substances Act of 1970 gave the FDA & DEA control over drug coding & enforcement. Drugs with abuse potential are called controlled substances.
Drug Enforcement Agency Schedules of Controlled Substances
Schedule I (C-I): High abuse potential and no accepted medical use (heroin, marijuana, LSD)
Schedule II (C-II): High abuse potential with severe dependence liability (narcotics, amphetamines, and barbiturates)
Schedule III (C-III): Less abuse potential than schedule II drugs and moderate dependence liability (nonbarbiturate sedatives, nonamphetamine stimulants, limited amounts of certain narcotics)
Schedule IV (C-IV): Less abuse potential than schedule III and limited dependence liability (some sedatives, antianxiety agents, and nonnarcotic analgesics)
Schedule V (C-V): Limited abuse potential. Primarily small amounts of narcotics (codeine) used as antitussives or antidiarrheals. Under federal law, limited quantities of certain schedule V drugs may be purchased without a prescription directly from a pharmacist. The purchaser must be at least 18 years of age and must furnish suitable identification. All such transactions must be recorded by the dispensing pharmacist.
Generic Drugs:
FDA grants time-limited patent for drug formulas. When patent expires, drug can be produced by other manufacturers. FDA monitors development of generic medications, which must have same active ingredient, dosage form, route of administration, and safe inactive & packaging/storage. Generic medications are bioequivalent to brand name, but can affect some people differently. Most find generic medications safe & cost-effective alternatives to brand name.
States often require drugs be dispensed in generic form to reduce costs. Prescribers may require brand name drug be used ("dispensed as written"), for drugs such as Lanoxin and Coumadin, which have narrow safety margins. Initial cost may be higher, but some prescribers believe long-term cost to patient will be less.
Orphan drugs:
Are discovered but not financially viable and have not been adopted by a drug company. They can be useful in treating rare diseases but may have adverse effects. Orphan drugs are often abandoned after preclinical trials or phase I studies. Incentives from the 1983 Orphan Drug Act encourage drug companies to develop them, even if the market is small. Some drugs in this book have listed orphan drug uses.
Over-The-Counter
OTC drugs are available without a prescription for self-treatment. Some were approved as prescription drugs and some were not rigorously tested. Many were "grandfathered" in due to long-term use. The FDA is currently testing their effectiveness and evaluating all of them. Although generally safe, nurses should be aware of potential problems related to OTC drug use.
Taking these drugs could mask the signs and symptoms of underlying disease, making diagnosis difficult.
Taking these drugs with prescription medications could result in drug interactions and interfere with drug therapy.
Not taking these drugs as directed could result in serious overdoses.
Patients often don't report OTC drug use. Nurses must enquire about it & provide info about avoiding use while taking prescription drugs or consulting a healthcare provider.
 Knowt
Knowt