Lecture 08 2024 09 26 Ch12 p2
Oxidative Phosphorylation Overview
Complexes Involved in Oxidative Phosphorylation:
Complex I: NADH dehydrogenase
Catalyzes the reaction: NADH → NAD+ + H+ + e-
Initiates the electron transport chain by receiving electrons from NADH, contributing to proton pumping.
Complex II: Fumarate reductase
Converts fumarate to succinate and participates in the Krebs Cycle by facilitating the transfer of electrons.
Complex III: Cytochrome bc1 complex
Plays a vital role in the transfer of electrons from ubiquinol to cytochrome c; involved in pumping protons into the intermembrane space.
Complex IV: Cytochrome c oxidase
Reduces molecular oxygen to water while transferring electrons; this is the last enzyme of the electron transport chain.
Complex V: ATP synthase
Enzyme responsible for ATP production, catalyzing the conversion of ADP and inorganic phosphate (Pi) to ATP.
Electron Transport Chain (ETC)
H+ Pumping: Protons (2H+, 3H+) are pumped into the intermembrane space (IMS) during electron transfer, generating a proton gradient essential for ATP production.
Learning Objectives
Role of Accessory Proteins: Understand how accessory proteins assist in energetic processes and contribute to complex assembly, stability, and function.
Glycolytic vs. Lipolytic Reactions: Differentiate between glycolysis (the breakdown of glucose) and lipolysis (the breakdown of fats), recognizing their pathways and energy outputs.
Energetic Steps in the Kreb's Cycle: Identify key steps such as the conversion of pyruvate to acetyl-CoA and the multiple reactions leading to the production of NADH, FADH2, and GTP.
Proton Motive Force: Grasp the concept of proton motive force in driving ATP synthesis through chemiosmosis.
ATP Synthase Function: Describe the structural components of ATP synthase and explain how it harnesses energy from the proton gradient to synthesize ATP.
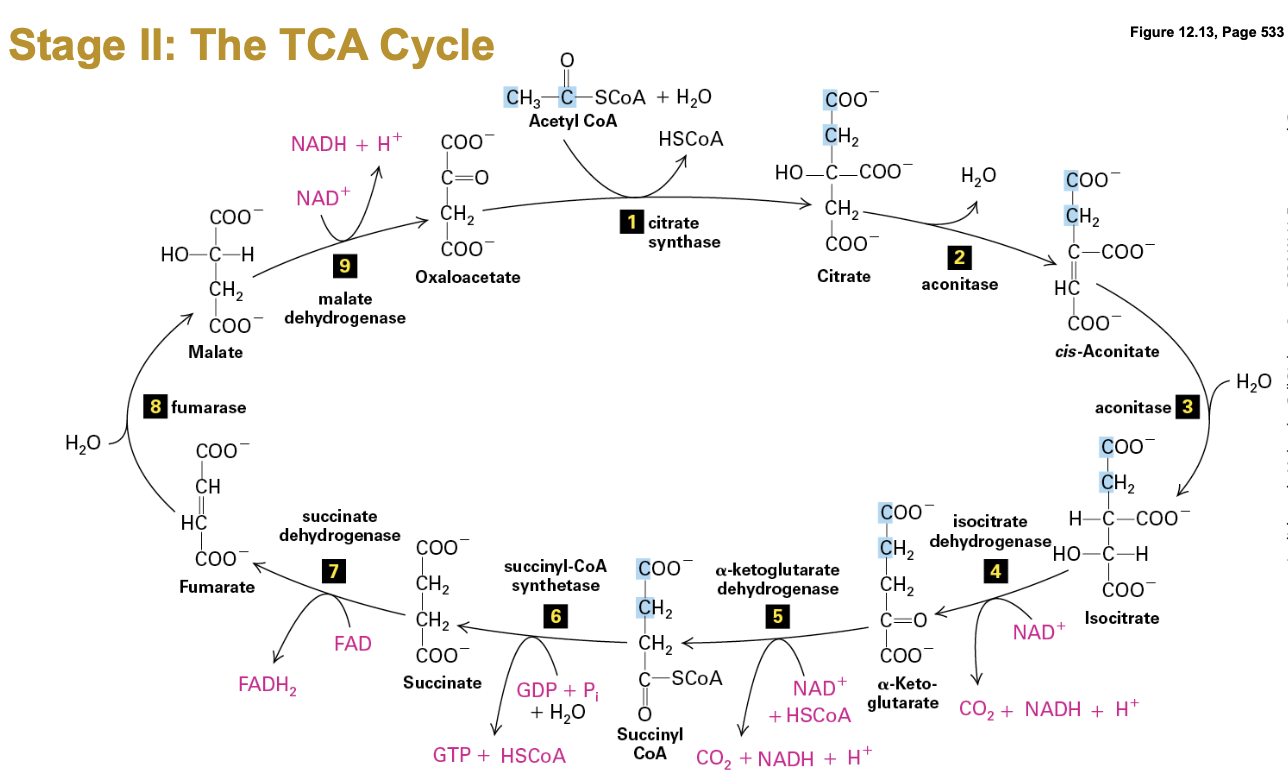
Stage II - The TCA Cycle
General Overview: Also known as the Tricarboxylic Acid Cycle or Citric Acid Cycle, this crucial metabolic pathway occurs in the mitochondrial matrix, where acetyl-CoA is oxidized.
