Bio Chapter 2 - The Cell
“But the plans of the Lord stand firm forever, the purposes of his heart through all generations” - Psalm 33:11
Lesson 2.1 - Atoms + Other Building Blocks of Life
Electron - a ==negative== charge on the ==outside== of an atom
Neutron - @@no charge@@ and @@inside@@of the atom
Proton - a positive charge inside the atom and bonded to neutrons
Compound - consisting of %%two or more%% simple parts or individuals in %%combination%%
What is the compound H2SO4 made of - ^^two hydrogen^^ atoms, ^^one sulfur^^ atom, and ^^four oxygen^^ atoms
Why Does Ice Float - Ice floats because when it freezes, ==it expands== and ==binds to other water molecules== doing the same. These ==stick together== and work to ==form ice,== which is ==less dense than water.== This is important to the organisms underneath because they are ==protected from the harsh elements== by this ice and therefore can live through the winter.
Adhesion - The act of water sticking to @@other surfaces@@
Cohesion - The act of water sticking to itself
Capillary Action - The act of water using %%both cohesion and adhesion%% to supply water to the leaves of a plant
Surface Tension - Tension created by tension ^^inflicted upon the bonds of water^^ molecules
Differences - Water sticking to ==itself versus other surfaces,== water using this to ==perform an action==, and the ==use of cohesion in surface tension==
Lesson 2.2 - Water Molecule
Polar Covalent Bond - These are formed when there is an @@unequal sharing of electrons@@ between atoms
Covalent Bond - These are formed when there is an equal sharing of electrons between atoms
Why is Water a Polar Molecule - Water is polar because the hydrogen and oxygen atoms share electrons. However, the %%oxygen atom has more protons%% in its nucleus (which are positive) and %%therefore attracts more electrons%% (making it negative), although the hydrogen keeps some (making it positive). These %%uneven amounts of electrons create poles%%, which makes water polar.
How Hydrogen Bonds Form with Water Molecules - Since ^^oxygen atoms have slightly negative charges^^ and ^^hydrogen atoms have slightly positive charges^^, the two can attach to one another. This is essentially the bonding of two water molecules
Solution - A type of ==homogenous mixture== in which the particles of one or more substances (the solute) are ==distributed uniformly throughout== another substance (the solvent). The particles are too small to be seen by the naked eye, unlike in a ==suspension==, where the particles ==don’t settle== are ==big enough for the naked eye to see==
Solute - A substance that is @@dissolved@@ in a solution - Sugar in Tea
Solvent - A liquid that is able to dissolve a solid - Acetone
Acid - A compound that %%forms H+ ions%% in a solution. pH %%scale range 0-6%%, weak 4-6, strong 0-3
Base - A compound that produces ^^hydroxide ions^^ in a solution. pH ^^scale range 8-14^^, weak 8-10, strong 11-14
Neutral - A compound with a ==pH of 7==, or perfectly neutral, without leaning acidic or basic
Homeostasis - The tendency toward a relatively @@stable equilibrium@@ between interdependent elements
Buffer - Weak acids or bases that can react with strong acids or bases to prevent sharp, sudden changes in pH. These help the body to remain in homeostasis
Lesson 2.3 - Carbon Compounds
Carbohydrates - Compounds made of %%carbon, hydrogen, and oxygen%%
- Used as a ^^main source of energy^^ in living things
- Plants use it for ==structural purposes==
- Breakdown of carbs (sugars) @@provide immediate energy@@ to cells
- Store extra sugar as complex carbs called starches
- Monosaccharides - anything that %%ends in “ose”,%% and %%includes sugars and starches%%
Lipid - Compounds made from ^^carbon and hydrogen atoms^^
- ==Not soluble== in water
- @@Fats@@, @@oils@@, @@waxes@@, and @@steroids@@
- Used to store energy and create waterproof cell membranes
- Formed when %%glycerol molecules%% are combined with %%fatty acids%%
