Exam 3 Lecture Notes
Chapter 11: Cell Membranes
Membrane Structure
All cells have them.
Prokaryotes: 1 membrane → the cytoplasmic membrane (plasma membrane)
Eukaryotes: Multiple membranes → cytoplasmic membrane + all the various internal membranes (membrane-bound organelles)
General Properties
Fluid
Able to change shape very easily; flexible. Individual components that make up the membrane are usually free to move around in various ways.
Ex: Fibroblasts - Assume these really extreme angles.
Self-annealing.
Selectively Permeable
Selective about which molecules they allow to permeate the membrane.
How they maintain certain chemical environments.
Mosaics
Mixtures of different kinds of molecules (lipids, proteins, carbohydrates, etc.)
Ex: Human RBC, plasma membrane
~43% lipids by weight
~49% proteins by weight
~8% carbohydrates by weight
Ex: Human neuron, plasma membrane
~79% lipids
~18% proteins
~3% carbohydrates
Asymmetric
The two halves of the bilayer are chemically distinct; two layers with different compositions.
Cytoplasmic side (inner side)
Non-cytoplasmic side (outer layer)
AKA the luminal side
AKA the extracellular side
Components of Cell Membranes
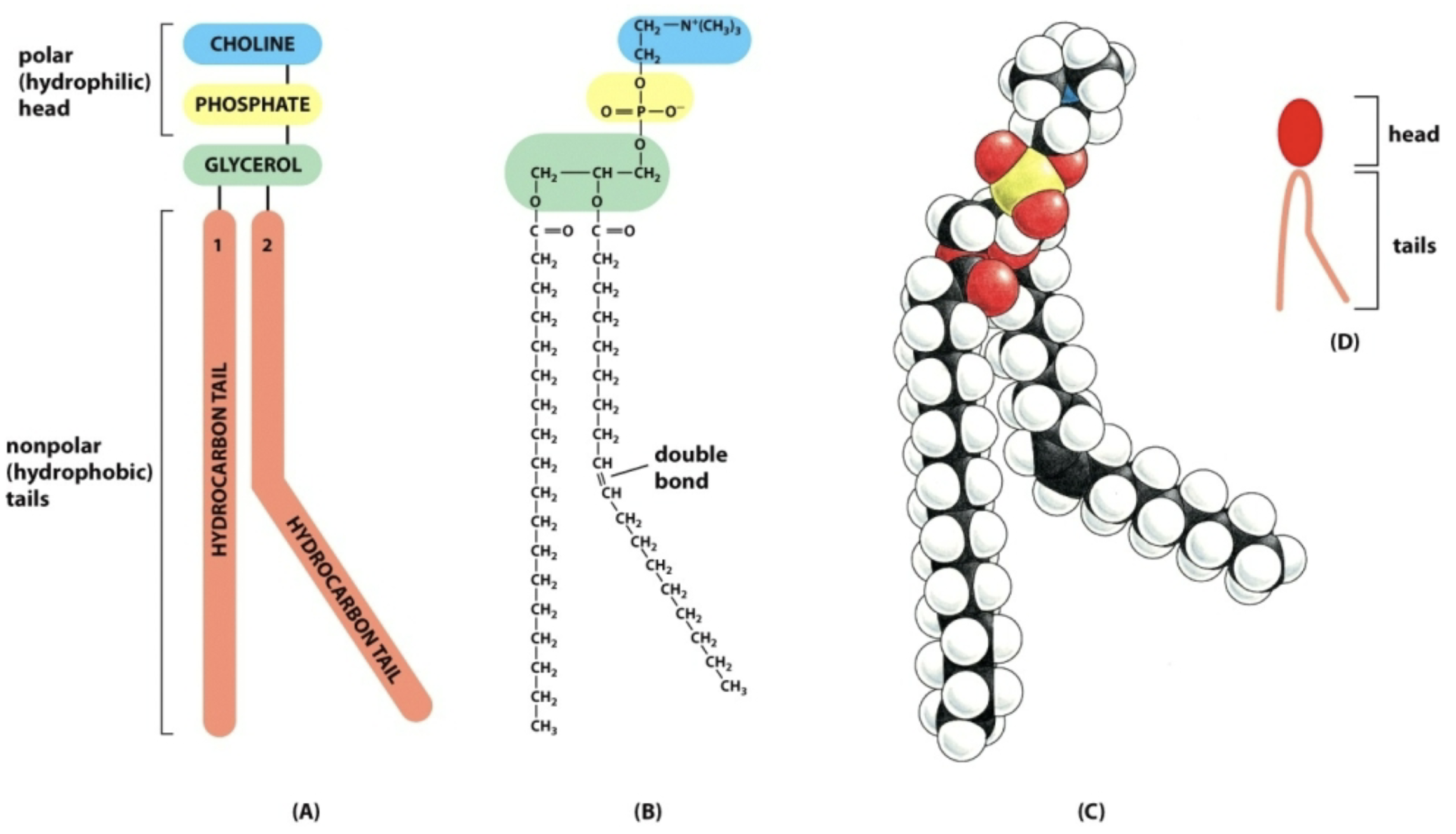
Phospholipids
Primary structural components
Amphipathic (partially hydrophilic and partially hydrophobic)
Head: choline, phosphate, glycerol, polar, charged; Hydrophobic: Fatty acid chains, nonpolar, uncharged.