Initiation: Pyruvate is transported from the cytosol to the mitochondrial matrix, where it undergoes decarboxylation to form acetyl-CoA. This step releases CO2 and generates NADH, a high-energy electron carrier.
Step 1: Conversion of Pyruvate to Acetyl-CoA. 3 carbon sugar is decarboxylated (CO2) and sugar harvested (NADH)
Acetyl-CoA is fed into a cyclical enzymatic pathway. One Acetyl-CoA gives a turn of the wheel.
Oxidation of the sugar is completed plus energy harvest (NADH, FADH2, GTP)
This stage also involves 8 enzymes, one part of the ETC
Continued TCA Cycle
Cyclical Pathway: Acetyl-CoA enters the cycle, undergoing a series of enzymatic reactions. Key intermediate products include citrate, alpha-ketoglutarate, succinate, and malate.
Energy Harvesting: Each cycle processes one acetyl-CoA, producing NADH, FADH2, and GTP that will later feed into the electron transport chain to generate more ATP.
Enzymatic Utilization: The cycle utilizes eight enzymes, each playing a specific role in facilitating the conversion of metabolites, ultimately contributing to energy production and metabolic processes.
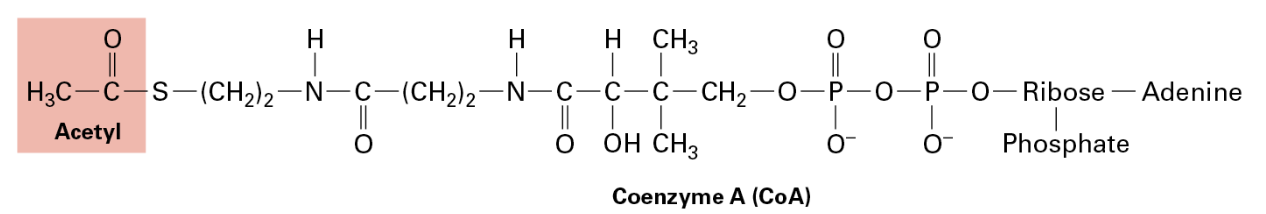
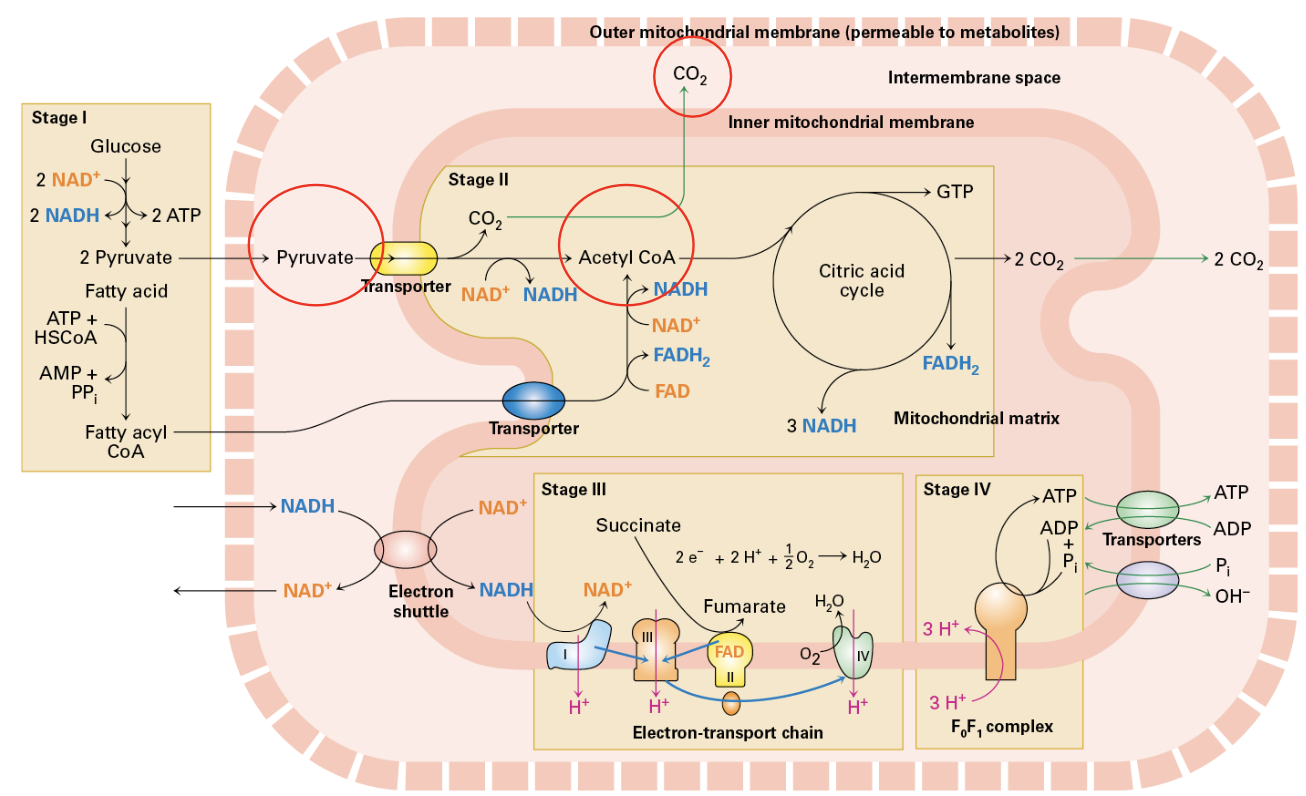
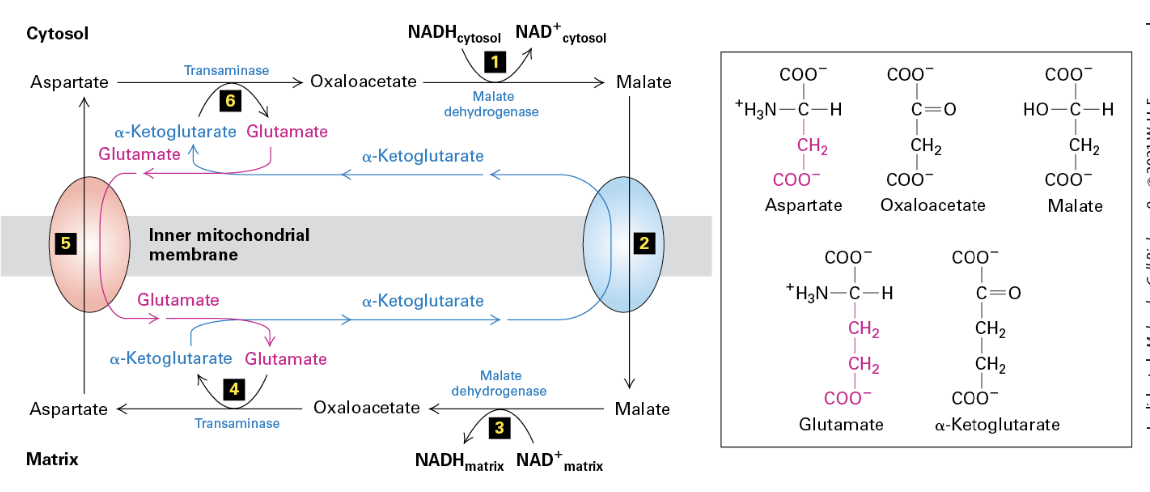
Feeding Mitochondria
Role of NADH: NADH, generated in glycolysis and the TCA cycle, enters mitochondrial metabolism, serving as an electron donor.
Transamination Reactions: These key processes allow for the conversion of amino acids, indicating integration of the amino acid pool into the Krebs Cycle, specifically through the conversion with oxaloacetate and malate.
