2.1.2 Proteins
2.1.2 Biological molecules (k-o and part of q)
Proteins - Notes
2.1.2k: the general structure of an amino acid
Draw and label a diagram of an amino acid. (F)
Identify the part of an amino acid which is variable.
State how many amino acids occur in life.
Describe the different types of amino acid in life.
2.1.2l: the synthesis and breakdown of dipeptides and polypeptides, by the formation and breakage of peptide bonds
Draw a labelled diagram demonstrating the condensation and hydrolysis of peptide bonds. (F)
Draw a dipeptide and label the peptide bond.
Explain how the variety of amino acids leads to a wide range of dipeptides and very quickly to an incredible variety of polypeptide chains.
Define the terms “polypeptide chain” and “protein”.
Describe how one end of a polypeptide chain differs from the other end.
2.1.2m: the levels of protein structure
Define the term “primary structure” of a protein and describe how it is held together. (F)
Define the term “secondary structure” of a protein, describe the two different types and describe how the secondary structure is held in place. (F)
Define the term “tertiary structure” of a protein, and describe how it is held in place (include all the different possible bonds). (F)
Explain how the primary structure of a protein determines its tertiary structure.
Define the term “quaternary structure” of a protein, and describe how it is held in place (include all the different possible bonds).
2.1.2n: the structure and function of globular proteins including a conjugated protein
Define the terms “globular protein” and “fibrous protein”. (F)
Define the terms “prosthetic group” and “conjugated protein”. (F)
Draw a table to show an example of a conjugated protein, an enzyme and a peptide hormone and for each identify key structural components, properties and functions.
2.1.2o: the properties and functions of fibrous proteins.
Give 3 examples of fibrous proteins.
Draw a table to compare the structure, properties, location and functions of collagen, keratin and elastin.
Draw a table to compare the structure, properties and functions of globular and fibrous proteins.
Use the protein data bank to describe the structure and function of 10 interesting proteins. (S+C)
2.1.2q: how to carry out and interpret the results of the following chemical tests: biuret test for proteins, Benedict’s test for reducing and non-reducing sugars, reagent test strips for reducing sugars, iodine test for starch, emulsion test for lipids.
Draw a table that shows the chemicals used, an outline of the method, the appearance of a negative results and the appearance of a positive result for the biuret test for proteins, the Benedict’s test for reducing sugars, the Benedict’s test for non-reducing sugars, the iodine test for starch and the emulsion test for lipids. (F) [protein one only]
An introduction to proteins
Proteins contain the elements C, H, O, N and S.
Proteins are polymers. They are made of many monomers joined together in a chain. The monomers for a protein are amino acids. The bond that links amino acids together into a chain is called a peptide bond.
One chain of amino acids is called a polypeptide. Technically, the term protein is used for something that is a functional unit – i.e. it can do a job. Some proteins are single polypeptides but other proteins are made of more than one polypeptide joined together. In this second case the polypeptides themselves are not proteins but when joined together they for a protein.
Proteins are the molecules of life that allow the genetic code (in DNA) to control what’s going on in the cell. This is mainly because life is just a bunch of chemical reactions called metabolism, each reaction is catalysed by an enzyme, all enzymes are proteins, and DNA codes for which enzymes can be made and regulates the production of each of them.
Proteins have many other functions. As well as some of them being enzymes, some are antibodies, hormones, channel proteins, carrier proteins, receptor proteins on the surface of cells, transport proteins, structural proteins, and proteins that allow movement.
All the possible proteins produced by any of the different cells of an organisms at any stage of its life is called an organism’s ‘proteome’.
Amino acids
All amino acids have a central carbon (called the alpha carbon) that is attached to 4 groups: a hydrogen atom, a carboxylic acid (carboxyl) group, an amine group, and another group that varies between amino acids called the R-group.