Spontaneously form a bilayer in water
If enough of them, they will seal off and form a ring (artificial cell membranes/liposomes).
Usually free to move around in various ways within one half of the bilayer
If they have any thermal energy: Flexion, which is the movement of the tails
Rotation is rotating on their axis/tails spin on head
Lateral Diffusion = drifting
Flipping = Rarely occurs, but means a phospholipid could jump from one half to the other half of a bilayer
New phospholipids are made in the luminal space of the endoplasmic reticulum
“Scramblases” - Scramble, or randomly distribute, the phospholipids from one monolayer to another
Golgi apparatus is where phospholipid asymmetry is generated
“Flippases” - Catalyzes the transfer of specific (scrambled) phospholipids to the cytosolic monolayer
One fatty acid hydrocarbon tail is straight (saturated) and one is kinked or bent (unsaturated)
Saturated: No double bonds (flexible)
Unsaturated: Have one or more double bonds (rigid, more inflexible) which causes kinks
This reduces/eliminates the possibility of “phase separation”
If both tails were saturated (straight), there would be less fluidity but the membrane would be easier to freeze (lose transport capacity and ability to transport vesicles).
If both tails were unsaturated (kinked), the membrane would be more fluid or even too flexible. It would also be harder to freeze.
Overall, this causes congregations/patches of phospholipids that freeze unevenly.
Ex: phosphatidylcholine
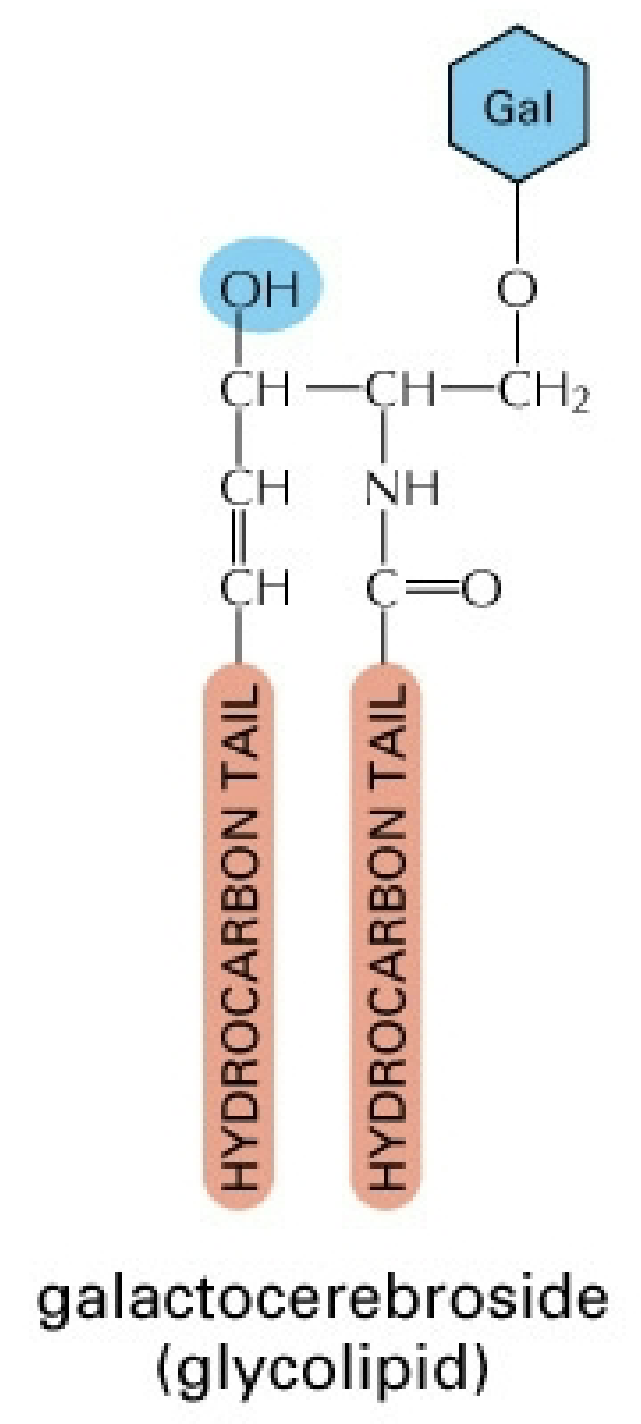
Glycolipids
Secondary structural & functional components
Ex: Galactocerebroside - In neuron plasma membranes
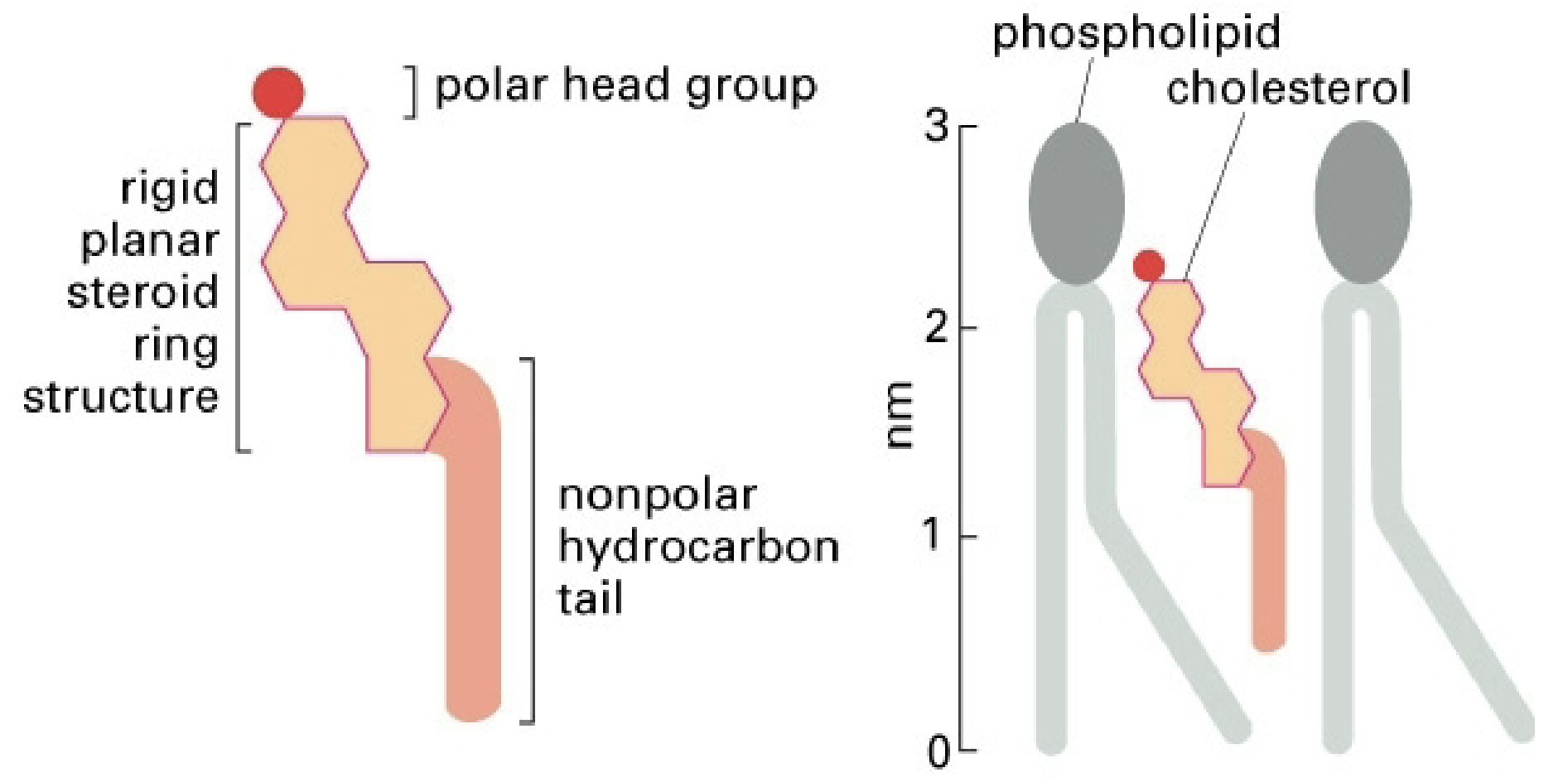
Cholesterol
Secondary structural component in animal cell membranes mainly
Affects membrane fluidity (more cholesterol = less flexible; less cholesterol = more flexible)