Beta Oxidation
Energy Source from Fat: Beta-oxidation is the metabolic pathway that breaks down fatty acids to acetyl-CoA, serving as a significant energy source during prolonged fasting or intense exercise.
Fat Storage: Fat is stored as triacylglycerol, a water-insoluble compound that enables efficient energy storage and transportation within the body.
Fatty Acid Oxidation
Process Overview: Fatty acids are converted to fatty-acyl CoA to facilitate their transport into the mitochondrial matrix for oxidation.
Oxidation Mechanism: Fatty-acyl CoA undergoes cyclic oxidation, yielding acetyl-CoA and generating NADH and FADH2, which power ATP production through the electron transport chain.
Peroxisomes and Energy
Oxidative Reactions: Peroxisomes play a key role in oxidative reactions, particularly in the metabolism of very-long-chain fatty acids and the detoxification of hydrogen peroxide. Peroxisomes are protein dense.
Beta-Oxidation Location: While some beta-oxidation occurs in peroxisomes (in other animals), the majority happens in mitochondria (for humans), highlighting the organelle's importance in energy metabolism.
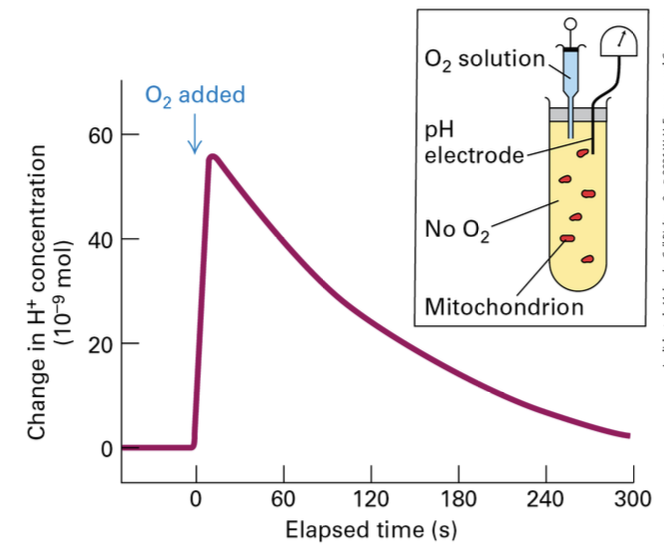
Experimental Evidence
Functionality of Mitochondria: Isolated mitochondria have been used to demonstrate the functionality of electron transport chain components, essential for testing hypotheses regarding oxidative phosphorylation.
pH Stability: Observations of pH stability at rest even in the presence of NADH.
