Note
0.0(0)
Explore Top Notes Note
Note Studied by 41 people
Studied by 41 people Note
Note Studied by 30 people
Studied by 30 people Note
Note Studied by 18 people
Studied by 18 people Note
Note Studied by 150 people
Studied by 150 people Note
Note Studied by 116 people
Studied by 116 people Note
Note Studied by 72 people
Studied by 72 people
Chapter 10 - Understanding work teams
4.5(2)
2.7: binary molecular compounds
5.0(1)
APUSH REVIEW (1788-1817)
4.0(1)
Lord of the Flies
4.9(7)
Chapter 7: Biological Bases: The Brain and Nervous System
5.0(3)
Transcription
4.0(1)
1.3B Orbital Diagrams and Electron Configs
Electron orbitals arranged by energy
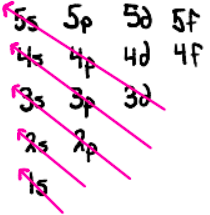
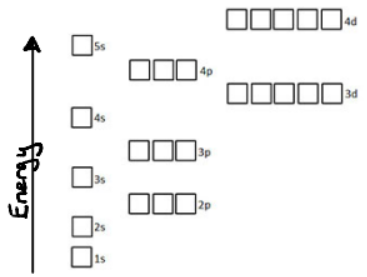 Order for filling electron orbitals
Order for filling electron orbitals
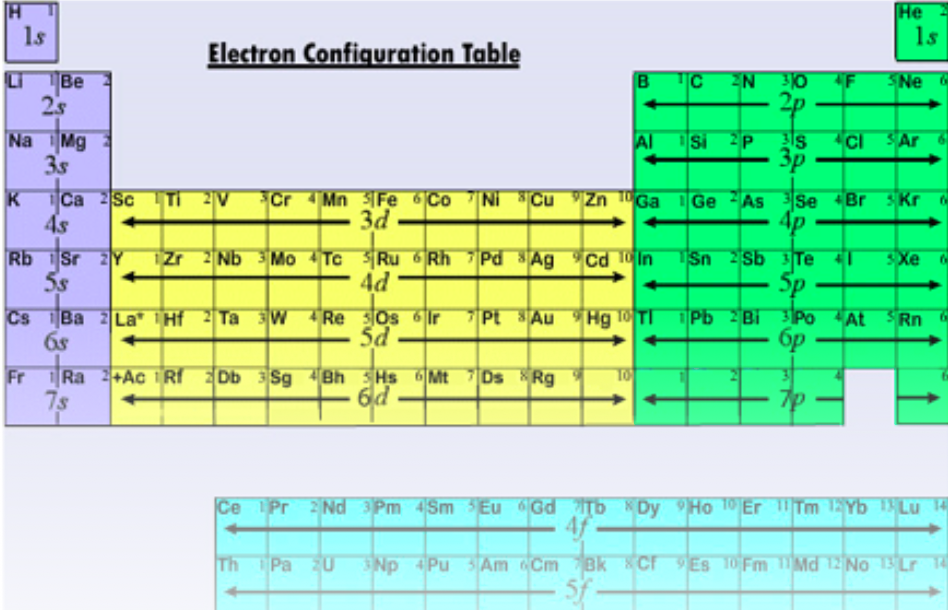
 Electron Configurations and the Periodic Table
Electron Configurations and the Periodic Table
Electron configurations can be written using the periodic table
Steps
Start at H
Move through the rows, top to bottom, left to right until you get to your given element
Each box adds one electron
Electrons go in orbital listed for that row
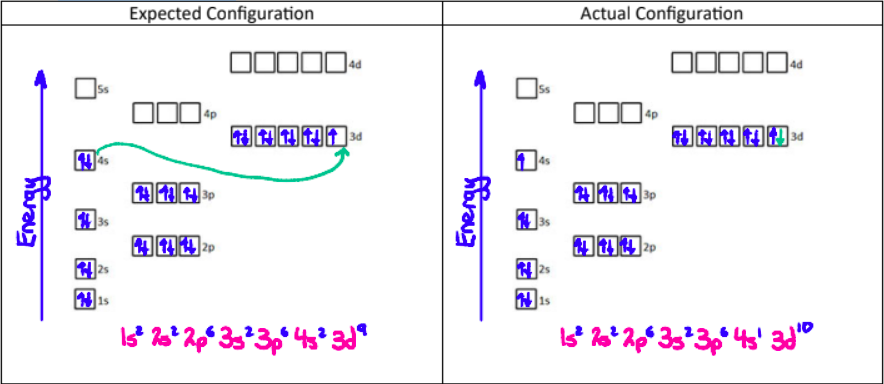
 Electron Configuration Exceptions
Electron Configuration Exceptions
1. Chromium (24 electrons)
Chromium takes on this configuration because a half-filled orbital has extra stability.
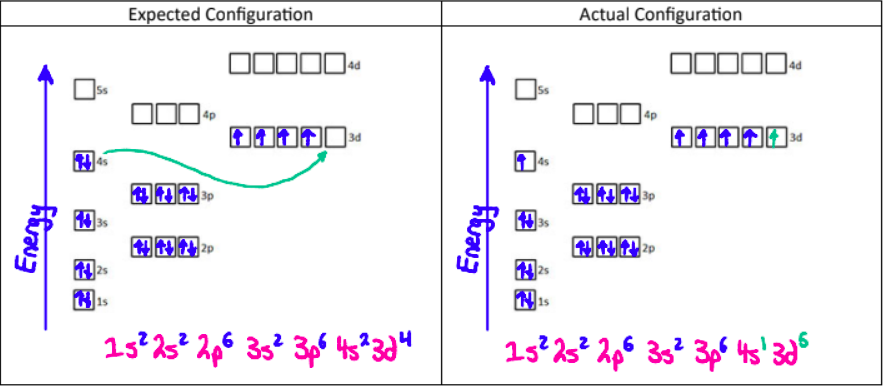
2. Copper (29 electrons)
Copper takes on this configuration because a totally filled orbital has extra stability.
Note
0.0(0)
Explore Top Notes Note
Note Studied by 41 people
Studied by 41 people Note
Note Studied by 30 people
Studied by 30 people Note
Note Studied by 18 people
Studied by 18 people Note
Note Studied by 150 people
Studied by 150 people Note
Note Studied by 116 people
Studied by 116 people Note
Note Studied by 72 people
Studied by 72 people
Chapter 10 - Understanding work teams
4.5(2)
2.7: binary molecular compounds
5.0(1)
APUSH REVIEW (1788-1817)
4.0(1)
Lord of the Flies
4.9(7)
Chapter 7: Biological Bases: The Brain and Nervous System
5.0(3)
Transcription
4.0(1)