Able to relieve tension in the membrane by flipping
This is why cholesterol is so abundant in animal cells and not so much in plant cells—animal cells are exposed to much more extreme bending and movement
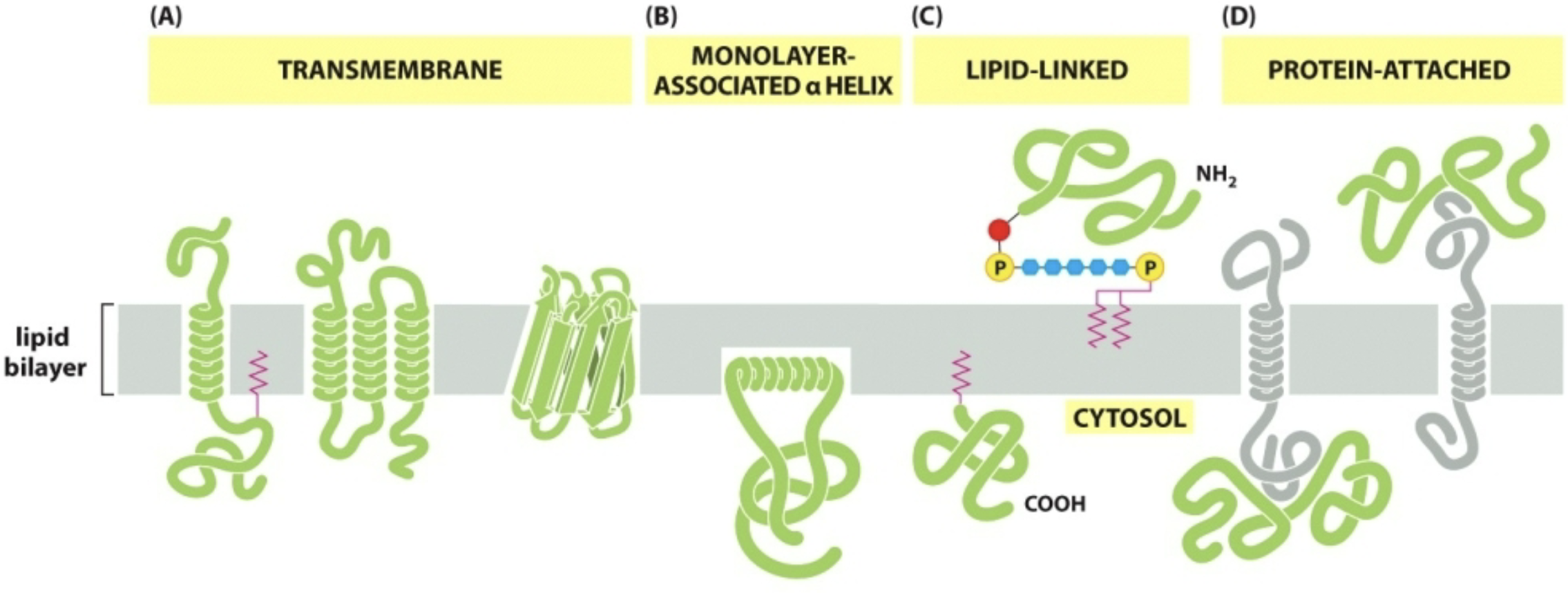
Proteins & Glycoproteins
Primary functional components (not so much structural)
Integral Membrane Proteins
Proteins that are integrated directly into the phospholipid bilayer
Transmembrane proteins
Span across/embedded in both halves of the bilayer
Membrane-associated proteins
Embedded in only one half of the bilayer
Lipid-linked proteins
Covalently attached to lipids that are part of one half of the bilayer
Peripheral Membrane Proteins
Noncovalently associated with integral membrane proteins
Signal transduction proteins and kinases
Functionally:
Transport
Receptors (hormone, cytokine, growth factor)
Enzymes (in mitochondria involved in cellular respiration)
Recognition
Up to 50% of membrane by weight
Carbohydrates
Attached to membrane lipids (glycolipids) + attached to membrane proteins (glycoproteins) → form the glycocalyx (outer layer of carbohydrates)
Noncytoplasmic side only (= extracellular side = lumenal side)
Attached to membrane lipids and proteins in the ER and the Golgi (done by enzymes)
Functions: Protection, lubrication, adhesion (helping cells stick to things or other cells), communication, cellular migration, etc.
Ex: Lymphocyte
Has extremely large nuclei
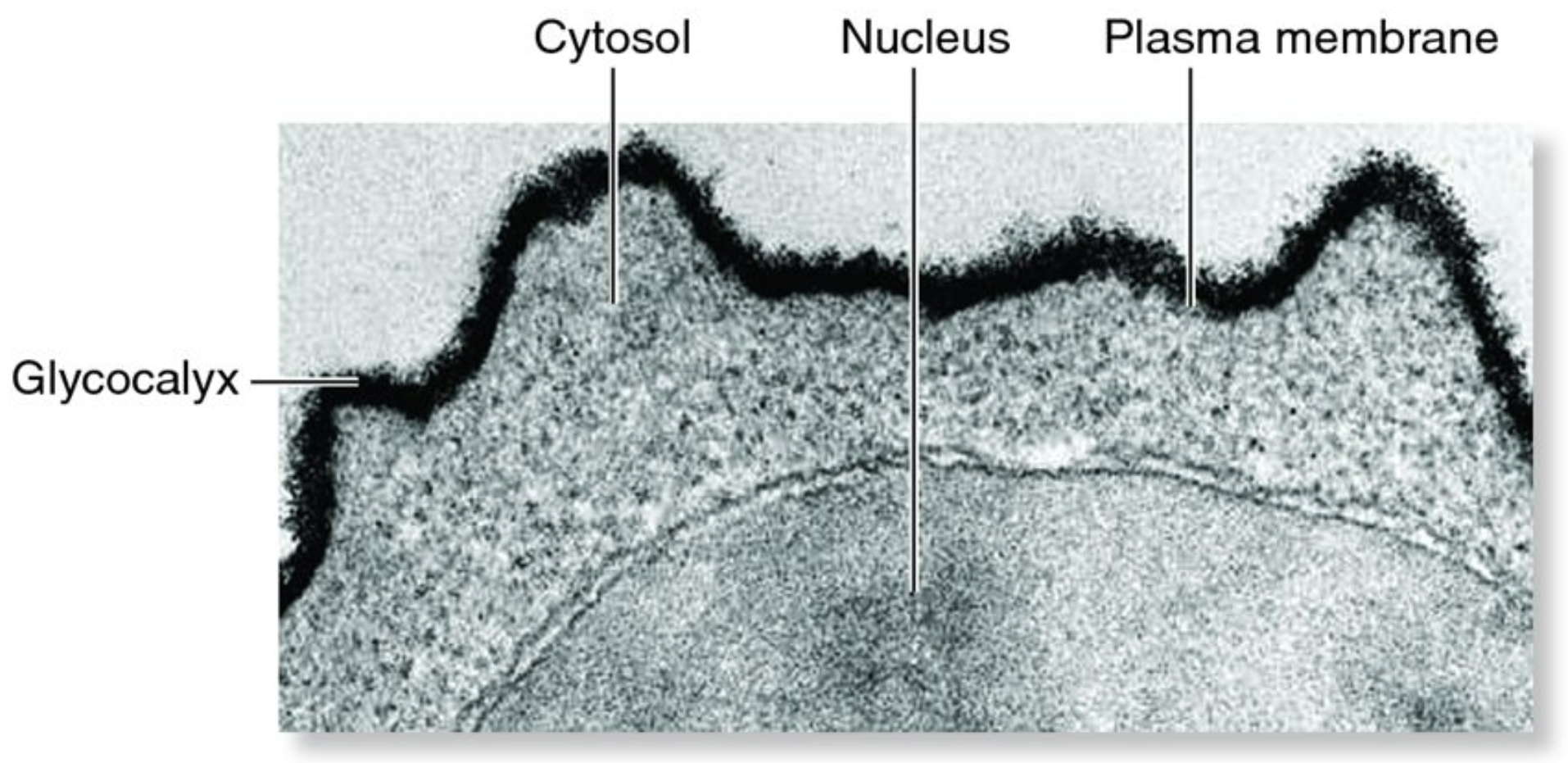
Membrane Proteins
Membrane Domains
Portion of an integral membrane protein that is actually embedded in the bilayer; consists of all or mostly hydrophobic amino acids
Ex: Single alpha helix
Monolayer - one half of bilayer
Transmembrane domain - span both halves of bilayer (~19 amino acids)
Hydrophobic side chain amino acids have to face outward
