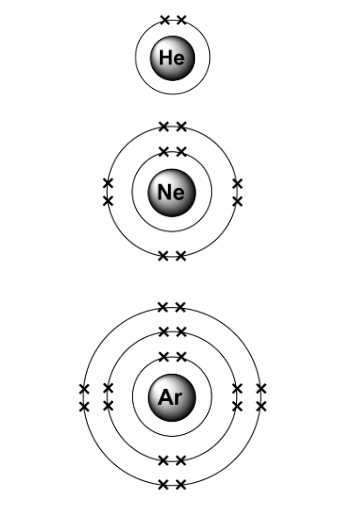
Groups in Periodic Table
The periodic table is a list of elements arranged in order of increasing atomic number. The electronic configuration of the first 20 Elements is shown below:
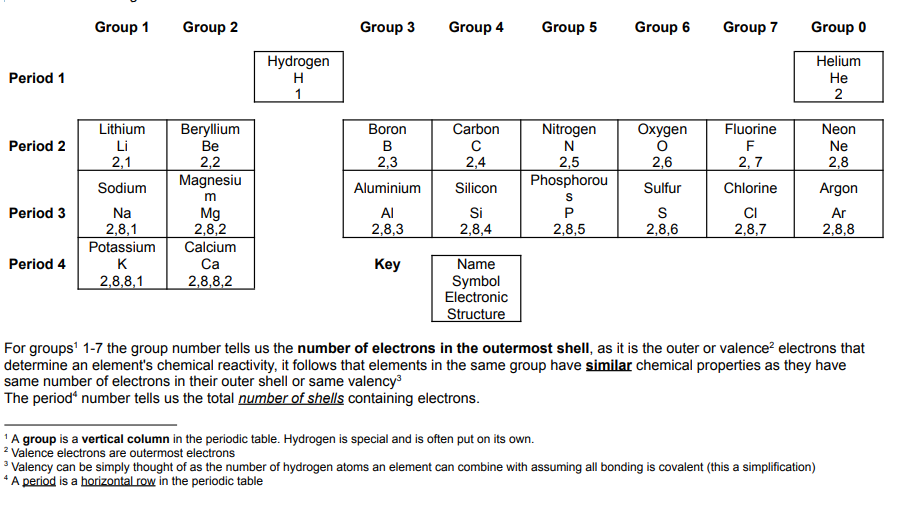
Metal and Non-metals:
The periodic table can be split into metals and non-metals by the zig-zag line that starts between group 2 and 3 in period 3.
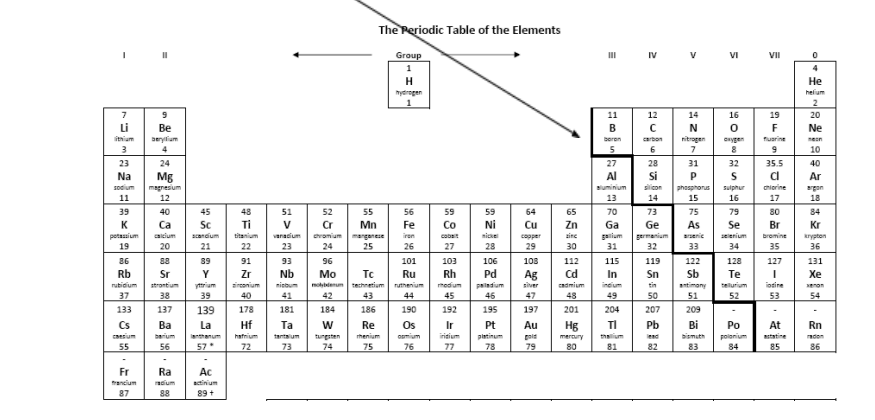 You may wonder who decided where to draw the line. There are numerous ways to classify elements as metals and non-metals and you may previously have used physical properties such as appearance to distinguish between them. E.g. metals are often shiny when freshly cut, metals are malleable etc.
You may wonder who decided where to draw the line. There are numerous ways to classify elements as metals and non-metals and you may previously have used physical properties such as appearance to distinguish between them. E.g. metals are often shiny when freshly cut, metals are malleable etc.
Elements can also be classified by their chemical properties, in this case the acid/base nature of their oxides.
Oxides of metals form bases e.g. magnesium oxide, MgO
Oxides of non-metals form acids e.g. sulphur dioxide, SO2
Group 1, The Alkali Metals
The alkali metals can be found in group 1 of the periodic table.