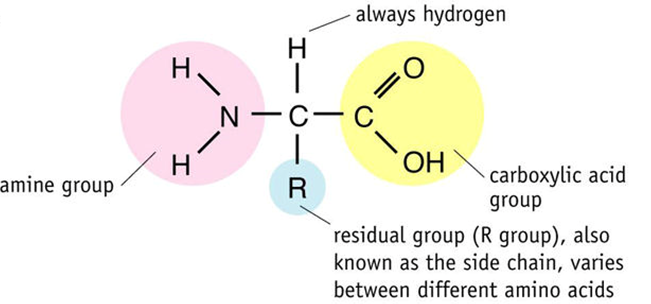 There are 20 different amino acids coded for by the DNA of life. They are all identical except for the R-group. R-groups can be:
There are 20 different amino acids coded for by the DNA of life. They are all identical except for the R-group. R-groups can be:
Non-polar (and so hydrophobic)
Polar
Negatively charged
Positively charged
Ones that contain a sulfur atom
These properties are all important in understanding how proteins hold their shape.
Joining amino acids together to make a chain
As with all joining reactions in biology joining two amino acids together is a condensation reaction. The OH from the carboxyl group of one amino acid is removed as is one of the H atoms from the amine group of the other amino acid. This OH and H combine to produce water. The C from the carboxyl group then joins with the N from the amine group and a peptide bond is formed.
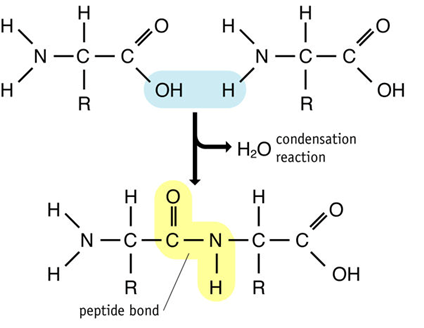 When two amino acids join together the new molecule produced is called a dipeptide. It has an amine group at one end and a carboxyl group at the other. Polypeptides are many amino acids long (often 100s) but no matter how long they are they will always have an anime group at one and a carboxyl group at the other.
When two amino acids join together the new molecule produced is called a dipeptide. It has an amine group at one end and a carboxyl group at the other. Polypeptides are many amino acids long (often 100s) but no matter how long they are they will always have an anime group at one and a carboxyl group at the other.
Because each amino acid only has one amine group and one carboxy group it is not possible to have branching polypeptides. All polypeptides are unbranched.
Given that there are 20 different amino acids coded for by life, that any amino acid can follow any other amino acid in the chain, and that each end of the chain is different, how many different dipeptides are there?
Answer:
What about a chain of:
3 amino acids Answer:
4 amino acids Answer:
5 amino acids Answer:
50 amino acids Answer:
This diversity is what ultimately creates the diversity of life and is the raw material that evolution ‘plays with’ to generate the diversity of life.
The different ‘levels’ of protein structure
There are 4 levels of protein structure. These ‘levels’ are just terms people have invented to describe certain aspects of a protein’s structure. They are:
Primary structure: the number and sequence of amino acids in the polypeptide chain.
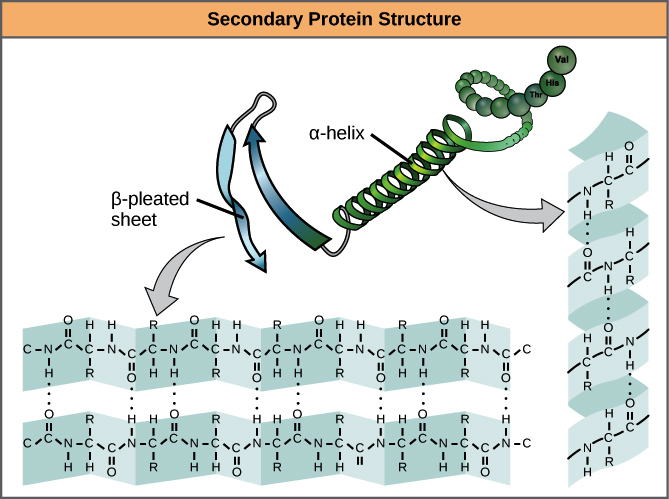
Secondary structure: the coiling of part of a polypeptide chain into an alpha-helix or the folding of part of a polypeptide chain into a beta-pleated sheet.
Tertiary structure: the irregular folding of parts of a polypeptide chain to generate the overall 3-dimensional shape of the polypeptide chain.
Quaternary structure: the binding of two or more polypeptide chains into one functional unit – a protein.
Primary structure
All proteins have a primary structure. It is the sequence of amino acids in the polypeptide chain(s). The primary structure is held together by peptide bonds.