Includes many receptors, enzymes, and recognition proteins
Ex: mHCI - Major histocompatibility complex; almost no chance that 2 people have the same one
Ex: mHCII - Heterodimer (two different subunits/alpha and beta); disulfide bonds facing outward to help hold shape of molecules together
Ex: CD4 and CD8 - Co-receptor on T cells (CD4) and activated t-lymphocytes when you get an infection. CD8 is a heterodimer with disulfide bonds anchoring to the plasma membrane
Ex: Golgi sialyltransferase - Enzyme embedded in the Golgi membrane by way of an alpha helix that adds sugars to proteins in the Golgi
Multiple alpha helices
Includes many in ion channels, receptors, and membrane-embedded enzymes
Often ½ of each alpha helix is hydrophobic and ½ is hydrophilic
Ex: bacteriorhodopsin-7 transmembrane alpha helices
Light absorption + proton pumping (photosynthesis in Halobacteria, more specifically Halobacterium salinarum).
Ex: G-Protein-coupled Receptor
Large family of receptors for cytokines, hormones, neurotransmitters, pheromones, etc… all have 7 transmembrane alpha-helical domains
β-adrenergic receptor = epinephrine receptor = adrenaline receptors
Smell and taste receptors
Opsins + rhodopsins (vision)
Ex: Voltage-gated Na+ ion channel involved in action potential in neurons (24 transmembrane alpha helices)
One or more “rolled sheets” (β-barrels)
Ex: Porin in outer membranes of gram-negative bacteria + outer membrane of mitochondria
Kyte-Doolittle Hydropathy Plot
Method to identify membrane domains in membrane proteins; graph of amino acids at each position vs. hydropathy score
Hydropathy Score:
More positive = more hydrophobic
More negative = more hydrophilic
Each position represents an amino acid
Each score above 0 is hydrophobic; each score less than 0 is hydrophilic
LSS = Leader Sequence
Acetylcholine