[H+] levels increase with addition of O2 , which correlates with proton pumping into the intermembrane space, establishing the proton gradient necessary for ATP synthesis. Once O2 is used up H+ slowly moves back into the matrix.
Prosthetic Groups
Definition: Prosthetic groups are non-peptide molecules that assist in the function of proteins; examples include heme in hemoglobin and in myoglobin (muscle) to coordinate oxygen (O2)
Structure: They come in various structural forms, here vs Fe-S clusters.
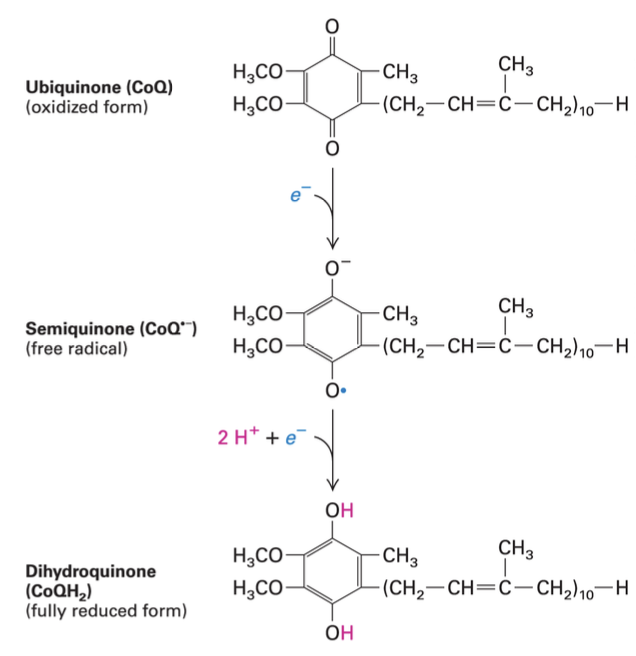
Ubiquinone (CoQ)
Role in Mitochondria: Ubiquinone is a hydrophobic molecule functioning primarily as an electron and proton acceptor, facilitating electron transfer between complexes. It resides in the inner membrane of the mitochondria.
Mechanism: It accepts two electrons and protons which helps prevent hydrophobicity issues during electron transport, reinforcing the functionality of the electron transport chain.
Electron Transport Chain
Overview: The pathways from NADH and succinate lead to ATP production, with detailed explanation of electron movement through various cytochromes and complexes, highlighting the critical interactions involved in energy production.
Proton Translocation
Mechanism: Complexes I, III, and IV are integral to the movement of protons from the mitochondrial matrix to the inter membrane space, a process enhanced by the coupled electron flow, contributing to the proton gradient vital for ATP synthesis.