Physical Properties
Silvery/shiny when freshly cut
Soft (easily cut with a knife) – become softer as you move down the group
Low density – float on water
Conduct electricity
Chemical Properties
They react with water to give a metal hydroxide and hydrogen
Eg. metal+ water → metal hydroxide + hydrogen
They become more reactive with increasing atomic number (as you go down the group)
The reaction of Alkali Metals with Water
The alkali metals all react rapidly with water producing hydrogen gas and the corresponding metal hydroxide. This similarity in reactivity creates a family of elements referred to as a group. The similarity in chemical properties within a group is due to all the members having the same number of electrons in the outer shell.
Observation for Li
Li is stored in oil to prevent it reacting with air
Li is soft enough to cut with a knife
Li Foats on water (its density must be less than that of water)
Li moves slowly around the surface of the water
Li fizzes when in reacting with water (hydrogen gas is given off)
The piece of Li disappears once it has all reacted
The water has become alkaline due to the formation of lithium hydroxide
2Li(s) + 2H_2O(l) → 2LiOH + H_2(g)
Observations for Na:
Na is stored in oil to prevent it reacting with air
Na is softer than Li
Na floats on water (its density must be less than that of water)
The heat of the reaction melts the Na into ball
Na moves quickly around the surface of the water
Na fizzes rapidly when in reacting with water (hydrogen gas is given off)
The piece of Na disappears once it has all reacted
The water has become alkaline due to the formation of sodium hydroxide
2Na(s) + 2H_2O → SNaOH(aq) + H_2(g)
Observations for K:
K is stored in oil to prevent it reacting with air
K is softer than Na
K floats on water (its density must be less than that of water)
The heat of the reaction melts the K into ball
K moves very quickly around the surface of the water
K fizzes violently when in reacting with water (hydrogen gas is given off)
The K ignites and burns with a lilac flame
The piece of K disappears once it has all reacted
The water has become alkaline due to the formation of potassium hydroxide
2K(s) + 2H_2O(l) → 2KOH(aq) + H_2(g)
Group 7, The Halogens and Redox
The halogens are all non-metals and found in group 7 of the periodic table. As elements they all occur as diatomic molecules. There are trends in some of the physical properties as you go down the group:
They become darker in colour
Their melting point/ boiling point increases (hence the gradual change in state at room temperature from gas to liquid to solid)
Key physical properties that you must learn have been highlighted in bold type. The other you can predict based on the trends listed above.
Name | Formula | Colour | Physical state at rtp | Colour in solution |
|---|---|---|---|---|
Fluorine | F2 | Yellow | Gas | - |
Chlorine | Cl2 | Green | Gas | Pale Yellow |
Bromine | Br2 | Red/Brown | Liquid | Orange |
Iodine | I2 | Dark Grey | Solid | Dark reddish brown |
Astatine | At2 | Black | Solid | - |
Relative reactivities of the Halogens:
The halogens become less reactive as you go down the group. This can be illustrated by comparing their reactions with iron or hydrogen. As a result a more reactive halogen will displace a less reactive halogen from a solution of one of its salts.
Eg. If you add colourless chlorine water to a colourless solution of potassium bromide the chlorine will displace the bromine from the potassium bromide as chlorine is more reactive. The solution turns orange as bromine is formed.
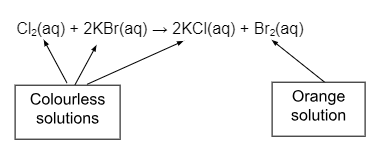
To form the ionic equation we rewrite the equation splitting ionic compounds into their ions
Cl_2(aq) + 2K^-(aq) +2Br_2(aq) → 2K^+(aq) +2Cl^-(aq) + Br_2(aq)
Remove any ions that appear on both sides of the arrow, these are called spectator ions
Cl_2(aq) + 2K^+(aq) +2Br^-(aq) → 2K^+(aq) +2Cl^-(aq) + Br_2(aq)
What remains is the ionic equation
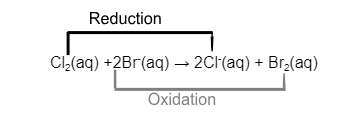
From the ionic equation we see that these displacement reactions are also redox reactions.
In the above example:
Cl2 has gained electrons and been reduced to form Cl- ions
Br- ions have lost electrons and been oxidised to form Br2
Group 8, The Noble Gases
The noble gases are a family of inert gases found in group 0 of the periodic table. They are very unreactive because they all have full valence shells and hence have a valency of 0.
The atoms hold onto their electrons too tightly to form covalent bonds or +ve ions. They don’t gain electrons to form –ve ions because the electrons would have to go into the next shell but as this is further away from the nucleus the nuclear attraction would be insufficient.