Four transmembrane alpha helices (M1, M2, M3, and M4)
LSS is hydrophobic
Chapter 12: Membrane Transport
Intracellular vs. Extracellular Environment
Phospholipid bilayers are selectively permeable.
Small, hydrophobic molecules are able to cross the membrane very quickly and easily
O2, CO2, N2, benzene
Makes cell respiration easy as it does not have to have any specific dedicated carrier or transporter due to the concentration gradient created
Small polar (partial charge) molecules are able to cross the membrane easily on their own, just takes a little more time to wedge through the phospholipids
H2O, glycerol, ethanol
Larger (3 carbons or more) polar molecules are not able to cross on their own because they are bulky and polar (hydrophilic)
Amino acids, glucose
Ions + Charged molecules do not cross on their own
H+, Na+, HCO3-, K+, Ca2+, Cl-, Mg2+, amino acids, nucleotides
Types of Membrane Transporters
Channel Protein - Creates a hydrophilic pore to allow ions to move in or out of the cell
Passive only → Facilitated diffusion (high to low concentration through the hole)
Selective based on size and charge
Constitutive (always open) and Gated (open or closed) channels
Voltage-gated ion channels (open/close based on charge/electrolyte balance or imbalance)
Ligand-gated
Mechanically-gated
Ex: K+ ion channels
Most common type
Present in most cell types
Many subtypes
K+ leak channels - Constitutive; help maintain resting membrane potential
Ca2+ Gated - Open only in response to high levels of [Ca2+]
Voltage-Gated K+ Channel - Open in response to changes in resting membrane potential
Ex: Voltage-Gated Na+ Channels
Help propagate action potential in neurons
Carrier Proteins
Behave like enzymes
Michaelis-Menten Graph: the ½ V-max on the y-axis (rate of transport) corresponds with the Km on the x-axis (concentration of transported molecule).
