AP Biology unit 1: chemistry of life
1.1 structure of water and hydrogen bonding
atoms: the smallest fundamental unit of matter. there are 120 different kinds of atoms (elements). Biology is made of 4 major (CHON) -10 minor and -30-50 trace elements.
energy & atoms:
Energy interacts with atoms in different ways.
energy holds e’s to the nucleus.
when atoms absorb energy, e’s move to higher energy levels.
the movement of e’s back to lover energy levels releases energy (as EM radiation)
the move energy shells you have the mover energy is absorbed in that system. vise versa.
atoms bonds
bonding is accomplished by electrons interacting between atoms ( due to balance considerations).
2 major kinds of bonds hold atoms together.
ionic bonds: transfer e’s, not many possible combinations. kind of boring.
covalent bonds: sharing of e’s functionally infinite combinations. (ex: glucose, DNA) are covalently bonded.
not all bonds are created equal.
polarity: the unequal sharing of electrons in a covalent bond. leads to unequal distribution of charge in the molecule.
hydrogen bonds: The strongest attraction between the most polar molecules. common in biological systems.
polar molecules are attracted to other polar molecules
LIKE attracts LIKE
bonds determine shape and shape determines function. structure and function are always related. Usually seen in proteins. Molecules have different folds, bends, and shapes.
all chemical reactions result in the breaking and forming of bonds. in any reaction, mass, energy, and charge are conserved. matter can not be created or destroyed.
compounds and emergence: the properties of a compound can be very different frpm the properties of the elements that make them emergence: increasing levels of complexity in a system can demonstrate novel properties not seen in the levels below them.
Radioactivity: Nuclei are unstable in the atoms. Radioactive atoms spontaneously emit high-energy particles until stability is reached. excess radiation is damaging to biological systems. why? because radiation is used in biology as molecular labels.
water
The chemistry of life is a solution-based chemistry. The majority of any organism is water.
water is one of the few substances that exists in all 3 phases at normal terrestrial conditions.
water’s unique properties: All of them are due to the polarity of water (and its resulting hydrogen bonds).
cohesion: sticking together (water to water)
adhesion: sticking to other things. (water on glass)
the cohesiveness of water gives it a very high surface tension.
helps photosynthesis. water travels up trees with cohesion and adhesion.
transpiration: the movement of water through trees.
water has a high specific heat: How much heat is absorbed/released before an increase/decrease in temperature.
evaporative cooling: how we cool our body down.
ice floats because solid water is less dense than liquid water.
Water is a great but not “universal solvent”
water dissociates because it is so polar, that water can easily break apart. This produces a hydronium (H3)=) and hydroxide (OH-). in pure water, the concentration of these ions is equal.
Acids: donate protons: [H3O+] > [OH-]
Bases: accept protons: [H3O+] < [OH-]
PH: a measure of acidity
biological systems can only tolerate a narrow of PH. Ocean is pretty neutral and our body is pretty acidic. Extreme variations in pH have bad effects at all levels of organization.
Carbon
Carbon is abundant and versatile. Carbon is tetravalent, it makes 4 bonds to get stable, which leads to infinite variety. Everything has carbon.
Isomers: Molecules with the same molecular formula, but different structures. any molecule more complex than propane (C3H8) has at least one isomer. An example of an emergent property.
3 kinds of isomers
Structural- same formulas, different order.
Cis-Trans- same formula, different positioning around a double bond.
Enantiomers- same formula, mirror image positioning around a central carbon. just reflected, but still changes the function.
functional groups modify the properties of organic molecules
Hydroxyl: (R/ -OH) (O-H) polar, dissolves in water, and alcohol. example: Ethanol.
Carbonyl: (C=O) polar, dissolves in water example: acetone and propanal
Carboxyl: (R-COOH) not polar or nonpolar, just charged and acidic. can donate H+ . example: Acetic acid.
Amino: (NH2) charged, basic can pick up an H+ from the surrounding solution. example: Glycine.