Secondary structure
There are two very commonly occurring shapes that parts of polypeptide chains form that are held together by H-bonds between the oxygen in one peptide bond and the hydrogen in another peptide bond further down the chain. These are alpha-helices and beta-pleated sheets.
Tertiary structure
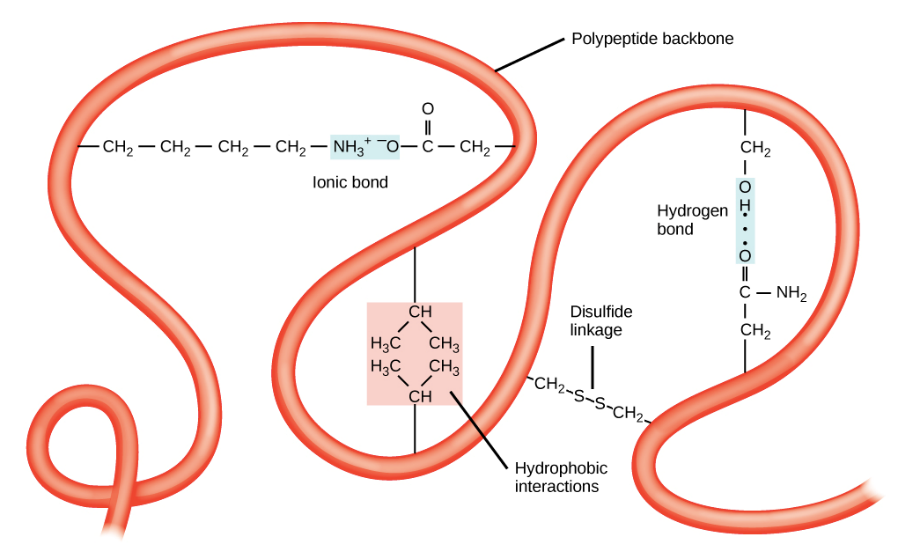
The overall 3-D shape of a polypeptide chain is determined by irregular folding of the chain. This shape is held together by bonds between R-groups. There are 4 types of bond involved in holding the tertiary structure in place:
Hydrogen bonds between polar R-groups.
Ionic bonds between positively and negatively charged R-groups.
Disulfide bonds (or bridges) between two R-groups that each contain sulfur atoms.
Hydrophobic associations where hydrophobic R-groups cluster together in the centre of a polypeptide/protein away from water.
Hydrophilic associations between R-groups and the aqueous environment around the protein.
Quaternary structure
Sometimes, a protein is composed of more than one polypeptide chain held together to create a functional unit. In this case each polypeptide chain is called a ‘subunit’ (e.g. the alpha and beta subunits in haemoglobin). The bonds that hold two or more subunits together are the same bonds between R-groups as those in the tertiary structure of a polypeptide chain.
The importance of shape for protein function
Proteins can only perform their function because they hold a very specific shape. For example:
Enzymes need to have an active site that is a complementary shape to the substrate(s).
Hormones need to have a complementary shape to the binding site of the receptor protein.
Receptor proteins need to have a binding site that is a complementary shape to the molecule that they detect.
Channel proteins need to have a channel that is shaped in such a way as to allow one specific molecule/ion through but not others.
Carrier proteins need to have a binding site that is a complementary shape to the molecule they transport.
Antibodies need to have binding sites that are complementary to the antigen that they bind to.
Structural proteins need to have a shape that gives them particular physical properties.
The 3-D shape of a polypeptide chain is determined by the sequence of amino acids in the polypeptide chain (i.e. by the primary structure).
The logic goes like this:
The sequence of amino acids determines which bonds between R-groups can form and where.
The location of the bonds between R-groups determines the shape that the polypeptide chain holds itself in.
Fibrous and globular proteins
Proteins fall into one of two categories depending on their shape.
Fibrous proteins are long fibres. They often have a simple repeated sequence of a limited number of amino acids. This gives them very regular structures. They are insoluble in water. They tend to have structural functions.
Globular proteins have a very irregular folding pattern and are ‘roughly’ spherical (only very roughly in most cases!). They often have a wide range of different amino acids in their polypeptide chains. They are soluble in water because they have hydrophilic R-groups on their outer surface. An important aspect of their ability to hold their shape is due to the fact that there are many hydrophobic R-groups internally and if these were to drift out of place they would be pushed back inside due to them being hydrophobic. They tend to have metabolic functions (e.g. enzymes, hormones, carrier proteins).