Direct diffusion across the membrane: Straight line (/)
Passive (work down a concentration gradient) or Active (needs energy, forcing an ion or molecule against a concentration gradient)
3 Types:
Uniporter - Transport 1 type of ion or molecule in one direction either into or out of the cell
Symporter - Transporting two types of ions or molecules in the same direction simultaneously
Antiporter - Transporting 2 types of ions or molecules in opposite directions
Sources of energy for active transport:
ATP hydrolysis, concentration gradient (couple the downhill transfer of a molecule or ion to the uphill transfer of another molecule or ion; downhill process endergonic and uphill process exergonic; “coupled transport”), sunlight (bacteriorhodopsin)
Ex: Na+/K+ Pump
ATP-driven active antiporter
Ouabain binds to this pump to prevent K+ binding
In cytoplasm membranes of all animal cells (including humans)
Account for ~30% of all ATP use in animal cells
For every ATP molecule they hydrolyze, they pump 3 Na+ ions out of the cell and 2 K+ in
Generate Na+ gradient (High [Na+] outside the cell), K+ gradient (High [K+] inside the cell), and electrochemical gradient (“resting membrane potential”; positive on outside, negative on inside; ~70 mV difference)
Other carriers can tap into these gradients, which is how we get “action potential”
Temporary rapid depolarization, followed by a repolarization
Help animal cells regulate osmotic pressure, since they don’t have cell walls
Ex: Glucose Transporters (GLUTs); ~15 types
Passive glucose uniporter
Takes advantage of a preexisting glucose concentration to passively bind to those glucose molecules and transport glucose down a concentration gradient
Gradient-Driven Active Glucose Symporters
Transports glucose against the concentration gradient
Transports Na+ down its concentration gradient at the same time
Ex: Ca2+ Pump
ATP-driven active uniporter
Plasma membranes of eukaryotic cells; pump Ca2+ out of the cell
Animals muscle cells = in sarcoplasmic reticulum pump Ca2+ into lumen of S.R.
Keeps Ca2+ in cytoplasm low
Ex: Bacteriorhodopsin
Light driven active transporter
7 transmembrane alpha helices
Plasma membrane of Halobacteria (archaea)
Performs light reactions of photosynthesis
Absorbs green light; transmits purple light
Pumps H+ out of the cell
Creates H+ gradient used by ATP synthases to make ATP
Summary of Action Potential
Action Potential - Electrical impulse that neurons use to rapidly deliver messages to the body; change in the neuron’s “resting membrane potential”.
Na+/K+ pumps in all animal cells generate Na+ gradient (high on outside, low on inside), a K+ gradient (high on inside, low on outside), and electrical gradient.
Neurons tap into the energy of these gradients to generate action potential.
Neuron stimulated by pressure, sound waves, light waves, chemicals, and a variety of initial stimuli → Gated passive Na+ channels in plasma membrane of the neuron open up.
Na+ diffuses into the cell → Depolarization begins (#1 on graph)
Threshold: ~ -50 mV. If threshold is not reached, depolarization will subside (failed initiations spot on graph)
If threshold is achieved, voltage-gated Na+ channels open → more Na+ rushes into the cell; membrane completely depolarizes (up to +35-40 mV) (#2 on graph)
Voltage-gated Na+ channels close after ~0.5 ms
Gated Passive K+ channels open → K+ diffuses out of the cell and repolarization occurs (#3 on graph)
~5 ms must pass before gated passive K+ channels can be reactivated → “refractory period”
Gated K+ cannot be activated during this period. The purpose of this 5 ms delay is to trigger the propagation of action potential (self-propagating wave of depolarization) by triggering the opening of Na+ voltage-gated channels. By the time the refractory period has elapsed, the wave is further down and the Na+ has already been pumped out. This makes sure that the action potential goes in one direction.
Na+/K+ pumps restore resting membrane potential (#5 on graph)
Na+ which diffuses into cell at step 6 (#2 in graph) diffuses laterally and triggers neighboring voltage-gated Na+ channels to open → Propagates action potential along the length of the axon or dendrite.