Sulfhydryl: (-SH) polar, dissolves in water, neither acidic nor basic. example: cysteine.
Methyl: nonpolar, does not dissolve in water, example: methyl cytidine.
Phosphate: Acidic and negatively charged, not polar or nonpolar. example: Glyceral phosphate
structure (hydroxyl, carbon) function, polar or nonpolar, acidic/basic, or charged
Macromolecules
made up of few, common atoms.
accomplish all life functions.
put together in a special way.
can be incredibly complex.
4 main kinds: Carbohydrates, lipids, proteins, and nucleic acids.
building macromolecules: except for lipids, macromolecules exist in two forms
monomer- the simplest unit
polymer-a large molecule made of repeating monomers.
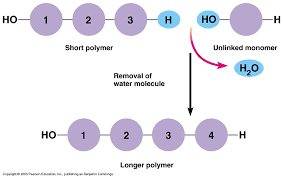
the movement between monomers and polymers is facilitated by adding/removing water.
Dehydration Synthesis: Builds more complex molecules from smaller ones by removing 2 H and 1 O and replacing it with a bond ➡ water is produced.
Anabolic: build complexity
endergonic: requires energy.
requires enzymes.
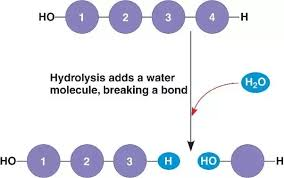
Hydrolysis: reverse of dehydration synthesis.
-lysis = “breaking”
water is needed
Catabolic: reduces complexity
exergonic: releases energy
requires enzymes
Carbohydrates
starches and sugars
made of C & H & O (1:2:1 ratio in monomers)
used for short-term energy storage and structure.
monomer = “monosaccharides”
different sugar monomers can have different numbers of carbon
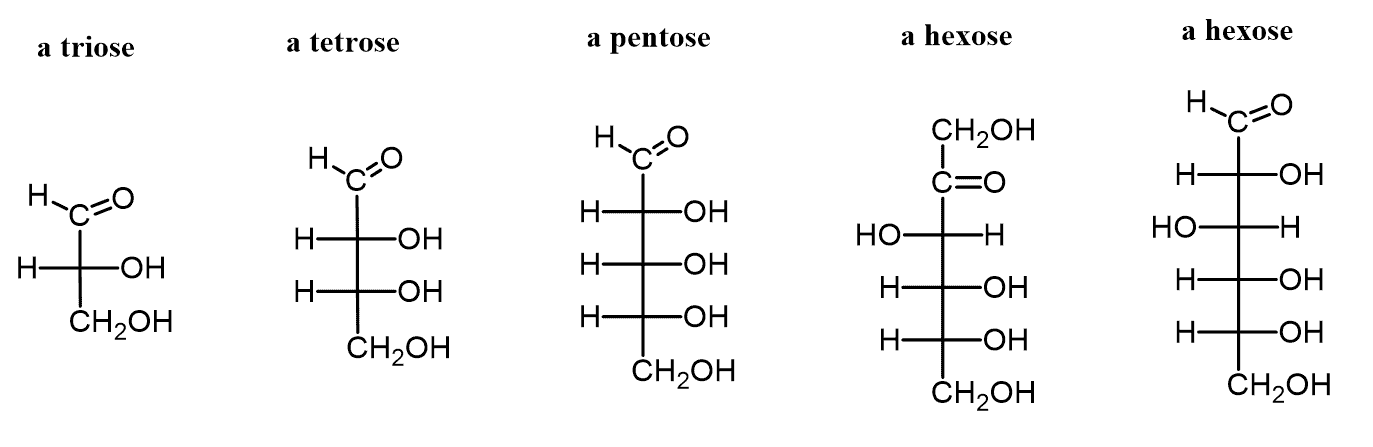
Monosaccharides and Disaccharides: Major carbohydrates used for energy.
hexose sugars are most common: glucose, galactose, and fructose. they are typically shown as carbon rings.
combine 2 by dehydration synthesis and you get a disaccharide.