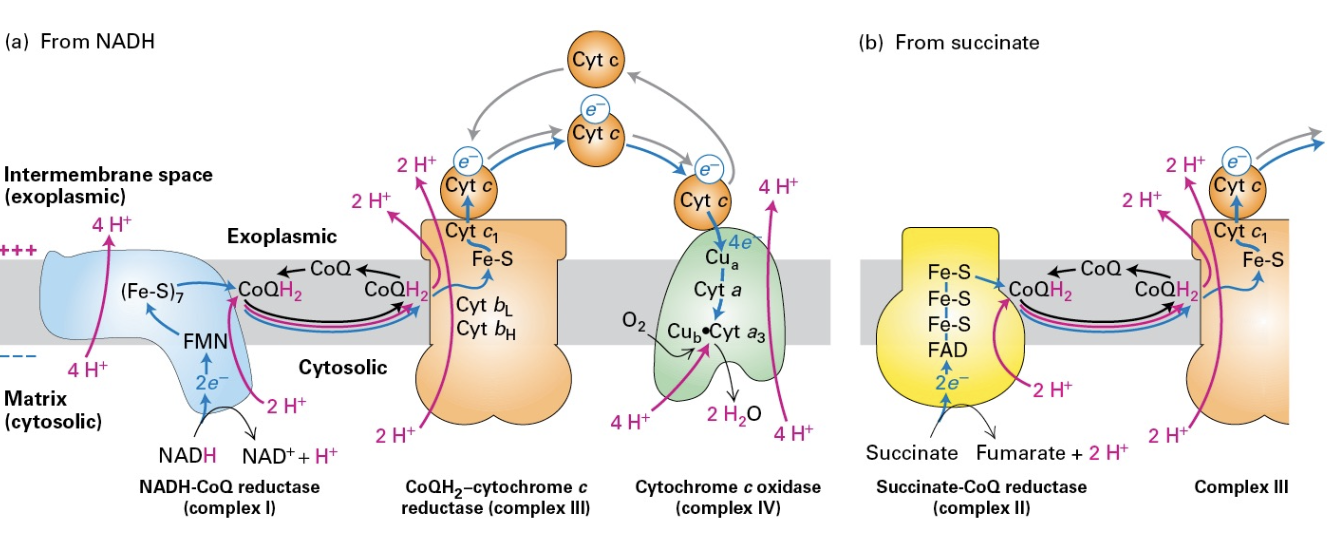
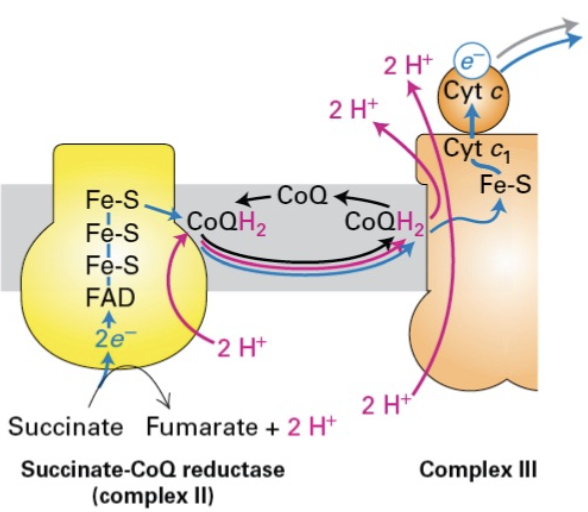
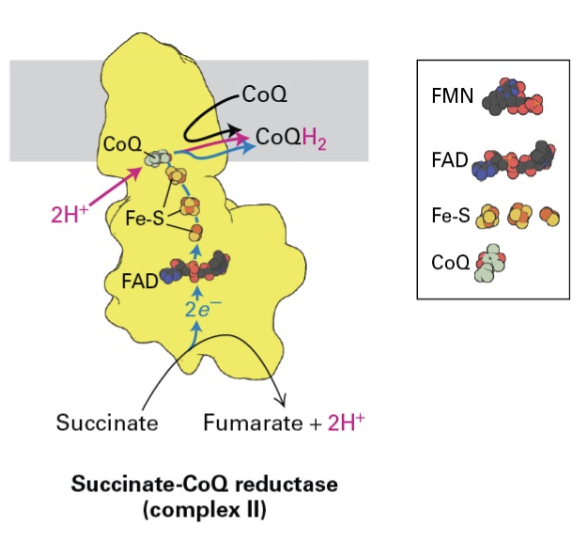
Complex II: Succinate-CoQ Reductase
Function: Succinate-CoQ reductase is involved in the Krebs cycle. Although it does not transport protons, it aids in electron transfer and reduction of CoQ.
Succinate dehydrogenase is oxidation can reduce FAD to FADH2 but cannot move protons across the membrane… Protons are picked up from the matrix by CoQ to form CoQH2.
complex II feeds complex III
Prosthetic Groups in Complexes
Comparative Analysis: Examining the structure and function of prosthetic groups in complexes I and II demonstrates their role in electron transport mechanics and enzyme functionality.
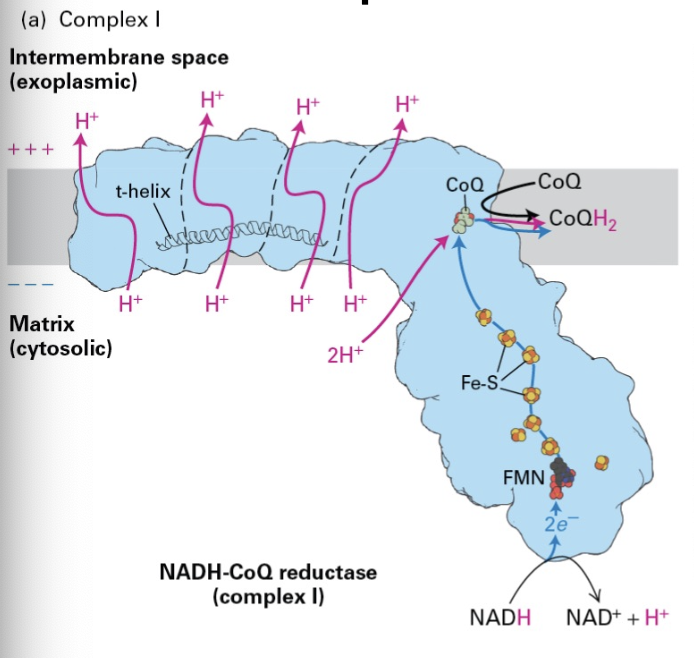
Complex I Function
Key Role: Complex I's primary role is to reduce NADH and translocate protons, thereby contributing substantially to the establishment of the proton motive force essential for ATP production.
Complex I stripds NADH of electrons. Electrons are transferred from the FMN prostheic group to Fe-S prosthetic groups finally to CoQ to form CoQH2.
Movement of e’ across the complex causes 4 protons to be moved across the complex into the iMS, 2 more are attached to CoQ.
Complex II Function
Low Energy Interaction: Complex II reduces FAD to FADH2 but lacks the ability to translocate protons; it instead engages CoQ, linking it to the electron transport pathway.