Self-propagating wave of depolarization
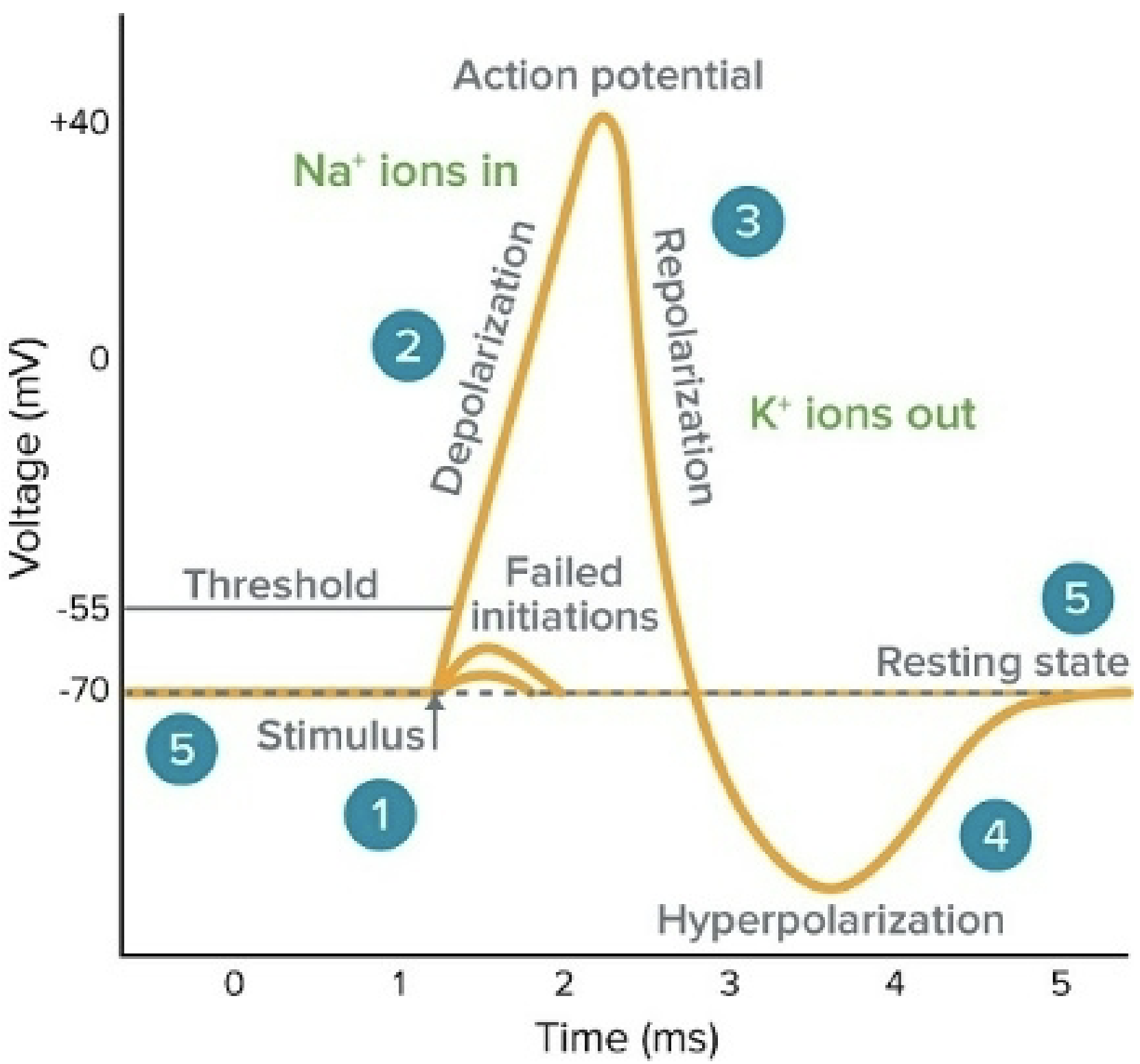
The dashed line on the -70 is the resting membrane potential
The line on ~ -50 is the threshold
Dashed line at top of graph
Y-axis says ‘membrane potential (mV)’ instead of voltage (mV)
Depolarization begins at #1 in the graph (step 4)
Add #5 (for step 5) to failed initiations spot
Chapter 14: Energy Generation in Mitochondria + Chloroplasts
Mitochondria
A typical eukaryotic cell contains ~1000 mitochondria
May change shape + change location in a cell
Do not have the ability to move on their own, so the cell itself moves it around (microtubules, motor vesicles)
Reproduce inside cells by binary fission
Contains two membranes
Acquired the second when they were originally engulfed by endosymbiosis
Outer membrane - smooth
Inner membrane - highly folded into structures called “cristae”
Contain 4 “compartments”
Mitochondrial Matrix (Inner Compartment)
Contains 67% of total mitochondrial protein
Enzymes for citric acid cycle (where it takes place; includes pyruvate dehydrogenase complex
Enzymes for fatty acid oxidation (digestion of fats)
Enzymes for amino acid oxidation (converting them to energy)
Where mitochondrial DNA, mitochondrial ribosomes, and mitochondrial tRNAs are located
Inner Mitochondrial Membrane
Folded into “cristae” to increase surface area for packing in more proteins and enzymes
Impermeable to most molecules (if a molecule is not small and hydrophobic, it is not going to get across the membrane on its own)
Contains ~21% of total protein in mitochondria
Enzymes for oxidative phosphorylation (where chemiosmosis takes place) — includes proteins making up the ETC, the proton pumps, and ATP synthases.
Carrier proteins (for pyruvate, ADP, inorganic phosphate; ATP out)
Outer Membrane
Contains ~6% of total mitochondria protein
Contains porin (channel formed by a beta sheet that rolled over on its side for a wide diameter hole. The holes are embedded in the outer membrane to allows things like pyruvate, ADP, and ATP out/in of the mitochondria). It is permeable to most small/medium size molecules.