Prosthetic groups
Some proteins need other molecules (not made from amino acids) in order to hold the correct shape and perform the correct function. If these are permanent parts of the structure of the protein they are called prosthetic groups.
Proteins that need prosthetic groups to be functional are called conjugated proteins.
One of the roles of the Golgi apparatus is to add prosthetic groups to proteins (i.e. to modify proteins).
Examples of prosthetic groups are the haem groups in haemoglobin and the carbohydrate parts of glycoproteins.
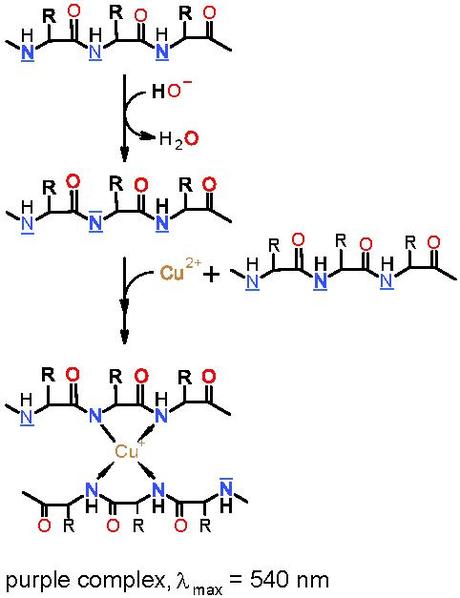 The Biuret test for proteins
The Biuret test for proteins
Biuret reagent contains copper sulfate (amongst other things). The Cu2+ ions give the Biuret reagent its blue colour.
This test is a test for peptide bonds. The Cu2+ ions are reduced to Cu+ ions which, when in an alkaline solution, form a complex with the nitrogen component of four peptide bonds. This complex is purple and so a positive result for proteins in the Biuret test is a change from pale blue to purple (or lilac).
Types of proteins – named examples in the “proteins” and “enzymes” topics
Protein | Function | Globular or fibrous | Number of Polypeptide chains | Non-protein component? | Properties | Quaternary structure? | Conjugated? |
Haemoglobin | Transport oxygen. | Globular | 4 (2 alpha and 2 beta) | 4 haem groups containing Fe2+ | Structure allows for reversible binding with O2. It is soluble in water. | y | y |
Elastin | Allows structures to stretch when necessary whilst also returning them to their original size (such as blood vessel walls and alveoli of the lungs). | Fibrous | Many | n | Elastic and flexible. Insoluble in water. | y | n |
Catalase | Enzyme: Catalyses the breakdown of hydrogen peroxide (a harmful by-product of metabolism). | Globular | 4 | 4 haem groups containing Fe2+ | It has a specific shape so that its active site increases the rate of reaction of this specific reaction only. It is soluble in water. | y | y |
Collagen | Resists a change in length under force – provides a high tensile strength to structures. Used in skin, tendons, ligaments, the nervous system, bone, cartilage. | Fibrous | 3 | n | Inelastic and flexible. High tensile strength. Insoluble in water. | y | n |
Amylase | Enzyme: catalyses amylose and amylopectin hydrolysis. | Globular | 1 | Chloride ion and calcium ion | It is soluble in water. It has a specific shaped active site to catalyse amylose and amylopectin hydrolysis. | n | y |
Carbonic anhydrase | Enzyme: catalyses the conversion between carbon dioxide and water, and carbonic acid. | Globular | 1 | Zinc ion | It is soluble in water. It has a specific shaped active site to catalyse the conversion between carbon dioxide and water, and carbonic acid. | n | y |
Insulin | Hormone: Travels around the body in the bloodstream. Released by the pancreas when blood sugar levels are high. It causes more glucose to be taken up from the blood and stored as glycogen. | Globular | 2 | n | Soluble in water. It is a specific shape to bind to receptors on liver (and other) cells. | y | n |
Keratin | Provides a high tensile strength and toughness to structures such as hair, skin and nails. | Fibrous | Many | n | Inelastic and inflexible (although the number of disulfide bonds a particular molecule contains determines the flexibility). High tensile strength. Insoluble in water. | y | n |