Succinate-CoQ Reducatse is part of the Krebs Cycle. Instead of NADH, the lower energy reaction is enough to reduce FAD to FADH2 but not translocate protons.
FADH2 —> FeS —> CoQH
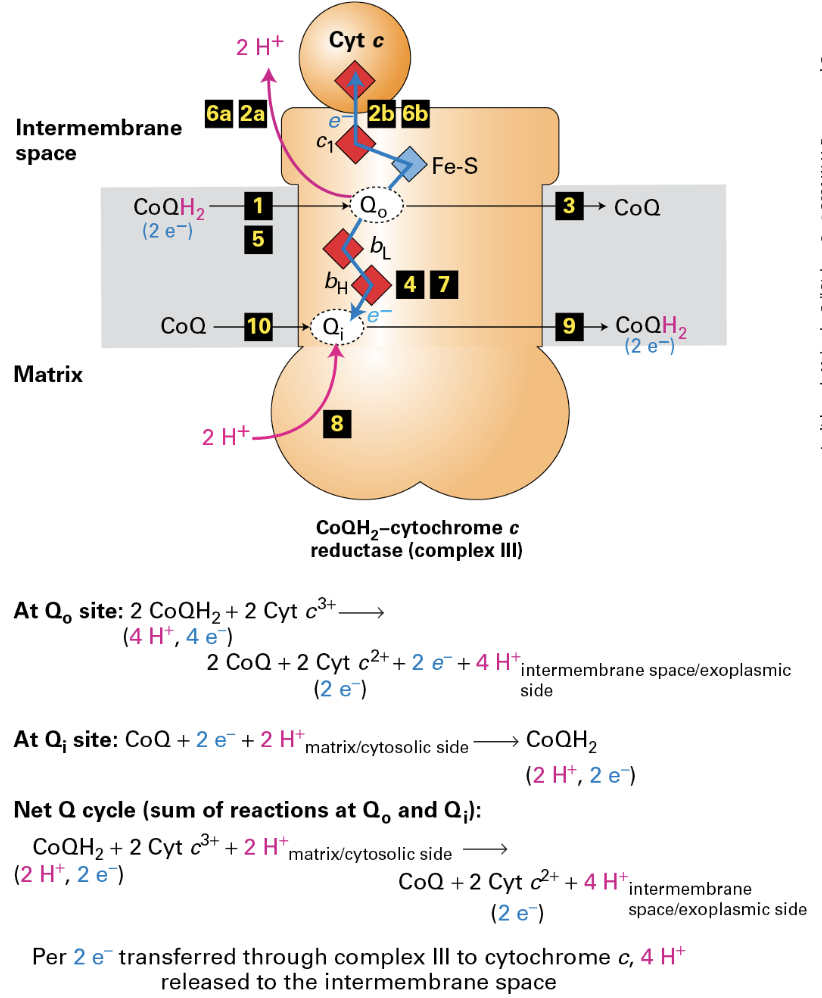
Complex III: The Q Cycle
Mechanism: Oxidation of CoQH2 involves a complex interaction of electron transfer to cytochrome c, with concurrent translocation of H+ ions across the membrane, crucial for maintaining the gradient used by ATP synthase.
CoQH2 is oxidixed feeding one electron to Cytochrome c and the other to reduce CoQ
When 2 CoQH2 are oxidized, 2 e- are fed to cytochrome c and 4 H+ are translocated.
The Q cycle allows recycling and maximum energy harvest at Complex III per 2e’
Energy Harvesting
Process: Increasing reduction potential through the electron transport chain significantly contributes to both proton translocation and the eventual production of water at the end of the chain.
In the ETC, the movement of high energy electrons moves stepwise allowing energy harvest as proton translocation events.
Electron Sink: ½ O2 and 2 H+ from the matrix to form metabolic water.
Supercomplexes
Efficiency: The concept of supercomplexes suggests that the aggregation of ETC complexes enhances the efficiency of metabolite exchange and accelerates the overall process of oxidative phosphorylation.
Cardiolipin is an important factor for supercomplexes appear. It is also known as diphosphatidyl glycerol.
Importance: Cardiolipin is essential for the structural stability of supercomplexes; it is located in the inner mitochondrial membrane and is linked to processes such as mitophagy (mitochondrial eating) ensuring mitochondrial health.
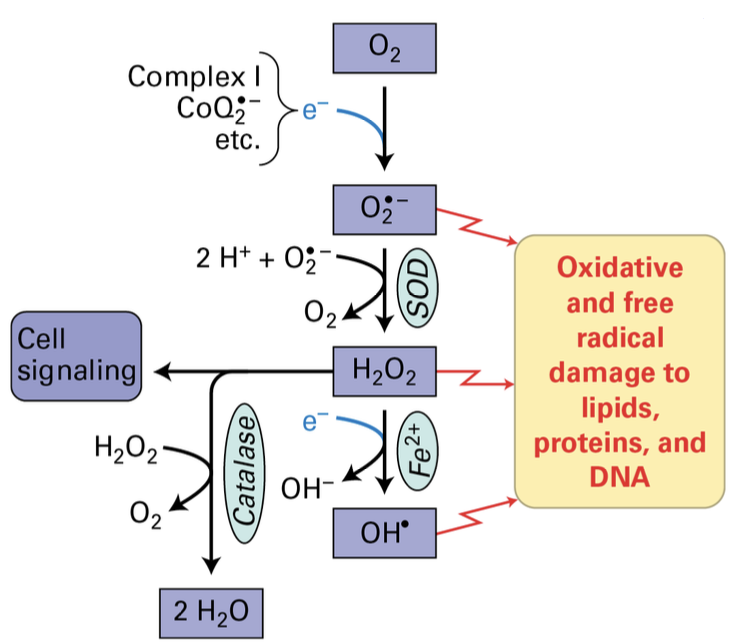
Reactive Oxygen Species (ROS)
Production Mechanism: The generation of reactive oxygen species occurs during the electron transport process, potentially leading to cellular damage if not properly regulated, mitigated by enzymes such as superoxide dismutase (SOD).