- Fatty acids chains are formed when a ^^carbon molecule is joined with two carbon molecules^^
- A ==satured fatty acid== is formed when a ==chain is joined with a single bond==
- An @@unsatured fatty acid@@ is formed when a chain is joined with another chain or if there is @@at least one carbon-carbon double bond@@
Protein - Macromolcues that contain oxygen, nitrogen, carbon, hydrogen, and sulfur
- Made of %%amino acid monomers%%
- Amino acids have an ^^amino group on one end^^ and a ^^carboxyl group on the other^^
- There are ==20 different amino groups==
- @@Instructions@@ for amino acids to form are @@found in DNA@@
- Control the rate of reactions and regulate cell processes
- Used to %%form bones and muscles%%
- Used to ^^transport other substances^^ and ^^fight diseases^^
- There are ==four== steps to forming proteins
- 1: Amino acids @@form a chain@@
- 2: Amino acids are twisted to form a helix or a pleated sheet
- 3: The chain is then %%folded into a 3D object%%
- 4: If there is more than 1 object, the ^^two can join and create another object^^
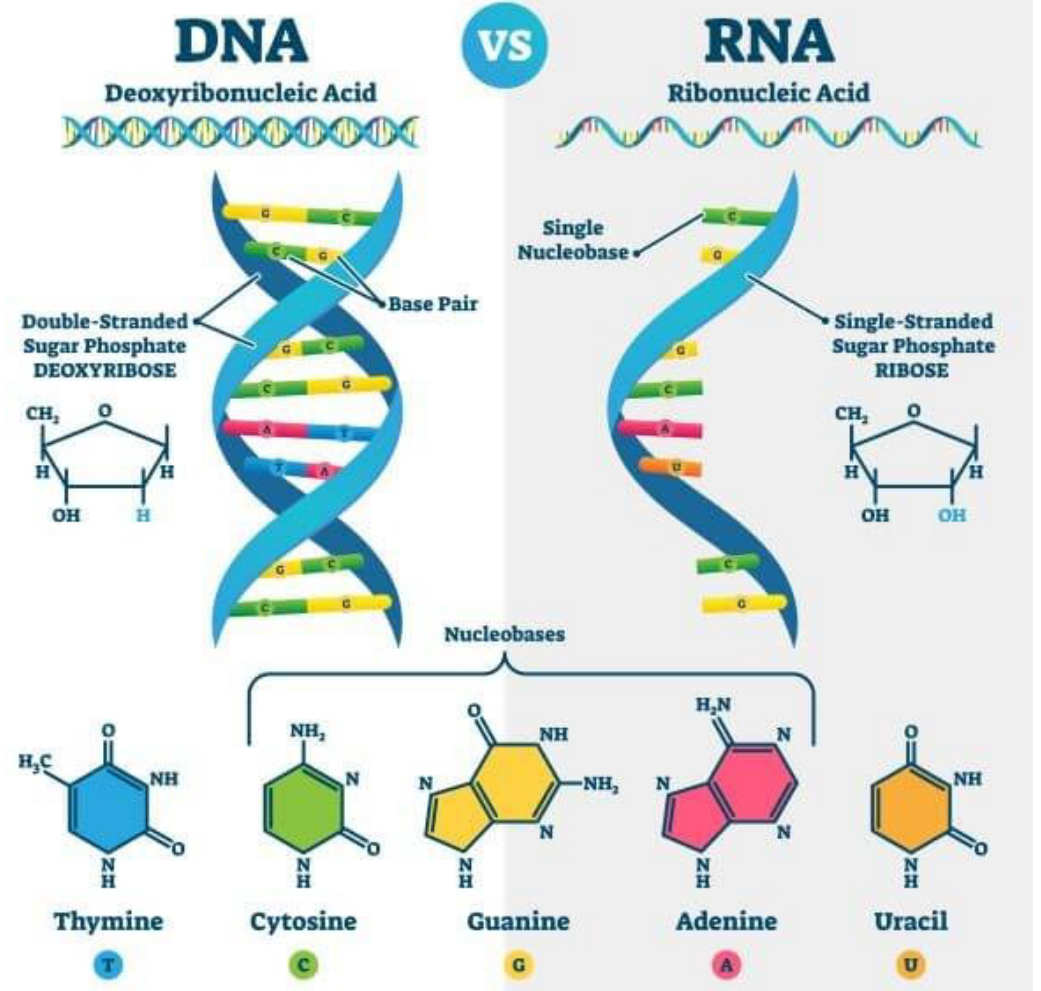
Nucleic Acid - Macromoclues that contain ==hydrogen, oxygen, nitrogen, carbon, and phosphours==
Made of @@nucleotide@@ monomers
Nucleotides contain/are made of:
- A %%5-carbon sugar%%
- A ^^phosphate^^ group
- A ==nitrogen base==
When @@multiple nucleotides@@ are @@joined by covalent bonds@@, they create @@nucleic acids@@
Used to store and transmit genetic info to cells
RNA - %%Single%% helix & DNA - %%double%% helix
Lesson 2.4 - Chemical Reactions + Enzymes
Chemical Reaction - A process that ^^changes one set of chemicals^^ into ^^another set of chemicals^^
During a Reaction Energy Can Be Released In - The form of ==energy==
Energy - Cannot be @@created or destroyed@@, but is @@transferred each time a chemical reaction is carried out@@. It is also needed in @@each and every chemical reaction,@@ as it is used to activate it
Plants - Get their energy from the sun (this is their activation energy)
Humans - Get their energy from %%eating plants and animals%% (this is their activation energy)
Endergonic - Energy ^^absorbed^^ in a reaction
Exergonic - Energy ==released== in a reaction
What Happens to ___ During a Chemical Reaction:
- Atoms - They are @@rearranged@@
- Compounds - They are also rearranged to fit the new set of chemicals
Product and Reactants of 2H + O > H2O:
- Products - The %%element H2O%%
- Reactants - ^^2 Hydrogen^^ molecules, ^^1 oxygen^^ molecule
Activation Energy - The energy that is ==needed to get a reaction started==
Catalyst - A substrate that @@speeds up the rate@@ of a @@chemical reaction@@. These work by @@lowering the nesscary amount of activation energy@@
Enzymes: “Ase”
- Proteins that act as biological catalysts
- %%Speed up chemical reactions%% in cells
- ^^Lower^^ing the ^^activation energy^^
- Are ==very specific== and only responsible for ==carrying out one reaction==
- @@Lact@@ase - @@Lact@@ose
Inhibitor - Something that prevents the action of an enzyme by blocking the active site so a substrate cannot enter as easily
Temperature and Enzymes - If enzymes are put in conditions that are %%too hot or cold for them to work easily,%% it will take them %%longer to produce new molecules%% and do their jobs properly
- ^^Hotter^^ Temp - Molecules move ^^too fast^^
- ==Lower== Temp - Molecules move ==too slow==
- Denaturation - 3D proteins @@losing their shape and size@@
2 Things Enzymes Do During Chemical Reactions:
- Enzymes lower activation energy
- Enzymes %%speed up a chemical reaction%%
The Steps an Enzyme Undergoes During A Chemical Reaction -
- Substrates ^^bind to active site^^ on enzyme
- Bond in substrate ==break/new bonds are created==
- Products are f@@ormed and released@@
- Enzyme is free to be used again
Enzymes Play Essential Roles In:
- %%Regulating%% cell pathways
- Making ^^materials^^ cells need
- ==Releasing== energy
- @@Transferring@@ information
“But as for you, be strong and do not give up, for your work will be rewarded” - 2 Chronicles 15:7