Glucose + Glucose = maltose “malt sugar”
Glucose + Fructose = sucrose “table sugar”
Glucose + Galactose = lactose “milk sugar'“
Polysaccharides
Polysaccharides: Massive polymers of sugar
Glucose polymers have two main functions in organisms
1. Energy storage: polysaccharides are great for short-term storage of energy. In plants, amylose “starch: is the major energy storage polysaccharide.
animals use glycogen for energy storage.
2. Structural support: Cellulose is the major component of plant-like cell walls.
The difference between starch and cellulose is in the linkages between glucose units. starch = alpha linked. cellulose = beta linked.
The problem with herbivores is that they need to digest cellulose.
Animals lack the enzymes necessary to break beta linkage. Several strategies are employed.
Other Carbohydrates
Chitin: A modified polysaccharide. Used in fungi cell walls, arthropod exoskeletons, and dissolving stitches.
Peptidoglycan: another modified polysaccharide. Used in bacterial walls.
Lipids
Fats, oils, waxes
Made of C, H and O
used for long-term energy storage and insulation
no polymers.
3 major groups: triglycerides, phospholipids, & steroids.
triglycerides: Made of one glycerol and 3 fatty acids.
connected by dehydration synthesis x 3 (ester linkages)
Saturated vs Unsaturated:
refers to the bonding of carbon in the fatty acids.
Saturated = no double dons between carbons.
Unsaturated = at least one double bond.
influences shapes which influence properties.
Oils vs Fats
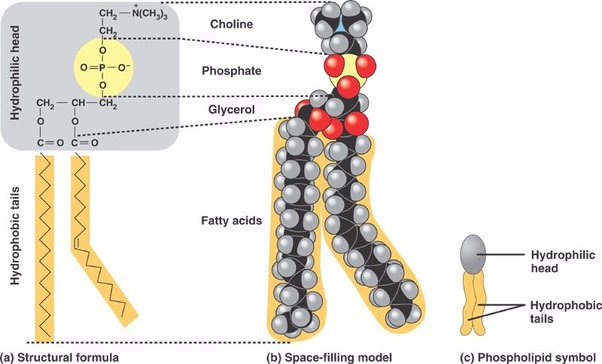
Phospholipids
modified triglycerides. replace fatty acid with a phosphate.
makes the molecule have a polar and non-polar region “amphipathic”
the major component of cell membranes (arranged as a “bi-layer”)
Steroids
1 class of hormones & cholesterol
notable structure = fused rings
The presence of different functional groups leads to different functions
Proteins
The most complex biological molecules.
Made of C,H,O,N and a little S
used to accomplish all life functions
all proteins are polymers of amino acid monomers
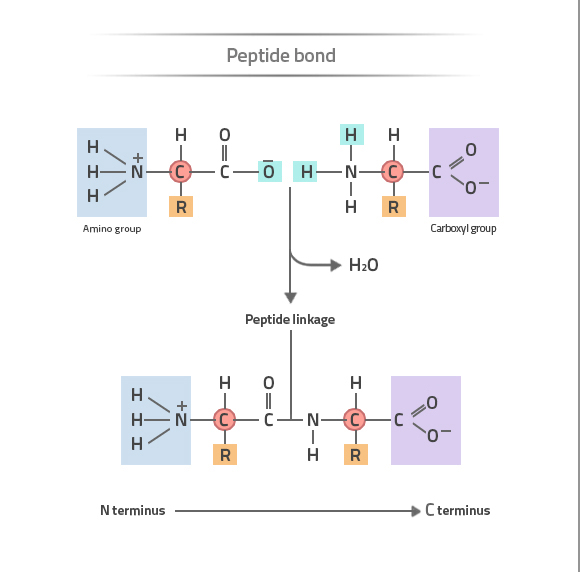
amino acids are joined by “peptide bonds”
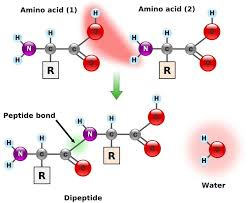
amino acids
There are 21 known amino acids used in biological systems
all amino acids contain an amino and carboxyl group, bonded to a central “alpha” carbon.