Oxidation reactions in the ETC have a small tendency to form reactive oxygen species (ROS)
ROS are destructive to molecules and systems if left unchecked
SOD exists to detoxify superoxide anion to form peroxide.
Catalase detoxifies H2O2 to form water
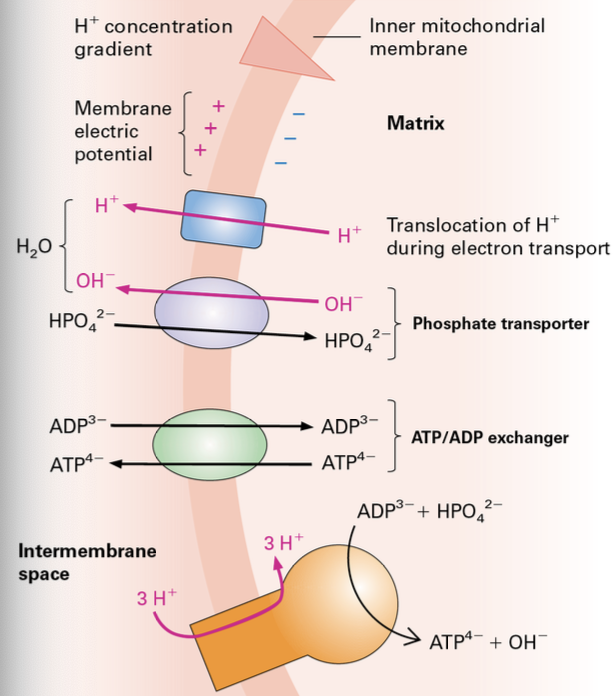
Proton Motive Force
Chemiosmotic Hypothesis: This hypothesis outlines how the established proton gradient across the inner mitochondrial membrane drives ATP synthesis, validated by experimental evidence demonstrating the linking of electron transport to ATP generation.
In 1961 Peter Mitchell proposed that proton motive force was the way that mitochondria made energy, called, Chemiosmatic Hypothesis.
Different in protons would drive the activity of the ATPase to form energy.
ETC Across Domains
Diversity: The diverse presentations of the electron transport chain across various life forms highlight functional similarities and adaptations, providing insight into the evolutionary conservation of this essential metabolic pathway. Mitochondria have ETCs in their inner membrane.
All domains of life have members with systems that contain some version of the ETC. Single cell bacteria ETCs exist at the cell membrane and they pump out H+ from the cell.
Thylakoid membranes use light energy to drive the movement of h+ into the inner most thylakoid lumen to drive ATP Synthase at thylakoid membranes
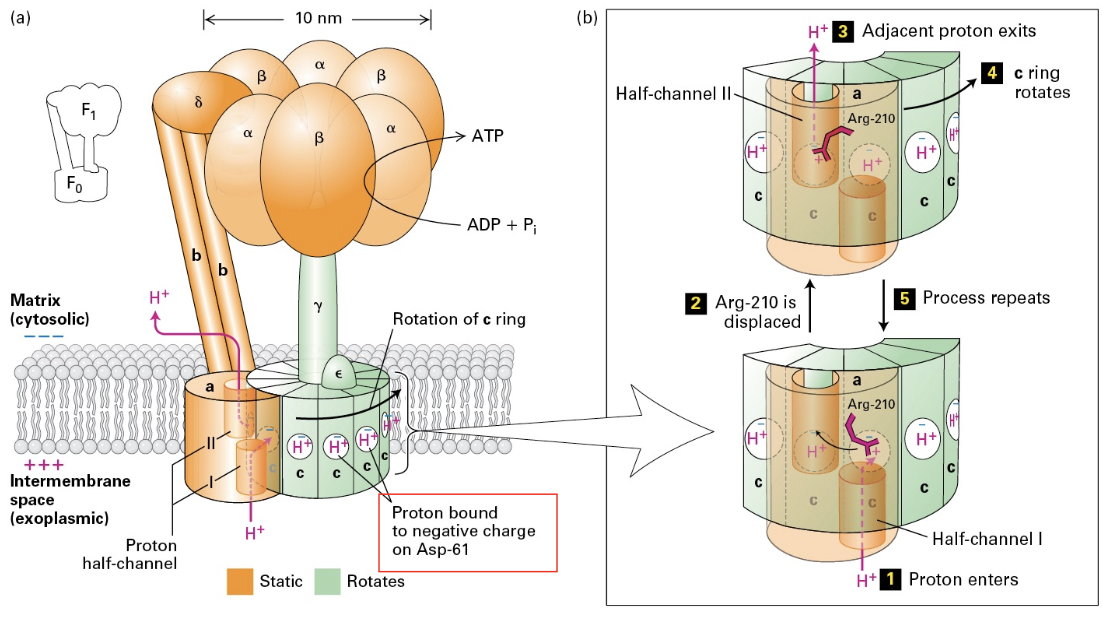
ATP Synthase Mechanism
Structure and Function: A structural analysis of ATP synthase elucidates the mechanisms of ion and proton movement that facilitate ATP synthesis and highlight the enzyme's intricate design.