Contains Bax and Bcl. 2 proteins → Involved in triggering apoptosis (cell suicide)
Outer Compartment (Inter-membrane Space)
Contains ~6% of total mitochondria protein
Similar composition to cytoplasm
Oxidative Phosphorylation (= chemiosmosis)
Indirect method of making ATP
Takes place in inner mitochondrial membrane
NADH + FADH2 deliver high energy electrons to the ETC in inner mitochondrial membrane
Modified nucleotide
High-energy electron carriers
Come from glycolysis → 2 NADH per glucose
Citric acid cycle → 8 more NADH + 2 FADH2 per glucose
Fatty acid oxidation (in mito. matrix) → 1 NADH + 1 FADH2 + 1 acetyl-CoA per each 2C unit on a fatty acid chain
a.) NAD+ = nicotinamide adenine dinucleotide
Coenzyme in all cells
Carries 2 e- + 1H+
NAD+ (oxidized) → NADH (reduced)
Delivers 2 e- to NADH dehydrogenase (1st component of ETC)
b.) FAD = Flavin adenine dinucleotide
Coenzyme in all cells
Carries 2 e- + 2 H+
FAD (oxidized) → FADH2 (reduced)
Delivers 2 e- to ubiquinone (2nd component of ETC)
ETC consists of 3 large complexes + 2 mobile e- carrier (~40 protein subunits)
Complex I = NADH Dehydrogenase Complex
e- bind to Fe at “Fe-S” complex (“electron cage”) attached to 4 cysteines (Fe3+ → Fe2+)
Pumps H+ across the inner mitochondrial membrane to build up proton concentration gradient
Mobile carrier I = ubiquinone = Q = “coenzyme Q” = “CoQ10”
Not a protein
Carries 2e-
Complex II = Cytochrome C Reductase Complex (= cytochrome b-c complex)
e- binds to Fe in heme group (nonprotein Fe + porphyrin ring) attached to methionines (Fe3+ → Fe2+)
Pumps H+ across the membrane
Mobile Carrier II = cytochrome C
e- binds to Fe in heme group (Fe3+ → Fe2+)
Highly conserved in evolution
Complex III = Cytochrome C Oxidase Complex
Binds 4 e- at a time (2 Fe in heme group + 2 Cu) (Fe3+ → Fe2+) (Cu2+ → Cu+)
Transfers e-’s to final electron acceptor (aerobic respiration) = O2 → H2O
Pump H+ across the membrane
e-’s move automatically through the ETC
High energy (low stability) → Low energy (high stability)
ATP Synthases tap into H+ gradient across membrane to indirectly make ATP (chemiosmosis)
Efficient because each time the electrons are transferred, they are going into a more stable state
Symporters in the inner mitochondrial membrane tap into H+ gradient to move pyruvate + inorganic phosphate into the mitochondrial matrix
Drugs that Block ETC
Rotenone (pesticide/piscicide) + amytal (amobarbital = a barbituate = tranquilizer “truth serum”)
Block transfer of electrons to ubiquinone
Antimycin A (piscicide/furgicide)
Blocks transfer of electrons from ubiquinone to cytochrome c reductase
Cyanide (CN-), azide (N3- molecule), carbon monoxide (CO)
Blocks transfer of electrons to O2 (cytochrome oxidase) at the last step
ATP Synthase
Highly conserved (bacteria, archaea, plants, animals, fungi, etc.)
Fully reversible:
ATP Synthesis: ADP + Pi → ATP
ATP Hydrolysis: ATP → ADP + Pi
2 Domains:
F0 = transmembrane domain; creates H+ channel
3 H+ → 1 ATP
F1 = “lollipop head” = catalytic domain (creates ATP molecule)
Acts like a molecular “turbine”: flow of H+ through F0 generates rotational energy
Produces ~100 ATP/second
Drugs that Block ATP Synthase
Oligomycin
Antibiotic-produced Streptomyces bacteria
Poisons ATP synthase and blocks F0 H+ ion channel
Prevents chemiosmosis from occurring
Can still make ATP by glycolysis
However, builds up too much lactic acid and acidifies blood pH and urine (fatal)
DNP = dinitrophenol
Affects ATP synthase indirectly by inserting itself into the inner mitochondrial membrane and acts as a H+ channel
Dissipating H+ gradient, preventing ATP synthase from tapping into a pool of energy that would have been made if all the H+ was transported across the membrane/H+ bypasses the ATP synthase; more heat is generated
Metabolic rate will increase in oder to compensate for the less amount of ATP being made (more fatty acid oxidation)
Very easy to overdose on it because if used as a weight loss drug, the therapeutic dose is very close to the toxic dose (now banned in U.S.)
Chloroplast vs. Mitochondria
Light reactions of photosynthesis by chemiosmosis, using an ETC, H+ gradient, and ATP synthases.
Chloroplast has more compact spece
Mitochondria permeable to water
Chloroplast
2 pH unit difference
100x more H+ in thylakoid lumen vs. stroma