every amino acid differs in the structure of a variable group (symbolized as R) bonded to the alpha carbon
the structure of the R-group varies widely.
chains of amino acids have a directionality, with an amino end (“N-terminus”) and a carboxyl end (“C-terminus”)
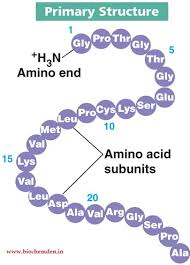
Protein structure: because of the diversity of amino acids, proteins have very complex 3-D structures. Generally, we can consider 4 levels of protein structure.
primary structure: The sequence of amino acids in one polypeptide chain. how does it happen? peptide bonds between amino acids. how does the celled know the order of amino acids? transcription and translation
secondary structure: Regular, repeating 3D structures found in all polypeptide chains.
Hydrogen bonding between atoms in the CN backbone of the polypeptide (no R-groups involved).
tertiary structure: the specific 3D shape of a particular polypeptide chain (aka the “confirmation”). How does it happen? interactions between R-groups and the local environments of the cell cause more unique folds.
quarternary structure: The specific 3D shape of any protein that is made of more than one polypeptide chain (many are). the only optional level of structure. how does it happen? the overall structure when multiple chains form a functional protein. why do some proteins consist of more than 1 polypeptide chain? they have different jobs.
protein function: proteins are responsible for all cell life activities (and by extension, the organism, and population).

Example: sickle cell anemia: one example of the relationship between protein structure and organismal physiology. Hemoglobin carries oxygen in your red blood cells.
Denaturation: Change to the structure of proteins. function changes too. if the conformation is altered, the function of the protein will also be altered. what sorts of conditions can denature proteins? temperature change and pH change.
visualizing proteins: computer modeling software is frequently used to help visualize important structural aspects.
nucleic acids
made of C,H,O, N & P
2 kinds of nucleic acids: DNA and RNA
all nucleic acids are polymers of nucleotides
nucleotides consist of a phosphate, a pentose sugar, and a nitrogenous base.
4 bases in DNA and RNA
DNA vs RNA: while similar in structure, there are a few key differences which lead to major differences in function. pentose: DNA = deoxyribose RNA = ribose bases: DNA = adenin, thymine, guanine, cytosine RNA = adenine, uracil, guanine, cytosine
strands: DNA = 2 strands (double helix) RNA = 1 strand (single helix)
DNA: serves 2 function in all life on earth.
stores information about the primary structure of proteins, and the sequences of RNA molecules.
is heritable
DNA structure:
2 chains of covelently bonded nucleotides, from sugar to phosphate (“phosphodiester bonds”)
chains are bonded to each other by hydrogen bonds between N Bases
A bonds to T : G bonds to C
Purine (A,G) always opposite Pyrimidine (T,C)
discovery of DNA structure:
Watson & Crick - published the paper
Maurice Wilkins & Rosalind Franklin - did the X-Ray diffraction work
RNA: Serves many functions for life
transmits and translates DNA information into protein
many enzymes and regulatory functions.
1 kind of DNA, 15 types of known RNA at current (3 main types)
RNA structure
less table than DNA
1 strand, but base-pairing can occur (A bond to U)
information in biology
biological systems are process matter, energy and information
the information stored in DNA moves to RNA before some of that information finally directs the construction of proteins.
this is knows as the “central dogma” of molecular biology,
it will be the underpinning of most important biological advances during your lifetime (it already is)
Make sure you can
identify the structure of the monomers and polymer of the four major classes of macromolecules.
diagram the synthesis and hydrolysis of carbohydrates and polypeptides
explain the biological functions of all the molecules discussed in these notes.
explain the emergence of all four levels of protein structure.
describe the role of general role of nucleic acids in living systems.
 Knowt
Knowt