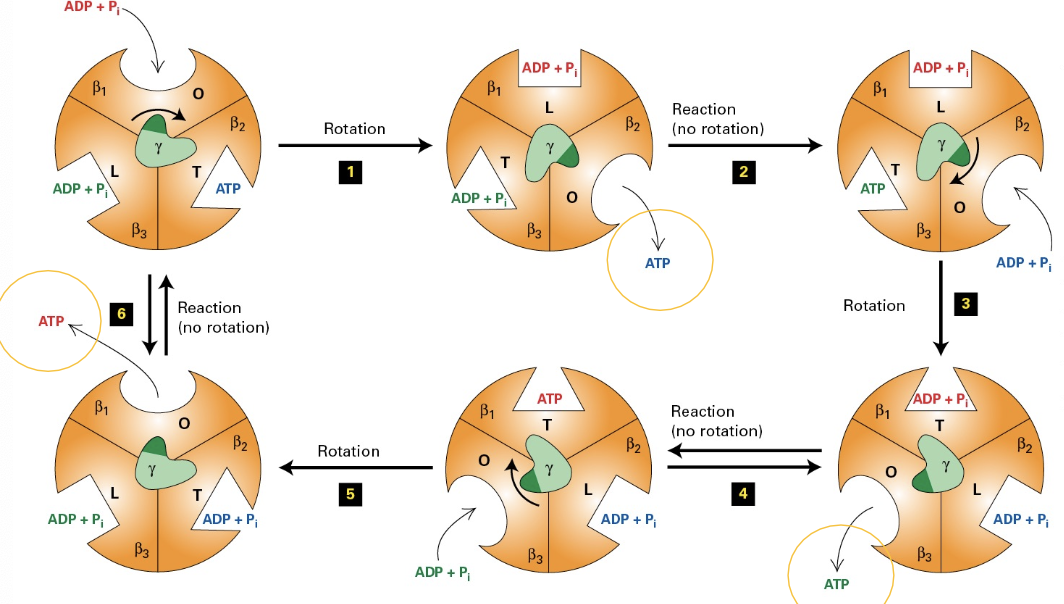
ATP Synthase Changes
Mechanism of Energy Generation: Detailed exploration of the ATP generation mechanism describes the changes that occur in the beta subunit states during cycles of ATP synthesis, illustrating the dynamic functionality of ATP synthase.
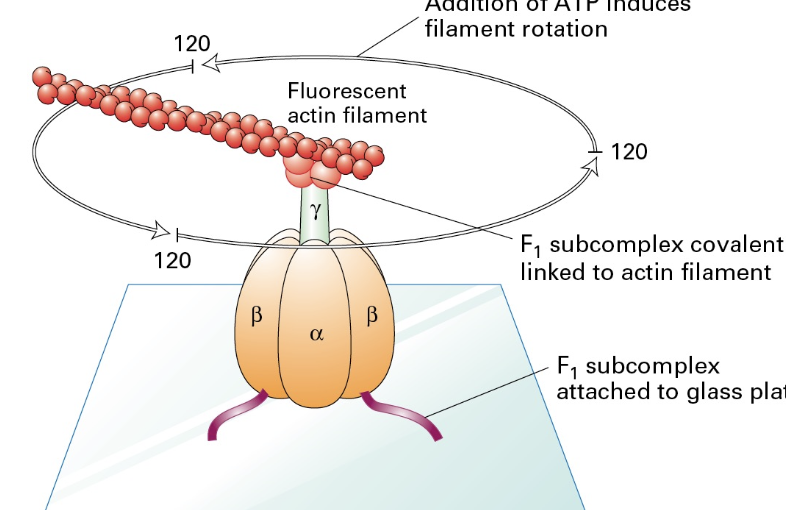
F1 Subunit Activity
Function: Isolation studies have confirmed the F1 subunit's role as an ATPase and explored its mechanical interactions with actin and other substrates, emphasizing its critical involvement in ATP production.
Isolation of the F1 subunit members shows chemical mechanical interplay.
it is isolated F1 = ATPase
Attached to an actin filament + ATP in the solution = rotation
ATP Turnover
Dynamics of Hydrolysis: Daily ATP hydrolysis rates far exceed the body's stored levels of ATP, illustrating the dynamic nature of energy exchange occurring at cellular membranes.